Abstract
Background
Intensive care unit (ICU) patients are exposed to many sources of discomfort. Although growing attention has been given to the detection and treatment of pain, very little has been given to the detection and treatment of dyspnea (defined as ‘breathing discomfort’).
Discussion
In this article, we review the published information on prevalence, mechanisms and potential negative impacts of dyspnea in mechanically ventilated patients. In addition, we review the most appropriate tools to detect and quantify dyspnea in ICU patients.
Conclusions
Growing evidence suggests that dyspnea is a frequent issue in mechanically ventilated ICU patients, is highly associated with anxiety and pain, and is improved in many patients by altering ventilator settings. Future studies are needed to better delineate the impact of dyspnea in the ICU, and to define diagnostic, monitoring and therapeutic protocols.
Dyspnea: An unrecognized cause of discomfort in mechanically ventilated patients?
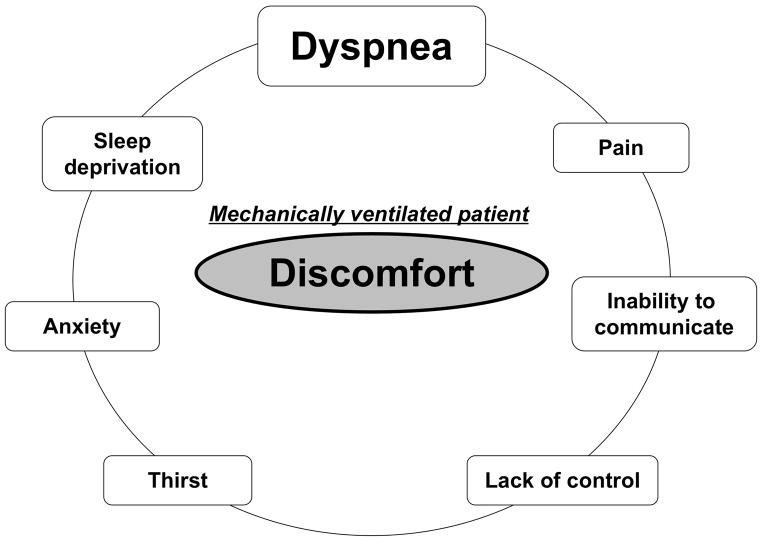
Optimizing patient comfort is a prominent concern in the intensive care unit (ICU). Alleviating immediate suffering is indeed a natural mission of all categories of caregivers. Optimizing patient comfort involves three steps: 1) the identification of potential discomfort, 2) the diagnosis of the reason for this discomfort, 3) the initiation of a therapeutic response to treat this discomfort. Although mechanically ventilated ICU patients cannot communicate easily, it is possible to communicate with many of them, and possible to guess at discomfort from signs in the remainder. Identifying the reason for discomfort can be challenging because discomfort in these patients can have many causes (Figure 1). Identifying the right one is crucial since each of the potential causes of discomfort leads to a different therapeutic response. Among the causes of discomfort in ICU patients, pain has received a major attention during the past decade with beneficial effects on long-term outcomes. Awareness of pain and its proactive management has resulted in improved ICU outcomes [1, 2].
Figure 1.
Possible reasons for discomfort in mechanically ventilated patients
Although dyspnea and pain share many similarities [3–5], little attention has been given to assessing and managing dyspnea; there is little rigorous research data and there are no clinical guidelines for managing dyspnea in the ventilated patient. However, dyspnea can be assessed and available data show that it frequently causes discomfort in ICU mechanically ventilated patients.
ICU patients are exposed to many stimuli that can generate or exacerbate dyspnea. In addition to underlying cardiopulmonary abnormalities, respiratory discomfort may be caused by therapeutic management strategies that have been adopted in recent years. These include lowering sedation [6], preserving spontaneous breathing activity [7], and the use of low tidal volumes [8] even in the absence of severe lung disorders [9–11]. It cannot be ruled out that sedation may give a falsely reassuring outward appearance of comfort in patients actually suffering from undiminished, or even increased respiratory discomfort, as in the case of pain [12, 13]. For example, pain ratings and pain-related cortical activations in response to cutaneous pain stimuli were increased by moderate propofol sedation. Strong pain-related activations remained in some cortical regions even during heavy propofol sedation that rendered subjects unresponsive.
This review is intended to promote awareness in ICU caregivers of the unrecognized problem of dyspnea. It summarizes current knowledge about the prevalence of dyspnea in mechanically ventilated ICU patients and about the corresponding risk factors. It also suggests possible approaches to detect and quantify dyspnea in these patients.
Simplified physiological basis of dyspneic sensations
Extensive reviews on dyspnea physiology can be found elsewhere [14–17]. The neurophysiological basis of dyspnea is more complex than for many sensations, involving both excitatory and inhibitory afferent inputs from sensory nerves as well as perception of motor commands (so-called corollary discharge). In mechanically ventilated patients, it seems reasonable to focus on two main dyspnea modalities, namely “air hunger” and “excessive work/effort of breathing”.
Air hunger
Air hunger (an unpleasant, unsatisfied urge to breathe) is perhaps the most distressing dyspnea modality [18, 19]. This dyspnea modality corresponds to verbal expressions such as “I am not getting enough air”, “I feel that I am suffocating”, “I need more air”. Experiments in paralyzed subject (complete neuromuscular block in normal volunteers [18] or C1-C2 quadriplegic patients [20]) have shown that an acute rise in PaCO2 suffices to induce air hunger independent of any respiratory muscle activity. Air hunger also increases when tidal volume is decreased under mechanical ventilation and PaCO2 is held constant [21, 22]. Air hunger appears to arise from a “corollary discharge” or copy of the automatic efferent command from the brainstem respiratory motor center. The brainstem respiratory center is excited by various stimuli that ‘drive’ respiration hypercapnia, hypoxia, exercise, and hyperthermia. Even when respiratory drive is held constant, air hunger is relieved by tidal expansion of the lungs [23]. The principle pathway for this volume expansion relief is pulmonary stretch receptor afferents [22], although a contribution from chest wall afferents has not been ruled out [24]. Thus, air hunger develops when there is an imbalance between the efferent and the inhibitory afferent messages. This imbalance triggers activity in interoceptive areas of the brain involved in unpleasant sensations (such as visceral pain, thirst, or hunger), particularly the insular cortex, amygdala, and anterior cingulate [21]. In contrast, when increased ventilatory drive is matched by an adequate stretch receptor return from the lungs respiratory sensations will not necessarily be perceived as uncomfortable and will therefore not qualify as dyspnea (e.g. during moderate exercise in healthy persons).
