Abstract
OBJECTIVE
The purpose of this study was to demonstrate that digital polymerase chain reaction (PCR) enables rapid, allele independent molecular detection of fetal aneuploidy.
STUDY DESIGN
Twenty-four amniocentesis and 16 chorionic villus samples were used for microfluidic digital PCR analysis. Three thousand and sixty PCR reactions were performed for each of the target chromosomes (X, Y, 13, 18, and 21), and the number of single molecule amplifications was compared to a reference. The difference between target and reference chromosome counts was used to determine the ploidy of each of the target chromosomes.
RESULTS
Digital PCR accurately identified all cases of fetal trisomy (3 cases of trisomy 21, 3 cases of trisomy 18, and 2 cases of triosmy 13) in the 40 specimens analyzed. The remaining specimens were determined to have normal ploidy for the chromosomes tested.
CONCLUSION
Microfluidic digital PCR allows detection of fetal chromosomal aneuploidy utilizing uncultured amniocytes and chorionic villus tissue in less than 6 hours.
Keywords: aneuploidy, digital PCR, rapid prenatal diagnosis
The incidence of fetal aneuploidy and other chromosome abnormalities is approximately 9 per 1000 live births.1 It is difficult to estimate their true incidence among all pregnancies due to the strong association with fetal miscarriage and stillbirth. The prevalence of chromosomal abnormalities in clinically recognized early pregnancy loss is greater than 50%, and fetuses with aneuploidy account for 6–11% of all stillbirths and neonatal deaths.2 Aneuploidy rates increase with advancing maternal age, yet despite advances in noninvasive prenatal screening, diagnosis of fetal chromosomal abnormalities is the most common indication for invasive prenatal testing.2
Conventional cytogenetics is currently the gold standard for determining fetal karyotype. Fetal cells obtained from amniotic fluid or chorionic villi are cultured, and the karyotype is analyzed with condensed chromosomes during metaphase stage. While conventional cytogenetics can provide accurate information regarding chromosomal aberrations, it requires approximately 1–2 weeks for patients to obtain results. This time delay may result in both increased anxiety for expectant parents, and greater maternal morbidity should pregnancy termination be desired in the setting of abnormal results. Rapid and accurate molecular based detection of aneuploidy is thus highly desirable.
There have been several molecular diagnostic techniques developed for aneuploidy detection,3–5 but they tend to be labor intensive and some are allele dependent, so that the results depend on the underlying genetics of the population. We demonstrate here that digital polymerase chain reaction (PCR) enables rapid detection of fetal aneuploidy from uncultured amniocytes and chorionic villi. The results of the assay are obtained in 6 hours and are not allele dependent.
In conventional real-time PCR, one threshold cycle corresponds to a 2-fold change in copy number, making it exceedingly challenging to measure smaller changes,6 such as a 1.5-fold increase in number of a trisomic chromosome as compared to a normal disomic chromosome. Digital PCR is a method used to quantify the amount of nucleic acids by counting amplification from single molecules.7,8 In brief, a PCR reaction mixture containing a sample of DNA template is diluted and distributed into a large number of compartments such that, on average, there is less than 1 copy of template per compartment. PCR products are fluorescently detected. By counting the number of compartments that display fluorescent signals at the end of the PCR reaction, one can obtain the counts of the DNA template. Because digital PCR converts the exponential nature of PCR to linear signal, copy number changes less than 2-fold can easily be measured with high precision. In addition, unlike conventional real-time PCR, quantification with digital PCR is not affected by the efficiency of amplification.
Materials and Methods
Study design
Pregnant women presenting for clinically indicated amniocentesis or chorionic villus sampling (CVS) at the Lucile Packard Perinatal Diagnostic Center of Stanford University were offered enrollment. Patients were recruited between January and June 2008, and informed consent was obtained prior to each procedure. In cases of amniocentesis, 1–2 mL from the clinical sample was submitted separately for digital PCR analysis. If maternal blood was visually apparent, the first 2 mL of amniotic fluid were discarded. In the absence of obvious contamination, the first 2 mL were often retained, which was the case for many of the samples. The exact proportion for these cases was not tracked. In cases of CVS, 1–2 mg was submitted separately for digital PCR analysis. Both transabdominal and transvaginal CVS approaches were employed, and the decision to perform one rather than the other was based on placental location and operator preference.
