Abstract
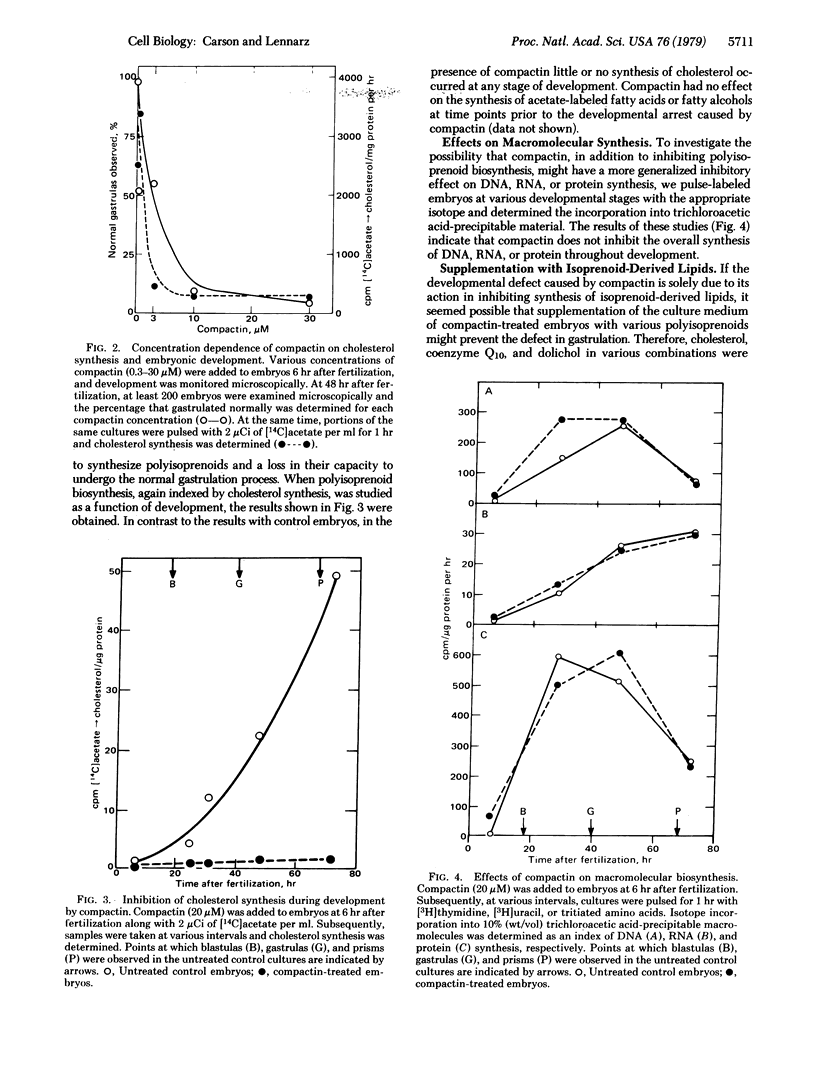
Compactin, a potent inhibitor of polyisoprenoid biosynthesis, induces abnormal gastrulation during sea urchin development at concentrations that have no effect on earlier embryonic development or on macromolecular synthesis. Three lines of evidence suggest that the developmental lesion caused by compactin results from inhibition of dolichol biosynthesis and a concomitant inhibition in the biosynthesis of the oligosaccharide chains of N-linked glycoproteins. (i) Embryos cultured in the presence of compactin gastrulate normally when supplemented with dolichol alone, whereas supplementation with cholesterol or coenzyme Q or both does not prevent the compactin-induced developmental lesion. (ii) Exogenously supplemented [3H]dolichol is incorporated into a compound with the chromatographic properties of oligosaccharide-pyrophosphoryldolichol. (iii) Embryos cultured in the presence of compactin exhibit a decreased capacity to synthesize mannose-labeled glycolipids and N-linked glycoproteins. This decrease in synthesis is abolished if the embryos are cultured in the presence of dolichol along with compactin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown M. S., Faust J. R., Goldstein J. L., Kaneko I., Endo A. Induction of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in human fibroblasts incubated with compactin (ML-236B), a competitive inhibitor of the reductase. J Biol Chem. 1978 Feb 25;253(4):1121–1128. [PubMed] [Google Scholar]
- D'Agnolo G., Rosenfeld I. S., Awaya J., Omura S., Vagelos P. R. Inhibition of fatty acid synthesis by the antibiotic cerulenin. Specific inactivation of beta-ketoacyl-acyl carrier protein synthetase. Biochim Biophys Acta. 1973 Nov 29;326(2):155–156. doi: 10.1016/0005-2760(73)90241-5. [DOI] [PubMed] [Google Scholar]
- Gustafson T., Wolpert L. Cellular movement and contact in sea urchin morphogenesis. Biol Rev Camb Philos Soc. 1967 Aug;42(3):442–498. doi: 10.1111/j.1469-185x.1967.tb01482.x. [DOI] [PubMed] [Google Scholar]
- Heifetz A., Lennarz W. J. Biosynthesis of N-glycosidically linked glycoproteins during gastrulation of sea urchin embryos. J Biol Chem. 1979 Jul 10;254(13):6119–6127. [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Comparative aspects of glycoprotein structure. Annu Rev Biochem. 1976;45:217–237. doi: 10.1146/annurev.bi.45.070176.001245. [DOI] [PubMed] [Google Scholar]
- Kozhina V. P., Terekhova T. A., Svetashev V. I. Lipid composition of gametes and embryos of the sea urchin Strongylocentrotus intermedius at early stages of development. Dev Biol. 1978 Feb;62(2):512–517. doi: 10.1016/0012-1606(78)90232-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lucas J. J., Waechter J., Lennarz W. J. The participation of lipid-linked oligosaccharide in synthesis of membrane glycoproteins. J Biol Chem. 1975 Mar 25;250(6):1992–2002. [PubMed] [Google Scholar]
- Mills J. T., Adamany A. M. Impairment of dolichyl saccharide synthesis and dolichol-mediated glycoprotein assembly in the aortic smooth muscle cell in culture by inhibitors of cholesterol biosynthesis. J Biol Chem. 1978 Aug 10;253(15):5270–5273. [PubMed] [Google Scholar]
- Ono T., Kesado T., Awaya J., Omura S. Target of inhibition by the anti-lipogenic antibiotic cerulenin of sterol synthesis in yeast. Biochem Biophys Res Commun. 1974 Apr 23;57(4):1119–1124. doi: 10.1016/0006-291x(74)90812-2. [DOI] [PubMed] [Google Scholar]
- SPERRY W. M., WEBB M. A revision of the Schoenheimer-Sperry method for cholesterol determination. J Biol Chem. 1950 Nov;187(1):97–106. [PubMed] [Google Scholar]
- Schneider E. G., Nguyen H. T., Lennarz W. J. The effect of tunicamycin, an inhibitor of protein glycosylation, on embryonic development in the sea urchin. J Biol Chem. 1978 Apr 10;253(7):2348–2355. [PubMed] [Google Scholar]
- Waechter C. J., Lennarz W. J. The role of polyprenol-linked sugars in glycoprotein synthesis. Annu Rev Biochem. 1976;45:95–112. doi: 10.1146/annurev.bi.45.070176.000523. [DOI] [PubMed] [Google Scholar]