Excessive work/effort of breathing
This dyspnea modality corresponds to verbal expressions such as “My breathing requires effort”, My breathing requires more work” [25], “I have difficulty breathing”, “I need to make an effort to get the air in”, “I must concentrate on my breathing”. It arises from a sense of excessive breathing effort, even when gas exchange needs are adequately met – this sense of work or effort arises both in muscle afferents of the chest wall and diaphragm, from volitional respiratory motor centers in the neocortex, and perhaps from automatic respiratory motor centers. Dyspnea of the excessive work/effort type characteristically occurs when there is an imbalance between the load imposed upon the respiratory muscle (respiratory impedance) and the capacity of these muscles to overcome this load [26]. This can correspond to deteriorated respiratory mechanics, a weakening of inspiratory muscles, or the combination of both. Dynamic hyperinflation in patients with chronic obstructive pulmonary disease (COPD) provides a typical example of such situations. Dynamic hyperinflation combines an increased respiratory impedance (increased lung elastance at high lung volume requiring high inspiratory muscle force) and a functional weakening of inspiratory muscles (hyperinflation-related shortening placing the muscles at disadvantage both mechanically —e.g. flattened diaphragm— and on the length-tension curve). In addition, patients must overcome the resultant intrinsic positive expiratory pressure —PEEPi, before beginning to move air and trigger the ventilator — imposing an inspiratory threshold load. Neuromuscular disorders involving the respiratory muscles can also give rise to dyspnea of the excessive work/effort type. Adequate ventilatory support should prevent this type of dyspnea, the very purpose of ventilatory assistance being to adjust the load/capacity balance of the respiratory system.
Complex sensations
Patients mechanically ventilated for acute respiratory failure generally experience mixed respiratory sensations. Increased respiratory impedance, often associated with decreased respiratory muscle strength, generates a sense of excessive respiratory work/effort, and the corresponding failure to maintain gas exchange combined with inadequate tidal volume is a source of air hunger. It is therefore expected that dyspneic ICU patients will report more than one dyspnea modality [27].
Differential affective components
Dyspnea, like pain, can be viewed as having an immediate unpleasantness as well as more complex emotional sequelae [28]. Recent experimental data have demonstrated that both unpleasantness and emotional response can be differentially altered with different dyspnea modality or drug treatment. At similar level of sensory intensity, air hunger elicits more unpleasantness than excessive work/effort and is associated with greater anxiety and fear [19]. Of clinical relevance is the experimental finding that an effective way to induce air hunger is to increase ventilatory demand via mild hypercapnia while hindering the normal ventilatory response [29, 30]. Likewise, therapeutic interventions can have different effects on the sensory and the affective component of dyspnea [31].
What are the main determinants of dyspnea in mechanically ventilated patients?
The determinants of dyspnea in mechanically ventilated patients are multifactorial, with contributions from the intrinsic bronchopulmonary status of the patients, ventilator settings, various care activities, extrinsic physiological stimulations of the ventilatory drive, and non-respiratory factors such as anxiety or pain. Several of these factors can coincide at any particular moment and they are likely to be synergistic.
Intrinsic cardiopulmonary status
Although the disease that has required ventilatory assistance is the usual cause of dyspnea, mechanical ventilation has the potential to reduce or eliminate the dyspnea. An increase in dyspnea during the course of mechanical ventilation can signal a problem with the mechanical ventilation or can signal an adverse event such worsening respiratory mechanics and/or the ventilation-perfusion equilibrium (pneumothorax, atelectasis, ventilator-associated pneumonia, hydrostatic pulmonary edema or ARDS) or a non-respiratory one (acute hemorrhage, sepsis, or worsening heart failure)[27].
Ventilator settings
The largest study dealing with dyspnea in ICU mechanically ventilated patients [27] showed that ventilator adjustments targeted at dyspnea reduction significantly improved respiratory comfort, often dramatically, in 35% of the mechanically ventilated patients who reported dyspnea. In addition, the data suggested that ventilator mode played a role, since dyspneic patients were more likely to be ventilated with assist-control ventilation (ACV) than non-dyspneic patients (69% vs. 45%) and multivariate analysis showed that ACV was independently associated with dyspnea (OR, 4.77; 95% CI, 1.60–14.3) [27]. This difference may be due to the fundamental characteristics of the ventilatory mode, but is more likely due to greater ease in optimizing ventilatory parameters during support. Unfortunately, little is known about the effect of ventilator mode per se, as the studies done on this problem have failed to measure or control for variables known to alter dyspnea such as tidal volume, arterial PaCO2, inspiratory flow rate among different modes [32].
It is likely that tidal volume changes alone can explain the observed differences between ventilator modes. Indeed, when we re-plotted the data from two studies to show dyspnea ratings vs. tidal volume [33, 34] we noted that ratings from different modes fell along the same line (Figure 3). This is an important issue since recent publications argue that tidal volume should be minimized in mechanically ventilated patients, even in those without risk factors for ARDS [9–11]. When setting tidal volume one should also be aware of the resultant discomfort and its potentially deleterious effects. Optimization of flow rate also influences respiratory comfort [27], consistent with earlier findings in healthy subjects [35, 36].