Study samples were labeled with specially assigned coded numbers and submitted for digital PCR analysis. The rest of each specimen was submitted to the Stanford cytogenetic laboratory for routine fetal karyotyping. Digital PCR analysis was performed with blinding to patients’ personal information and without prior knowledge of the clinical karyotype results. Patients did not receive the digital PCR results but were notified of their cytogenetic karyotype results within 1 to 2 weeks as per Stanford University routine practice. The study was approved by the Stanford Institutional Review Board (IRB).
Procedures
A total of 40 samples, consisting of 24 amniotic fluid and 16 CVS samples, were processed. One twin pregnancy and 1 triplet pregnancy were enrolled. Amniotic fluid was centrifuged at 14,000 rpm. Supernatant was removed and the cell pellet was resuspended in phosphate buffered saline (PBS). Chorionic villi were suspended in PBS. Genomic DNA was extracted from amniotic fluid and chorionic villi with QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. DNA was eluted into 100 μL and 200 μL of buffer for amniotic fluid and chorionic villi samples, respectively.
Taqman PCR assays were designed to amplify 1 region on each of the following chromosomes: 1, 13, 18, 21, X, Y. Chromosome 1 was chosen to be the reference chromosome since it is not associated with any aneuploidy observed in ongoing pregnancies.9 The assay of chromosome 1 contained a probe labeled with a HEX fluorophore, while the assays for the target chromosomes (13, 18, 21, X, Y) each contained a probe labeled with a FAM fluorophore. The amplicon of each assay was chosen to lie outside of the regions with known copy number variation in healthy individuals.10 In particular, the amplicons of chromosomes 1, 13, and 18 cover ultraconserved regions,11 which are rarely found to be associated with copy number variation in healthy individuals.10 The amplicons were all 80–90 bp in length to reduce any amplification bias. The sequences of the primers and probes are listed in the Table, and were purchased from Integrated DNA Technology (Coralville, IA).
TABLE.
Sequences of primers and probes
| Chr | Gene | Location | Forward primer (5′ - 3′) | Reverse primer (5′ - 3′) | Probe (5′ - 3′) | 5′ label | 3′ label | Product size (bp) |
|---|---|---|---|---|---|---|---|---|
| 1 | EIF2C1 (u.c. 13)a | 1p34.3 | GTTCGGCTTTCACCAGTCT | CTCCATAGCTCTCCCCACTC | CGCCCTGCCATGTGGAAGAT | HEX | BHQ1 | 81 |
| 13 | MBNL2 (u.c. 356)a | 13q32.1 | CTCACCTATCCACAATGCAA | GGGATTCAAGCGAATTAACA | AGGTGCATCATGGGAACGGC | FAM | BHQ1 | 81 |
| 18 | EHZF (u.c. 422)a | 18q11.2 | CCAGCTGGTACTTGGAAGAG | TGTCGTATGTGGAGCCAAC | TCAGTGCCTGCCTGGTTCCC | FAM | BHQ1 | 87 |
| 21 | PRDM15 | 21q22.3 | ATGTTTCGCCAACTTCTGAG | AGAGCTATGGCACAAACCTG | TCCCAAACTCTCCTGCCCTGA | FAM | BHQ1 | 89 |
| X | Noncoding | Xp22.3 | GATGAGGAAGGCAATGATCC | TTGGCTTTTACCAAATAGGG | TGTTTCTCTCTGCCTGCACTGG | FAM | BHQ1 | 86 |
| Y | SRY | Yp11.3 | CGCTTAACATAGCAGAAGCA | AGTTTCGAACTCTGGCACCT | TGTCGCACTCTCCTTGTTTTTGACA | FAM | BHQ1 | 84 |
Ultraconserved element: annotation follows the online supplement18 (http://www.soe.ucsc.edu/~jill/ultra.html).
The concentration of extracted genomic DNA of each sample was estimated by quantitative real-time PCR with Taqman PCR assay designed for the locus on chromosome 1. A 5-point 10-fold dilution series of a commercially available genomic DNA sample (Promega, Madison, WI) was used to generate the standard curve for quantification.
The 12.765 Digital Array microfluidic chip (Fluidigm, South San Francisco, CA) was chosen as the digital PCR platform for this study. Each chip contains 12 panels, which are compartmentalized into 765 nanoliter chambers by micromechanical valves. Based on the estimation of DNA concentration with quantitative real-time PCR, genomic DNA samples were diluted such that when loaded onto the microfluidic chip (Fluidigm), there was on average 1 template copy per every 3 (or more) chambers. Nine microliters of PCR reaction mixture containing 1× iQ Supermix (BioRad, Hercules, CA) or 1× FastStart Universal Probe Master (Roche, Indianapolis, IN), 0.1% Tween-20, 300 nmol/L primers, and 150 nmol/L probes of chromosome 1 and 1 of the 5 target chromosomes was loaded onto each panel of the chip. Four panels were dedicated for each target chromosome. The reaction was performed on the BioMark System (Fluidigm) with the following thermal cycling protocol: 95°C for 10 minutes, 40 cycles of 95°C for 15 seconds, and 60°C for 1 minute. Fluorescent images of the microfluidic chip were taken at the beginning and the end of the PCR. A computer program (Matlab; Mathworks, Natick, MA) was written to subtract the initial image from the final image in each fluorescent channel and to count the number of positive compartments in each subtracted image.