Figure 3. Relationship between dyspnea and tidal volume.

Data replotted from Mols et al. [34] (left) and Leung et al. [33] (right). Although the authors of both papers reported a difference in respiratory comfort dependent on ventilator mode, these plots suggest that the main effect of changing mode is to change the tidal volume delivered by the ventilator. Consequent changes in pulmonary stretch receptor activity as well as blood gasses are in the correct direction to explain the observed effects on discomfort ratings.
PAV, proportional assist ventilation; PSV, pressure-support ventilation; IMV, intermittent mandatory volume ventilation; dyspn unassist, unassisted breathing.
Finally, during inspiratory pressure support, there seems to be a U-shaped relationship between the level of assistance provided and dyspnea; if assistance is either too high or too low discomfort increases [37].
Patient-ventilator interface; respiratory and non-respiratory care activities
The patient-ventilator interface can induce discomfort, be it an endotracheal tube [38, 39] or a face mask [40]. Beyond this, the small descriptive case study by Lush et al. [41] indicates that many other events can be associated with dyspnea, such as turns, transfers, or bathing, but also the presence of the physicians or external events such as shift changes or a death elsewhere in the unit.
Extrinsic physiological stimulations of ventilatory drive
Respiratory drive in excess of achieved ventilation is the cause of air hunger (see above, physiological basis). Fever, acidosis, or anemia are frequent causes of increased ventilatory drive in ICU patients, and should therefore be looked for in the presence of apparently unexplained dyspnea.
Anxiety and pain
Anxiety and pain may increase dyspnea by stimulating ventilatory drive [42] and it is also likely that they can also interact with the affective dimension of dyspnea. In the dyspnea-ICU study by Schmidt et al. [27], pain and anxiety were more frequent in dyspneic than in non-dyspneic mechanically ventilated patients. Anxiety was independently associated with dyspnea (OR 8.84; 95% CI 3.26-24-14.3), and it was responsive to ventilator settings adjustments when they relieved dyspnea. This points at a bidirectional causative relationship between anxiety and dyspnea.
Prevalence of dyspnea in mechanically ventilated patients
Because dyspnea can only be perceived by the person experiencing it, dyspnea should be assessed by questioning the patient; only when communication with the patient is impossible should the clinician rely primarily on outward signs. We describe the assessment of dyspnea in more detail below. There is a paucity of published data on dyspnea prevalence in mechanically ventilated patients, and clinical experience suggests that dyspnea is not routinely assessed and recorded. Studies in which patients have been asked to recall their ICU experience give insights into the magnitude of the problem. For example, Rotondi et al. [38] were able to explore the recollections of 150 patients who had survived an ICU stay during which they had been mechanically ventilated for two days or more. Two thirds of these patients remembered the endotracheal tube (ETT); 45% recalled “feeling choked by the ETT”; and 24% remembered “not getting enough air from the ETT”. ETT-related experiences were considered stressful, and strongly associated with the occurrence of spells of terror or “feeling nervous when left alone” [38]. Among 126 COPD patients who survived an ICU stay, 90% recollected traumatic events having occurred during their stay when interviewed at the time of discharge [40]. “Suffocation” was the second most frequently noted item (55% of the respondents) just behind “sleep disorders” (63%) [40]. To date, it seems that dyspnea in mechanically ventilated patients has been the main research focus of only 7 published prospective studies [41, 43–48] [27].
Four of these studies focused on breathing comfort in the context of weaning trials [44–46, 48], but they provide pre-weaning dyspnea assessments. In this setting, dyspnea was common although the exact proportion of patients who were dyspneic before weaning was not systematically reported. One study [46] explored the relationship between dyspnea and pre-weaning mood state in 21 mechanically ventilated patients using a shortened version of the profile of mood states (sPOMS)[49]. Weak relationships were found between dyspnea intensity (VAS scale) and mood states, with the “vigor” subscore of the sPOMS being the closest to reach a statistically significant negative correlation with the intensity of dyspnea (p = 0.07) [46].
The four other studies evaluated dyspnea in alert mechanically ventilated patients not having entered the weaning process [27, 41, 45, 47]. The study by Lush et al. [41] has a descriptive case design and pertains to a convenience sample of 5 individuals. Dyspnea was systematically assessed by specially trained nurses every 4 hours. All patients reported dyspnea at some time during the study, with a VAS intensity reaching 95% of full scale in some cases. Weak correlations were found between dyspnea intensity and several physiologic variables such as PaO2, PaCO2 or ventilation. Stronger correlations were found between dyspnea intensity and the density of events and activities occurring in the ICU at the time of dyspnea evaluation, whether or not these events were directly related to patient care (examples of variables recorded included “change of shift”, “engineers in the unit”, “death next bed” etc.). The study by Karampela et al. [47] tested the “feasibility of incorporating a dyspnea evaluation protocol into bedside assessments routinely performed by respiratory therapists”. Systematic dyspnea assessment (“Are you feeling short of breath right now”; “Is your shortness of breath mild, moderate or severe?”) was performed at 4-hour intervals in 238 patients, leading to a database of 2539 patient-respiratory therapist encounters. Dyspnea was evaluated according to protocol in 74% of the cases; 32.1% of the patients were adequately alert to answer questions during encounters (n=600). Dyspnea was present in 11% of the cases, and was characterized as moderate to severe in a third of these. The study by Powers et al. [45] was designed to evaluate the “test-retest reliability of 5 dyspnea rating scales” and to examine the “correlations between each of these 5 rating scales and physiological measures of respiratory function”. Within a convenience sample of 28 patients, 50% reported dyspnea, with a median VAS rating of 52% of full scale. Finally, Schmidt et al. [27] studied 96 alert mechanically ventilated patients cared for at two separate ICUs. Forty-seven percent of these patients reported dyspnea, with a median VAS measure of 50% of full scale. This study was the first to explore the modality of dyspnea experienced by ventilated patients. When asked to describe their dyspnea as either “air hunger” or “excessive work/effort”; 56% of the dyspneic patients chose “air hunger” only; 16 % chose “excessive work/effort” only; 13% chose both “air hunger” and “excessive work/effort”; the remaining patients were unable to make a choice (Figure 2).
Figure 2.
Modality of dyspnea experienced by ventilated patients (from reference [27]).
The available set of data on dyspnea in mechanically ventilated patient is very limited in size and is heterogeneous in quality. It suggests that the frequency of this clinical issue is sufficiently high to make it worthy of attention. The next important question relates to the clinical relevance of being dyspneic under mechanical ventilation.
Clinical relevance of dyspnea in mechanically ventilated patients Immediate suffering
Dyspnea is a noxious sensation. With visual analog scale (VAS) ratings generally around 50% of full scale (e.g. [27]), dyspnea in mechanically ventilated patients qualifies as “moderate to intense”. It can however reach unbearable levels [e.g. 40]. Similar pain ratings constitute clear indication for administration of analgesics [50].
Dyspnea causes anxiety [51]. In the study by Schmidt et al. [27] discussed above, dyspneic patients were more likely to present with anxiety than non-dyspneic patients (71% vs. 24%) and dyspnea was independently associated with anxiety (odd ratio [OR], 8.84; 95% confidence interval [CI], 3.26 – 24.0; p <0.0001). Experimental dyspnea produces anxiety even in healthy subjects who know they are safe and disease free [19, 52]. The interplay between anxiety and dyspnea is complex and causative relationships can exist in both directions.
Delayed psychological sequelae
As mentioned above, patients’ recollections of their ICU experience point to dyspnea during mechanical ventilation as a major ICU stressor [38, 40]. Dark “respiratory recollections” may persist for several weeks. Bergbom-Engberg and Haljamae [40], studied 158 patients who had been mechanically ventilated during an ICU stay and who could remember the treatment. When questioned 2–48 months after discharge, 47% of these patients reported having felt anxiety and/or fear during mechanical ventilation. Respiratory difficulties (e.g. “ to synchronize with the respirator…”) were among potent drivers of feelings of anxiety, fear, agony, panic and insecurity.
Post Traumatic Stress Disorder (PTSD) is now recognized as a common sequel of the ICU experience [53–56]. Recalled dyspnea is associated with post-traumatic stress (PTSD) in ICU survivors [54]. PTSD symptom score in the post-ICU population is significantly correlated with duration of mechanical [55].
Impact on ICU stay outcomes
Dyspnea during mechanical ventilation may be useful to predict weaning outcome and ICU length of stay. In patients whose dyspnea failed to recede after adjusting ventilator settings successful extubation within 3 days of the assessment of dyspnea was significantly less frequent than in the patients whose dyspnea improved after ventilator adjustment [27].
The impact of dyspnea on ICU outcomes and on PTSD is strongly suggested by correlative data, but needs to be clarified with interventional trials. Until this link is disproved, the sensible and compassionate approach is to identify and alleviate dyspnea in mechanically ventilated patients.
Assessing dyspnea in the mechanically ventilated patient
The 2012 statement on dyspnea by the American Thoracic Society emphasizes strongly that “… dyspnea per se can only be perceived by the person experiencing it. Perception entails conscious recognition and interpretation of sensory stimuli and their meaning. Therefore, as is the case with pain, adequate assessment of dyspnea depends on self-report” [14]. The first step in managing dyspnea is therefore to ask the patient what he or she feels. One may obtain responses verbal or written responses to appropriate questions, responses to rating scales, or if these fail, use indirect means to assess.
Direct approach: guided questioning and rating scales
Clinical practice shows that conscious mechanically ventilated patients are able to answer simple questions about their respiratory sensations, even when they are slightly confused, cognitively impaired, or unable to speak due to an endotracheal tube. It is useful to start with a qualitative approach using questions phrased to allow simple yes/no answers, such as “is your breathing OK?”, “do you have difficulties breathing?”, “is your breathing comfortable?”.
Most awake patients can respond appropriately to pain and dyspnea scales, which can give considerably more useful information than binary responses. Rating scales provide a quantitative measure that can be tracked in time in the same patient, and can be statistically tested when assessing treatment effects, clinical unit performance, etc. In mechanically ventilated patients, dyspnea can be measured using available psychometric tools. Single-dimension dyspnea scales including the classical visual analogue scale, the modified Borg scale, a numerical ordinal scale, and a faces scale have been tested in a small population of mechanically ventilated patients [45]. Test-retest reliability and intraclass correlation were satisfactory [45]. In patients who cannot manipulate those tools themselves for any reason, it is easy for caregivers to provide adequate help. This option was explicitly offered in the study by Schmidt et al. [27] and no problems were reported. Of note, visual analogue scales have been used in mechanically ventilated patients to assess pain, anxiety, and the sense of inspiratory effort under different ventilatory assistance modalities [57].
Indirect approach: clinical surrogates
When the patient is unable to report his or her dyspnea, clinical surrogates of dyspnea would be useful. In mechanically ventilated patients, individual clinical signs correlate poorly with dyspnea ratings [27], contrary to the case in spontaneously breathing patients with acute respiratory failure. A composite observation scale such as the Respiratory Distress Observation Scale (RDOS) may be useful [58, 59]. This scale comprises weighted measures of heart rate, respiratory rate, the use of inspiratory neck muscles to breathe, the presence of paradoxical abdominal movement during inspiration, the degree of restlessness, the presence of end-expiratory grunting and nasal flaring, and, importantly, the presence of a fearful facial display. In studies of pulmonary rehabilitation patients and palliative care patients the RDOS was shown to correlate with VAS ratings and exhibited internal consistency and discriminant validity with pain [58]. It responded to treatment, including in patients who had not been able to self-describe their dyspnea [59]. However, the RDOS has only been validated in spontaneously breathing patients and needs to be adapted and validated for ventilated ICU Patients because several of the vital sign variables are likely to be disturbed by medications and mechanical ventilation in ICU patients.