Statistical analysis
Counts of positive compartments were converted to counts of input template molecules based on the binomial approximation.12 This correction arises from the fact that there will be compartments containing more than a single copy of template as the concentration of the template increases, and the count of positive compartments is an underestimate of the true count of input template molecules.
The difference between the target and reference chromosome corrected counts was computed. For the case of disomy, one would expect the difference to be approximately zero. For the case of trisomy, the difference would be positive and about half of the reference chromosome count, and in the case of monosomy the difference would be negative and about half of the reference chromosome count. We used Poisson statistics to construct confidence intervals for the count differences for every reference chromosome count and different cases of ploidy. The width of the 99.9% confidence interval of the count differences was estimated as 3.29*√(N+N) for disomy, 3.29*√ (N+1.5N) for trisomy, and 3.29*√ (N+0.5N) for monosomy, where N is the count of the reference chromosome. We then determined the ploidy of the target chromosome by looking at which region the data point was located. At the conclusion of the study period, the ploidy for each chromosome of each sample determined by digital PCR was compared to that of conventional karyotyping results to evaluate the diagnostic accuracy of digital PCR.
Results
Sample fluorescence images of the microfluidic digital PCR chip are shown in Figure 1. Signal from the FAM channel (target chromosomes) is shown in green and that from the HEX channel (reference chromosome) is shown in red. Figure 1A is from a sample identified as a female disomic for chromosome 21. The green counts from chromosome 21 and chromosome X are approximately equal to the red counts from chromosome 1. There is no signal from chromosome Y. Figure 1B is from a sample identified as a male trisomic for chromosome 21. The green count from chromosome 21 is approximately 1.5 times greater than the red count from chromosome 1. The green counts from chromosome X and Y are approximately half of the red counts.
FIGURE 1. Sample false-color images of microfluidic digital PCR chips.
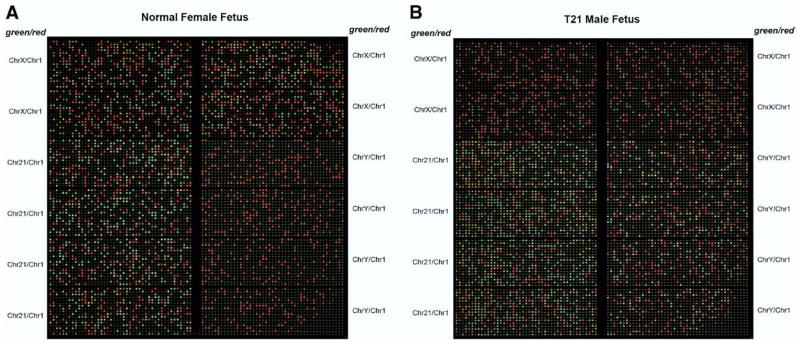
These images are produced by overlaying the subtracted images in both fluorescent channels. FAM signal is shown in green, and HEX signal is shown in red. A red square represents a compartment containing amplification products giving out signal in the HEX channel (chromosome 1 locus). A green square represents a compartment containing amplification products giving out signal in the FAM channel (chromosomes X, Y, or 21 loci, as labeled on the sides of the images). A yellow square is an overlap of a red and a green square. A, Normal female fetus (46 XX). The number of green squares is comparable to that of red squares in panels targeting chromosomes 21 and X. No green squares are present in panels targeting chromosome Y. B, Male fetus with trisomy 21 (47 XY +21). The number of green squares is approximately half that of red squares in panels targeting chromosomes X and Y. More than expected number of green squares is observed in panels targeting chromosome 21. Comparison of green and red square counts reveals a ratio of approximately 3:2.