Patient-ventilator dyssynchrony can be observed clinically and through inspection of the ventilator derived pressure and flow waveforms. How dyssynchrony relates to dyspnea is unclear. Indeed, patient-ventilatory asynchrony studies seldom mention dyspnea. Of note, situations typically associated with patient-ventilator asynchronies such as over-assistance in patients with COPD are likely to be associated with a strong central neural inhibition [60] that could, paradoxically, “protect” from dyspnea [61].
Electrophysiological surrogates
Physiological surrogates of neural respiratory drive based on flow, volume and respiratory muscle pressure generation usually underestimate levels of neural respiratory drive in patients with compromised respiratory mechanics [62]. Because they are not influenced by respiratory mechanics, electrophysiological indices may be better indices of the neural drive from respiratory motor centers. As mentioned above, a corollary copy of respiratory motor drive is thought to be an important excitatory input to dyspnea, thus a good measure of respiratory motor drive may help to infer dyspnea. It should be remembered, however, that severe discomfort arises not from drive alone, but from a failure of ventilation to match drive.
Electromyographic approach
The electromyographic activity (EMG) of the diaphragm and of the extra-diaphragmatic inspiratory muscles has often been used as a measure of respiratory center motor drive [63–65]. Several studies conducted in healthy subjects and in patients suggest that inspiratory muscle EMG mesurements could provide a surrogate neurophysiological biomarker of dyspnea [61, 64, 66–69]. These observations have been validated in ICU patients [61, 70].
Electroencephalographic approach
Normal individuals faced with external inspiratory loading exhibit a respiratory-related premotor cortical activity that can be described using electroencephalographic tools (EEG) [71]. This activity also becomes apparent during non-invasive mechanical ventilation when respiratory discomfort is induced through the use of inappropriate ventilator settings [72]. This premotor activity was correlated with respiratory discomfort under the circumstances of these experiments [72]. Although causal relationship between this cortical activity and dyspnea remains to be established and although ICU data have not yet been published, “respiratory-related EEG” could also provide a surrogate neurophysiological biomarker of dyspnea in the ICU.
Conclusions
Dyspnea in mechanically ventilated ICU patients is an under-recognized issue. We suggest that assessment and management of dyspnea in these patients has the potential to minimize suffering, reduce the use of sedation, and reduce ICU-related post-traumatic stress disorders. We suggest that future studies should aim to 1) provide tools to evaluate dyspnea in patients, who cannot respond to questioning; 2) directly evaluate the impact of dyspnea in of ICU patients and; 3) determine the efficacy of strategies aimed at minimizing dyspnea, ranging from improved ventilation strategies to pharmacologic interventions.
Table 1.
Prospective dyspnea studies in mechanically ventilated patients.
| Study | Number of patients | Frequency of dyspnea assessment | Total number of assessments | Nb of dyspneic patients (%) | Dyspnea Visual Analogue Scale (%FS) | Notes |
|---|---|---|---|---|---|---|
| Knebel and al. Connelly and al. [46] [44] | 21 | Repetitive | 40 | NA | 32 ± 22 | Compares dyspnea on SIMV and PSV during the weaning period. Dyspnea during SIMV/PSV predicted weaning success (p<0.05). Explores pre-weaning mood state and dyspnea in mechanically ventilated patients. |
| Bouley and al. [43] | 9 | Repetitive | 90 | NA | NA | Compares dyspnea experienced by ventilator-dependent patients receiving SIMV versus T-piece or PSV weaning. Findings indicated no difference in the degree of dyspnea experienced between weaning methods. |
| Karampela and al. [47] | 238 | Repetitive | 600 | 11 | NA | Tests feasibility of incorporating a dyspnea evaluation protocol into bedside assessments routinely performed by respiratory therapists on mechanically ventilated patients. |
| Twibell and al. [48] | 68 | Single time | 68 | NA | 35 ± 28 | Compares dyspnea on SIMV and PSV during weaning. Dyspnea was associated with physiological variables but not with weaning outcomes. |
| Lush and al. [41] | 5 | Repetitive | 189 | 100 | NA | Impact of environment variables as ICU environment, patient activities or events with dyspnea. |
| Powers and al. [45] | 28 | Repetitive | 53 | 50 | 52 ± 17 | Methodological study evaluating test-retest reliability of 5 dyspnea rating scale. |
| Schmidt and al. [27] | 96 | Single time | 96 | 47 | 50 (40–70) | Dyspnea was significantly associated with anxiety, assisted control ventilation and heart rate. Adjusting ventilator settings alleviated dyspnea in 35% of patients. |
%FS, % full scale; SIMV, Synchronized Intermittent Mandatory Ventilation; PSV, Pressure Support Ventilation; ICU, Intensive Care Unit; NA, not available. VAS results are given as mean ± standard deviation or median (25–75 interquartile range) when available.