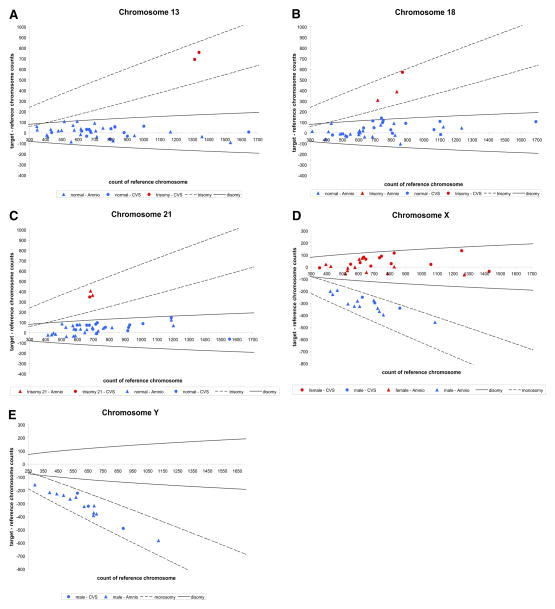
For each sample, the difference between target and reference chromosome counts were computed and plotted against the reference chromosome count (Figure 2). The 99.9% confidence interval for each cases of ploidy was constructed and used as a reference to classify the ploidy of each sample.
FIGURE 2. Digital PCR results.
For each sample, the difference between target and reference chromosome counts is plotted against the reference chromosome count. The boundaries represent 99.9% confidence interval of each cases of ploidy. A, Chromosome 13 as the target chromosome. All but 2 samples fell within the region of disomy. Two cases of trisomy 13 were detected. B, Chromosome 18 as the target chromosome. Three cases of trisomy 18 were detected. The rest were determined to be normal. C, Chromosome 21 as the target chromosome. Three cases of trisomy 21 were detected. The rest were determined to be normal. D, Chromosome X as the target chromosome. All female samples fell within the region of disomy, while all male samples lied within the region of monosomy. E, Chromosome Y as the target chromosome. All male samples fell within the region of monosomy. None of the female samples showed amplification for chromosome Y assay.
Digital PCR analysis accurately identified 2 cases of trisomy 13 (Figure 2, A), 3 cases of trisomy 18 (Figure 2, B), and 3 cases of trisomy 21 (Figure 2, C) in the 40 samples analyzed. No cases of monosomy X, XXY, and XYY were observed in our cohort. The rest of the samples were accurately identified as normal disomic for chromosome 13, 18, and 21, disomic and monosomic for chromosome X in the respective cases of female and male (Figure 2, D), and monosomic for chromosome Y for the cases of male (Figure 2, E).
Comment
Digital PCR was first used on a multi-well plate format to detect mutations and allelic imbalances associated with cancer development13–15, and this format has recently been applied to measure allelic imbalance in placental RNA with the goal of developing a noninvasive diagnostic for trisomy 21.16 A microemulsion platform was developed to increase the scale of the assay,17,18 and it is now being used as a sample preparation technique for massively parallel sequencing.19 However, previously described methods are cumbersome to implement and require significant labor. The emergence of microfluidics has led to the development of a commercially available microfluidic digital PCR platform that enables the simultaneous performance of ~9000 PCR reactions.20 It has been used to study the gene expression of single progenitor cells,12 to relate gene function to identity in environmental microbes,21 and to measure trisomy in human cell lines.22
We report here the use of microfluidic digital PCR for the rapid diagnosis of the most common fetal aneuploidies in ongoing pregnancies, specifically Down syndrome (trisomy 21), Edwards syndrome (trisomy 18), and Patau syndrome (trisomy 13). Our sample cohort set did not contain any cases of Turner syndrome (monosomy X), Klinefelter syndrome (XXY), and XYY syndrome, but based on our data, we would expect a larger clinical study with digital PCR to identify these cases with similar accuracy.
The ploidy of chromosome 18 for one of the samples was initially undetermined because it lied outside the threshold for normal ploidy (Figure 2, B). Further testing with a separate chromosome 18 specific assay correctly determined the ploidy of the sample (data not shown). This issue will be resolved in the future with further optimization and multiplexing of primer sets, and future clinical studies may benefit from more highly parallel chip formats that improve sensitivity and dynamic range.