Footnotes
Conflict of interest statement: On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- 1.Chanques G, Jaber S, Barbotte E, Violet S, Sebbane M, Perrigault PF, Mann C, Lefrant JY, Eledjam JJ. Impact of systematic evaluation of pain and agitation in an intensive care unit. Crit Care Med. 2006;34:1691–1699. doi: 10.1097/01.CCM.0000218416.62457.56. [DOI] [PubMed] [Google Scholar]
- 2.Payen JF, Bosson JL, Chanques G, Mantz J, Labarere J. Pain assessment is associated with decreased duration of mechanical ventilation in the intensive care unit: a post Hoc analysis of the DOLOREA study. Anesthesiology. 2009;111:1308–1316. doi: 10.1097/ALN.0b013e3181c0d4f0. [DOI] [PubMed] [Google Scholar]
- 3.Banzett RB, Mulnier HE, Murphy K, Rosen SD, Wise RJ, Adams L. Breathlessness in humans activates insular cortex. Neuroreport. 2000;11:2117–2120. doi: 10.1097/00001756-200007140-00012. [DOI] [PubMed] [Google Scholar]
- 4.Gracely RH, Undem BJ, Banzett RB. Cough, pain and dyspnoea: similarities and differences. Pulm Pharmacol Ther. 2007;20:433–437. doi: 10.1016/j.pupt.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morelot-Panzini C, Demoule A, Straus C, Zelter M, Derenne JP, Willer JC, Similowski T. Dyspnea as a noxious sensation: inspiratory threshold loading may trigger diffuse noxious inhibitory controls in humans. J Neurophysiol. 2007;97:1396–1404. doi: 10.1152/jn.00116.2006. [DOI] [PubMed] [Google Scholar]
- 6.Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 7.Henzler D, Pelosi P, Bensberg R, Dembinski R, Quintel M, Pielen V, Rossaint R, Kuhlen R. Effects of partial ventilatory support modalities on respiratory function in severe hypoxemic lung injury. Crit Care Med. 2006;34:1738–1745. doi: 10.1097/01.CCM.0000218809.49883.54. [DOI] [PubMed] [Google Scholar]
- 8.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 9.Serpa Neto A, Cardoso SO, Manetta JA, Pereira VG, Esposito DC, de Pasqualucci MO, Damasceno MC, Schultz MJ. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. JAMA. 2012;308:1651–1659. doi: 10.1001/jama.2012.13730. [DOI] [PubMed] [Google Scholar]
- 10.Lellouche F, Dionne S, Simard S, Bussieres J, Dagenais F. High tidal volumes in mechanically ventilated patients increase organ dysfunction after cardiac surgery. Anesthesiology. 2012;116:1072–1082. doi: 10.1097/ALN.0b013e3182522df5. [DOI] [PubMed] [Google Scholar]
- 11.Lellouche F, Lipes J. Prophylactic protective ventilation: lower tidal volumes for all critically ill patients? Intensive Care Med. 2013;39:6–15. doi: 10.1007/s00134-012-2728-4. [DOI] [PubMed] [Google Scholar]
- 12.Hofbauer RK, Fiset P, Plourde G, Backman SB, Bushnell MC. Dose-dependent effects of propofol on the central processing of thermal pain. Anesthesiology. 2004;100:386–394. doi: 10.1097/00000542-200402000-00031. [DOI] [PubMed] [Google Scholar]
- 13.Frolich MA, Zhang K, Ness TJ. Effect of sedation on pain perception. Anesthesiology. 2013;118:611–621. doi: 10.1097/ALN.0b013e318281592d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parshall MB, Schwartzstein RM, Adams L, Banzett RB, Manning HL, Bourbeau J, Calverley PM, Gift AG, Harver A, Lareau SC, Mahler DA, Meek PM, O’Donnell DE. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185:435–452. doi: 10.1164/rccm.201111-2042ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manning HL, Schwartzstein RM. Pathophysiology of dyspnea. N Engl J Med. 1995;333:1547–1553. doi: 10.1056/NEJM199512073332307. [DOI] [PubMed] [Google Scholar]
- 16.O’Donnell DE, Banzett RB, Carrieri-Kohlman V, Casaburi R, Davenport PW, Gandevia SC, Gelb AF, Mahler DA, Webb KA. Pathophysiology of dyspnea in chronic obstructive pulmonary disease: a roundtable. Proc Am Thorac Soc. 2007;4:145–168. doi: 10.1513/pats.200611-159CC. [DOI] [PubMed] [Google Scholar]
- 17.Banzett RB, Similowski T, Brown R. Addressing respiratory discomfort in the ventilated patient. In: Tobin MJ, editor. Principles and practice of mechanical ventilatio. 3. McGraw Hill; 2012. [Google Scholar]
- 18.Banzett RB, Lansing RW, Brown R, Topulos GP, Yager D, Steele SM, Londono B, Loring SH, Reid MB, Adams L, et al. ‘Air hunger’ from increased PCO2 persists after complete neuromuscular block in humans. Respir Physiol. 1990;81:1–17. doi: 10.1016/0034-5687(90)90065-7. [DOI] [PubMed] [Google Scholar]
- 19.Banzett RB, Pedersen SH, Schwartzstein RM, Lansing RW. The affective dimension of laboratory dyspnea: air hunger is more unpleasant than work/effort. Am J Respir Crit Care Med. 2008;177:1384–1390. doi: 10.1164/rccm.200711-1675OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banzett RB, Lansing RW, Reid MB, Adams L, Brown R. ‘Air hunger’ arising from increased PCO2 in mechanically ventilated quadriplegics. Respir Physiol. 1989;76:53–67. doi: 10.1016/0034-5687(89)90017-0. [DOI] [PubMed] [Google Scholar]
- 21.Evans KC, Banzett RB, Adams L, McKay L, Frackowiak RS, Corfield DR. BOLD fMRI identifies limbic, paralimbic, and cerebellar activation during air hunger. J Neurophysiol. 2002;88:1500–1511. doi: 10.1152/jn.2002.88.3.1500. [DOI] [PubMed] [Google Scholar]
- 22.Manning HL, Shea SA, Schwartzstein RM, Lansing RW, Brown R, Banzett RB. Reduced tidal volume increases ‘air hunger’ at fixed PCO2 in ventilated quadriplegics. Respir Physiol. 1992;90:19–30. doi: 10.1016/0034-5687(92)90131-f. [DOI] [PubMed] [Google Scholar]
- 23.Fowler WS. Breaking point of breath-holding. J Appl Physiol. 1954;6:539–545. doi: 10.1152/jappl.1954.6.9.539. [DOI] [PubMed] [Google Scholar]
- 24.Harty HR, Corfield DR, Schwartzstein RM, Adams L. External thoracic restriction, respiratory sensation, and ventilation during exercise in men. J Appl Physiol. 1999;86:1142–1150. doi: 10.1152/jappl.1999.86.4.1142. [DOI] [PubMed] [Google Scholar]
- 25.Simon PM, Schwartzstein RM, Weiss JW, Lahive K, Fencl V, Teghtsoonian M, Weinberger SE. Distinguishable sensations of breathlessness induced in normal volunteers. Am Rev Respir Dis. 1989;140:1021–1027. doi: 10.1164/ajrccm/140.4.1021. [DOI] [PubMed] [Google Scholar]
- 26.Killian KJ, Gandevia SC, Summers E, Campbell EJ. Effect of increased lung volume on perception of breathlessness, effort, and tension. J Appl Physiol. 1984;57:686–691. doi: 10.1152/jappl.1984.57.3.686. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt M, Demoule A, Polito A, Porchet R, Aboab J, Siami S, Morelot-Panzini C, Similowski T, Sharshar T. Dyspnea in mechanically ventilated critically ill patients. Crit Care Med. 2011;39:2059–2065. doi: 10.1097/CCM.0b013e31821e8779. [DOI] [PubMed] [Google Scholar]
- 28.Lansing RW, Gracely RH, Banzett RB. The multiple dimensions of dyspnea: review and hypotheses. Respir Physiol Neurobiol. 2009;167:53–60. doi: 10.1016/j.resp.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chonan T, Mulholland MB, Cherniack NS, Altose MD. Effects of voluntary constraining of thoracic displacement during hypercapnia. J Appl Physiol. 1987;63:1822–1828. doi: 10.1152/jappl.1987.63.5.1822. [DOI] [PubMed] [Google Scholar]
- 30.Banzett RB, Lansing RW, Evans KC, Shea SA. Stimulus-response characteristics of CO2-induced air hunger in normal subjects. Respir Physiol. 1996;103:19–31. doi: 10.1016/0034-5687(95)00050-x. [DOI] [PubMed] [Google Scholar]
- 31.Banzett RB, Adams L, O’Donnell CR, Gilman SA, Lansing RW, Schwartzstein RM. Using laboratory models to test treatment: morphine reduces dyspnea and hypercapnic ventilatory response. Am J Respir Crit Care Med. 2011;184:920–927. doi: 10.1164/rccm.201101-0005OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russell WC, Greer JR. The comfort of breathing: a study with volunteers assessing the influence of various modes of assisted ventilation. Crit Care Med. 2000;28:3645–3648. doi: 10.1097/00003246-200011000-00017. [DOI] [PubMed] [Google Scholar]
- 33.Leung P, Jubran A, Tobin MJ. Comparison of assisted ventilator modes on triggering, patient effort, and dyspnea. Am J Respir Crit Care Med. 1997;155:1940–1948. doi: 10.1164/ajrccm.155.6.9196100. [DOI] [PubMed] [Google Scholar]
- 34.Mols G, von Ungern-Sternberg B, Rohr E, Haberthur C, Geiger K, Guttmann J. Respiratory comfort and breathing pattern during volume proportional assist ventilation and pressure support ventilation: a study on volunteers with artificially reduced compliance. Crit Care Med. 2000;28:1940–1946. doi: 10.1097/00003246-200006000-00042. [DOI] [PubMed] [Google Scholar]
- 35.Manning HL, Molinary EJ, Leiter JC. Effect of inspiratory flow rate on respiratory sensation and pattern of breathing. Am J Respir Crit Care Med. 1995;151:751–757. doi: 10.1164/ajrccm/151.3_Pt_1.751. [DOI] [PubMed] [Google Scholar]
- 36.Fernandez R, Mendez M, Younes M. Effect of ventilator flow rate on respiratory timing in normal humans. Am J Respir Crit Care Med. 1999;159:710–719. doi: 10.1164/ajrccm.159.3.9709090. [DOI] [PubMed] [Google Scholar]
- 37.Vitacca M, Bianchi L, Zanotti E, Vianello A, Barbano L, Porta R, Clini E. Assessment of physiologic variables and subjective comfort under different levels of pressure support ventilation. Chest. 2004;126:851–859. doi: 10.1378/chest.126.3.851. [DOI] [PubMed] [Google Scholar]
- 38.Rotondi AJ, Chelluri L, Sirio C, Mendelsohn A, Schulz R, Belle S, Im K, Donahoe M, Pinsky MR. Patients’ recollections of stressful experiences while receiving prolonged mechanical ventilation in an intensive care unit. Crit Care Med. 2002;30:746–752. doi: 10.1097/00003246-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Shaw RJ, Harvey JE, Bernard R, Gunary R, Tiley M, Steiner H. Comparison of short-term psychological outcomes of respiratory failure treated by either invasive or non-invasive ventilation. Psychosomatics. 2009;50:586–591. doi: 10.1176/appi.psy.50.6.586. [DOI] [PubMed] [Google Scholar]
- 40.de Miranda S, Pochard F, Chaize M, Megarbane B, Cuvelier A, Bele N, Gonzalez-Bermejo J, Aboab J, Lautrette A, Lemiale V, Roche N, Thirion M, Chevret S, Schlemmer B, Similowski T, Azoulay E. Postintensive care unit psychological burden in patients with chronic obstructive pulmonary disease and informal caregivers: A multicenter study. Crit Care Med. 2011;39:112–118. doi: 10.1097/CCM.0b013e3181feb824. [DOI] [PubMed] [Google Scholar]