Currently, a number of rapid molecular diagnostic tests for fetal aneuploidy are available. The most widely validated ones are fluorescent in situ hybridization (FISH),23–25 quantitative-fluorescent PCR (QF-PCR),26–33 and multiplex ligation probe amplification (MLPA).34–38 Compared to these methods, digital PCR presents several advantages. In this study, the total time required for sample preparation and digital PCR analysis was approximately 6 hours (1 hour of manual sample preparation and 5 hours for instrument results). In terms of speed, this is comparable to FISH and QF-PCR,3,4 and better than MLPA, which requires overnight hybridization.34 Unlike QF-PCR and MLPA, digital PCR is a single-step procedure and does not require post-PCR analysis with electrophoresis. Since PCR products are measured fluorescently and are never removed from the microfluidic device, there is no risk of product contamination between PCR reactions. Furthermore, digital PCR assays are universal and are not dependent on genetic polymorphisms; in contrast, the most common type of QF-PCR requires multiple polymorphic markers per chromosome to ensure informative results.3 Digital PCR is also superior to FISH in that FISH is labor intensive and requires both trained personnel and intact cells for analysis.3,4
In recent years, array comparative genomic hybridization (CGH) has also been introduced for the rapid prenatal diagnosis of aneuploidy and diseases associated with copy number variation.39–44 While array CGH is able to provide genome-wide information on copy number variations at relatively high resolution, it requires several days for analysis and substantial amount of genetic materials.39,44 We anticipate that digital PCR and array CGH can be used in a complementary fashion in order to provide rapid results on the most common genetic disorders via digital PCR, followed by more detailed but slower analysis with CGH. It also may be the case that in the future digital PCR can be paired with other PCR based assays to provide equivalent diagnostic power to CGH.
Many amniotic fluid and CVS samples are contaminated with maternal DNA. While the incidence of fetal mosaicism is low (0.25% of amniocentesis specimens and 1% of chorionic villus specimens2), it has been shown that maternal cells are present in up to 20% of uncultured amniotic fluid samples.45 The presence of contaminating euploid DNA in a sample from an aneuploid fetus would interfere with the accurate diagnosis of fetal aneuploidy. With contaminating euploid DNA, the ratio of counts of the abnormal chromosome to the reference chromosome would move to an intermediate value between 1.5 and 1.0, and the presence of trisomy DNA should be measurable by digital PCR by sampling sufficient number of single DNA molecules. We have shown previously that digital PCR is capable of detecting trisomy in a background of contaminating euploid DNA.22 In our cohort, any significant maternal DNA contamination would be revealed by bias in the X chromosome signal from male samples; we did not observe any significant bias (Figure 2, D). One of our amniotic fluid samples has a low level mosaicism (1 out of 15 cultured colonies was karyotyped as 45X while the remaining colonies were karyotyped as 46XX) and was interpreted as disomic for chromosome X by digital PCR. Such low grade mosaicism would not be detectable with the current depth of sampling, but should in principle be detectable by sampling much larger number of single DNA molecules. Since the clinical and phenotypic ramifications of such mosaicisms, especially placental mosaicisms, are often difficult to predict, further clinical studies are necessary to determine the useful sampling rate for detecting mosaicism.
Another limitation of digital PCR for rapid prenatal diagnosis is similar to those of FISH, QF-PCR, and MLPA in that it is not yet able to detect structural chromosomal abnormalities such as balanced translocations or inversions.4,5 We observed this effect in one of our CVS samples with a Robertsonian (13: 14) translocation. Similarly, improvements in assay design are needed to detect 69, XXX triploidy, which is detectable by FISH and QF-PCR.46,47 Although rare, these genetic defects may occur in < 1% of cases presenting for invasive diagnostic procedures.48,49 We anticipate that further refinements of the primer and assay design will enable the detection of these cases.
The current cost of aneuploidy detection with microfluidic digital PCR is approximately USD400, of which the majority is the cost of the microfluidic chips. However, the cost of digital PCR continues to decline over time as the technology of chip fabrication advances. In addition, the throughput and scale of microfluidic digital PCR should also improve considerably as better fabrication techniques allow more microfluidic compartments to be incorporated on a single chip. The robustness and simplicity of microfluidic digital PCR make it an attractive tool for rapid prenatal diagnostics and warrants further validation in larger clinical studies.
Acknowledgments
We thank the Division of Perinatal Genetics and the General Clinical Research Center (GCRC) at Stanford University for their assistance with subject recruitment and enrollment.
This study was supported by the Wallace H. Coulter Foundation and NIH Grant 1R01 G002644-01A1. Ms Fan was supported by a Stanford Graduate Fellowship. Dr Quake is a consultant and shareholder of Fluidigm Corporation, the manufacturer of the microfluidic chips used in this study.
Footnotes
Presented at the 29th Annual Meeting of the Society for Maternal–Fetal Medicine, San Diego, CA, Jan. 26–31, 2009.
References
- 1.Cunningham F, Hauth J, Leveno K, Gilstrap L, Bloom S, Wenstrom K. Williams obstetrics. New York: McGraw-Hill Professional; 2002. p. 942. [Google Scholar]
- 2.ACOG Practice Bulletin No. 88, December 2007. Invasive prenatal testing for aneuploidy. Obstet Gynecol. 2007;110:1459–67. doi: 10.1097/01.AOG.0000291570.63450.44. [DOI] [PubMed] [Google Scholar]
- 3.Hulten MA, Dhanjal S, Pertl B. Rapid and simple prenatal diagnosis of common chromosome disorders: advantages and disadvantages of the molecular methods FISH and QF-PCR. Reproduction. 2003;126:279–97. doi: 10.1530/rep.0.1260279. [DOI] [PubMed] [Google Scholar]
- 4.Dudarewicz L, Holzgreve W, Jeziorowska A, Jakubowski L, Zimmermann B. Molecular methods for rapid detection of aneuploidy. J Appl Genet. 2005;46:207–15. [PubMed] [Google Scholar]
- 5.Shaffer LG, Bui TH. Molecular cytogenetic and rapid aneuploidy detection methods in prenatal diagnosis. Am J Med Genet C Semin Med Genet. 2007;145C:87–98. doi: 10.1002/ajmg.c.30114. [DOI] [PubMed] [Google Scholar]
- 6.Zimmermann B, Holzgreve W, Wenzel F, Hahn S. Novel real-time quantitative PCR test for trisomy 21. Clin Chem. 2002;48:362–3. [PubMed] [Google Scholar]
- 7.Kalinina O, Lebedeva I, Brown J, Silver J. Nanoliter scale PCR with TaqMan detection. Nucleic Acids Res. 1997;25:1999–2004. doi: 10.1093/nar/25.10.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogelstein B, Kinzler KW. Digital PCR. Proc Natl Acad Sci U S A. 1999;96:9236–41. doi: 10.1073/pnas.96.16.9236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lathi RB, Westphal LM, Milki AA. Aneuploidy in the miscarriages of infertile women and the potential benefit of preimplanation genetic diagnosis. Fertil Steril. 2008;89:353–7. doi: 10.1016/j.fertnstert.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 10.Redon R, Ishikawa S, Fitch KR, et al. Global variation in copy number in the human genome. Nature. 2006;444:444–54. doi: 10.1038/nature05329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bejerano G, Pheasant M, Makunin I, et al. Ultraconserved elements in the human genome. Science. 2004;304:1321–5. doi: 10.1126/science.1098119. [DOI] [PubMed] [Google Scholar]
- 12.Warren L, Bryder D, Weissman IL, Quake SR. Transcription factor profiling in individual hematopoietic progenitors by digital RT-PCR. Proc Natl Acad Sci U S A. 2006;103:17807–12. doi: 10.1073/pnas.0608512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang HW, Lee SM, Goodman SN, et al. Assessment of plasma DNA levels, allelic imbalance, and CA 125 as diagnostic tests for cancer. J Natl Cancer Inst. 2002;94:1697–703. doi: 10.1093/jnci/94.22.1697. [DOI] [PubMed] [Google Scholar]
- 14.Zhou W, Galizia G, Lieto E, et al. Counting alleles reveals a connection between chromosome 18q loss and vascular invasion. Nat Biotechnol. 2001;19:78–81. doi: 10.1038/83572. [DOI] [PubMed] [Google Scholar]
- 15.Zhou W, Goodman SN, Galizia G, et al. Counting alleles to predict recurrence of early-stage colorectal cancers. Lancet. 2002;359:219–25. doi: 10.1016/S0140-6736(02)07448-2. [DOI] [PubMed] [Google Scholar]
- 16.Lo YM, Lun FM, Chan KC, et al. Digital PCR for the molecular detection of fetal chromosomal aneuploidy. Proc Natl Acad Sci U S A. 2007;104:13116–21. doi: 10.1073/pnas.0705765104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dressman D, Yan H, Traverso G, Kinzler KW, Vogelstein B. Transforming single DNA molecules into fluorescent magnetic particles for detection and enumeration of genetic variations. Proc Natl Acad Sci U S A. 2003;100:8817–22. doi: 10.1073/pnas.1133470100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diehl F, Li M, Dressman D, et al. Detection and quantification of mutations in the plasma of patients with colorectal tumors. Proc Natl Acad Sci U S A. 2005;102:16368–73. doi: 10.1073/pnas.0507904102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Margulies M, Egholm M, Altman WE, et al. Genome sequencing in microfabricated high-density picolitre reactors. Nature. 2005;437:376–80. doi: 10.1038/nature03959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin J, Jones RC, Ramakrishnan R. Studying copy number variations using a nanofluidic platform. Nucleic Acids Res. 2008;36:e116. doi: 10.1093/nar/gkn518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ottesen EA, Hong JW, Quake SR, Leadbetter JR. Microfluidic digital PCR enables multigene analysis of individual environmental bacteria. Science. 2006;314:1464–7. doi: 10.1126/science.1131370. [DOI] [PubMed] [Google Scholar]
- 22.Fan HC, Quake SR. Detection of aneuploidy with digital polymerase chain reaction. Anal Chem. 2007;79:7576–9. doi: 10.1021/ac0709394. [DOI] [PubMed] [Google Scholar]
- 23.Kuo WL, Tenjin H, Segraves R, Pinkel D, Golbus MS, Gray J. Detection of aneuploidy involving chromosomes 13, 18, or 21, by fluorescence in situ hybridization (FISH) to interphase and metaphase amniocytes. Am J Hum Genet. 1991;49:112–9. [PMC free article] [PubMed] [Google Scholar]
- 24.Klinger K, Landes G, Shook D, et al. Rapid detection of chromosome aneuploidies in uncultured amniocytes by using fluorescence in situ hybridization (FISH) Am J Hum Genet. 1992;51:55–65. [PMC free article] [PubMed] [Google Scholar]
- 25.Ward BE, Gersen SL, Carelli MP, et al. Rapid prenatal diagnosis of chromosomal aneuploidies by fluorescence in situ hybridization: clinical experience with 4,500 specimens. Am J Hum Genet. 1993;52:854–65. [PMC free article] [PubMed] [Google Scholar]
- 26.Mansfield ES. Diagnosis of Down syndrome and other aneuploidies using quantitative polymerase chain reaction and small tandem repeat polymorphisms. Hum Mol Genet. 1993;2:43–50. doi: 10.1093/hmg/2.1.43. [DOI] [PubMed] [Google Scholar]
- 27.Pertl B, Yau SC, Sherlock J, Davies AF, Mathew CG, Adinolfi M. Rapid molecular method for prenatal detection of Down’s syndrome. Lancet. 1994;343:1197–8. doi: 10.1016/s0140-6736(94)92404-x. [DOI] [PubMed] [Google Scholar]
- 28.Pertl B, Kopp S, Kroisel PM, et al. Quantitative fluorescence polymerase chain reaction for the rapid prenatal detection of common aneuploidies and fetal sex. Am J Obstet Gynecol. 1997;177:899–906. doi: 10.1016/s0002-9378(97)70292-8. [DOI] [PubMed] [Google Scholar]
- 29.Pertl B, Kopp S, Kroisel PM, Tului L, Brambati B, Adinolfi M. Rapid detection of chromosome aneuploidies by quantitative fluorescence PCR: first application on 247 chorionic villus samples. J Med Genet. 1999;36:300–3. [PMC free article] [PubMed] [Google Scholar]
- 30.Pertl B, Pieber D, Lercher-Hartlieb A, et al. Rapid prenatal diagnosis of aneuploidy by quantitative fluorescent PCR on fetal samples from mothers at high risk for chromosome disorders. Mol Hum Reprod. 1999;5:1176–9. doi: 10.1093/molehr/5.12.1176. [DOI] [PubMed] [Google Scholar]
- 31.Levett LJ, Liddle S, Meredith R. A large-scale evaluation of amnio-PCR for the rapid prenatal diagnosis of fetal trisomy. Ultrasound Obstet Gynecol. 2001;17:115–8. doi: 10.1046/j.1469-0705.2001.00340.x. [DOI] [PubMed] [Google Scholar]
- 32.Mann K, Fox SP, Abbs SJ, et al. Development and implementation of a new rapid aneuploidy diagnostic service within the UK National Health Service and implications for the future of prenatal diagnosis. Lancet. 2001;358:1057–61. doi: 10.1016/S0140-6736(01)06183-9. [DOI] [PubMed] [Google Scholar]
- 33.Mann K, Donaghue C, Fox SP, Docherty Z, Ogilvie CM. Strategies for the rapid prenatal diagnosis of chromosome aneuploidy. Eur J Hum Genet. 2004;12:907–15. doi: 10.1038/sj.ejhg.5201224. [DOI] [PubMed] [Google Scholar]
- 34.Schouten JP, McElgunn CJ, Waaijer R, Zwijnenburg D, Diepvens F, Pals G. Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification. Nucleic Acids Res. 2002;30:e57. doi: 10.1093/nar/gnf056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slater HR, Bruno DL, Ren H, Pertile M, Schouten JP, Choo KH. Rapid, high throughput prenatal detection of aneuploidy using a novel quantitative method (MLPA) J Med Genet. 2003;40:907–12. doi: 10.1136/jmg.40.12.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gerdes T, Kirchhoff M, Lind AM, Larsen GV, Schwartz M, Lundsteen C. Computer-assisted prenatal aneuploidy screening for chromosome 13, 18, 21, X and Y based on multiplex ligation-dependent probe amplification (MLPA) Eur J Hum Genet. 2005;13:171–5. doi: 10.1038/sj.ejhg.5201307. [DOI] [PubMed] [Google Scholar]
- 37.Hochstenbach R, Meijer J, van de Brug J, et al. Rapid detection of chromosomal aneuploidies in uncultured amniocytes by multiplex ligation-dependent probe amplification (MLPA) Prenat Diagn. 2005;25:1032–9. doi: 10.1002/pd.1247. [DOI] [PubMed] [Google Scholar]
- 38.Boormans EM, Birnie E, Wildschut HI, et al. Multiplex ligation-dependent probe amplification versus karyotyping in prenatal diagnosis: the M.A.K.E. study. BMC Pregnancy Childbirth. 2008;8:18. doi: 10.1186/1471-2393-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sahoo T, Cheung SW, Ward P, et al. Prenatal diagnosis of chromosomal abnormalities using array-based comparative genomic hybridization. Genet Med. 2006;8:719–27. doi: 10.1097/01.gim.0000245576.47154.63. [DOI] [PubMed] [Google Scholar]
- 40.Miura S, Miura K, Masuzaki H, et al. Microarray comparative genomic hybridization (CGH)-based prenatal diagnosis for chromosome abnormalities using cell-free fetal DNA in amniotic fluid. J Hum Genet. 2006;51:412–7. doi: 10.1007/s10038-006-0376-7. [DOI] [PubMed] [Google Scholar]
- 41.Larrabee PB, Johnson KL, Pestova E, et al. Microarray analysis of cell-free fetal DNA in amniotic fluid: a prenatal molecular karyotype. Am J Hum Genet. 2004;75:485–91. doi: 10.1086/423288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rickman L, Fiegler H, Shaw-Smith C, et al. Prenatal detection of unbalanced chromosomal rearrangements by array CGH. J Med Genet. 2006;43:353–61. doi: 10.1136/jmg.2005.037648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lapaire O, Lu XY, Johnson KL, et al. Array-CGH analysis of cell-free fetal DNA in 10 mL of amniotic fluid supernatant. Prenat Diagn. 2007;27:616–21. doi: 10.1002/pd.1752. [DOI] [PubMed] [Google Scholar]
- 44.Bi W, Breman AM, Venable SF, et al. Rapid prenatal diagnosis using uncultured amniocytes and oligonucleotide array CGH. Prenat Diagn. 2008;28:943–9. doi: 10.1002/pd.2087. [DOI] [PubMed] [Google Scholar]
- 45.Winsor EJ, Silver MP, Theve R, Wright M, Ward BE. Maternal cell contamination in uncultured amniotic fluid. Prenat Diagn. 1996;16:49–54. doi: 10.1002/(SICI)1097-0223(199601)16:1<49::AID-PD808>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 46.Gersen SL, Carelli MP, Klinger KW, Ward BE. Rapid prenatal diagnosis of 14 cases of triploidy using fish with multiple probes. Prenat Diagn. 1995;15:1–5. doi: 10.1002/pd.1970150102. [DOI] [PubMed] [Google Scholar]
- 47.Schmidt W, Jenderny J, Hecher K, et al. Detection of aneuploidy in chromosomes X, Y, 13, 18 and 21 by QF-PCR in 662 selected pregnancies at risk. Mol Hum Reprod. 2000;6:855–60. doi: 10.1093/molehr/6.9.855. [DOI] [PubMed] [Google Scholar]
- 48.Peng HH, Chao AS, Wang TH, Chang YL, Chang SD. Prenatally diagnosed balanced chromosome rearrangements: eight years’ experience. J Reprod Med. 2006;51:699–703. [PubMed] [Google Scholar]
- 49.Association of Clinical Cytogeneticists Working Party on Chorionic Villi in Prenatal Diagnosis. Cytogenetic analysis of chorionic villi for prenatal diagnosis: an ACC collaborative study of U.K. data. Prenat Diagn. 1994;14:363–79. doi: 10.1002/pd.1970140506. [DOI] [PubMed] [Google Scholar]