- 41.Lush MT, Janson-Bjerklie S, Carrieri VK, Lovejoy N. Dyspnea in the ventilator-assisted patient. Heart Lung. 1988;17:528–535. [PubMed] [Google Scholar]
- 42.Borgbjerg FM, Nielsen K, Franks J. Experimental pain stimulates respiration and attenuates morphine-induced respiratory depression: a controlled study in human volunteers. Pain. 1996;64:123–128. doi: 10.1016/0304-3959(95)00088-7. [DOI] [PubMed] [Google Scholar]
- 43.Bouley GH, Froman R, Shah H. The experience of dyspnea during weaning. Heart Lung. 1992;21:471–476. [PubMed] [Google Scholar]
- 44.Knebel AR, Janson-Bjerklie SL, Malley JD, Wilson AG, Marini JJ. Comparison of breathing comfort during weaning with two ventilatory modes. Am J Respir Crit Care Med. 1994;149:14–18. doi: 10.1164/ajrccm.149.1.8111572. [DOI] [PubMed] [Google Scholar]
- 45.Powers J, Bennett SJ. Measurement of dyspnea in patients treated with mechanical ventilation. Am J Crit Care. 1999;8:254–261. [PubMed] [Google Scholar]
- 46.Connelly B, Gunzerath L, Knebel A. A pilot study exploring mood state and dyspnea in mechanically ventilated patients. Heart Lung. 2000;29:173–179. doi: 10.1067/mhl.2000.105689. [DOI] [PubMed] [Google Scholar]
- 47.Karampela I, Hansen-Flachen J, Smith S, Reily D, Fuchs BD. A dyspnea evaluation protocol for respiratory therapists: a feasibility study. Respir Care. 2002;47:1158–1161. [PubMed] [Google Scholar]
- 48.Twibell R, Siela D, Mahmoodi M. Subjective perceptions and physiological variables during weaning from mechanical ventilation. Am J Crit Care. 2003;12:101–112. [PubMed] [Google Scholar]
- 49.Shacham S. A shortened version of the Profile of Mood States. J Pers Assess. 1983;47:305–306. doi: 10.1207/s15327752jpa4703_14. [DOI] [PubMed] [Google Scholar]
- 50.Wilson PR. Clinical practice guideline: acute pain management. Clin J Pain. 1992;8:187–188. doi: 10.1097/00002508-199209000-00001. [DOI] [PubMed] [Google Scholar]
- 51.Bergbom-Engberg I, Haljamae H. Assessment of patients’ experience of discomforts during respirator therapy. Crit Care Med. 1989;17:1068–1072. doi: 10.1097/00003246-198910000-00021. [DOI] [PubMed] [Google Scholar]
- 52.O’Donnell CR, Schwartzstein RM, Lansing RW, Guilfoyle T, Elkin D, Banzett RB. Dyspnea affective response: comparing COPD patients with healthy volunteers and laboratory model with activities of daily living. BMC Pulm Med. 2013;13:27. doi: 10.1186/1471-2466-13-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scragg P, Jones A, Fauvel N. Psychological problems following ICU treatment. Anaesthesia. 2001;56:9–14. doi: 10.1046/j.1365-2044.2001.01714.x. [DOI] [PubMed] [Google Scholar]
- 54.Schelling G. Effects of stress hormones on traumatic memory formation and the development of posttraumatic stress disorder in critically ill patients. Neurobiol Learn Mem. 2002;78:596–609. doi: 10.1006/nlme.2002.4083. [DOI] [PubMed] [Google Scholar]
- 55.Cuthbertson BH, Hull A, Strachan M, Scott J. Post-traumatic stress disorder after critical illness requiring general intensive care. Intensive Care Med. 2004;30:450–455. doi: 10.1007/s00134-003-2004-8. [DOI] [PubMed] [Google Scholar]
- 56.Rattray JE, Hull AM. Emotional outcome after intensive care: literature review. J Adv Nurs. 2008;64:2–13. doi: 10.1111/j.1365-2648.2008.04767.x. [DOI] [PubMed] [Google Scholar]
- 57.Ranieri VM, Grasso S, Mascia L, Martino S, Fiore T, Brienza A, Giuliani R. Effects of proportional assist ventilation on inspiratory muscle effort in patients with chronic obstructive pulmonary disease and acute respiratory failure. Anesthesiology. 1997;86:79–91. doi: 10.1097/00000542-199701000-00012. [DOI] [PubMed] [Google Scholar]
- 58.Campbell ML. Psychometric testing of a respiratory distress observation scale. J Palliat Med. 2008;11:44–50. doi: 10.1089/jpm.2007.0090. [DOI] [PubMed] [Google Scholar]
- 59.Campbell ML, Templin T, Walch J. A Respiratory Distress Observation Scale for patients unable to self-report dyspnea. J Palliat Med. 2010;13:285–290. doi: 10.1089/jpm.2009.0229. [DOI] [PubMed] [Google Scholar]
- 60.Hopkinson NS, Sharshar T, Dayer MJ, Lofaso F, Moxham J, Polkey MI. The effect of acute non-invasive ventilation on corticospinal pathways to the respiratory muscles in chronic obstructive pulmonary disease. Respir Physiol Neurobiol. 2012;183:41–47. doi: 10.1016/j.resp.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schmidt M, Kindler F, Gottfried SB, Raux M, Hug F, Similowski T, Demoule A. Dyspnea and surface inspiratory electromyograms in mechanically ventilated patients. Intensive Care Med. 2013 doi: 10.1007/s00134-013-2910-3. [DOI] [PubMed] [Google Scholar]
- 62.Sinderby C, Spahija J, Beck J. Changes in respiratory effort sensation over time are linked to the frequency content of diaphragm electrical activity. Am J Respir Crit Care Med. 2001;163:905–910. doi: 10.1164/ajrccm.163.4.2005121. [DOI] [PubMed] [Google Scholar]
- 63.Murphy PB, Kumar A, Reilly C, Jolley C, Walterspacher S, Fedele F, Hopkinson NS, Man WD, Polkey MI, Moxham J, Hart N. Neural respiratory drive as a physiological biomarker to monitor change during acute exacerbations of COPD. Thorax. 2011;66:602–608. doi: 10.1136/thx.2010.151332. [DOI] [PubMed] [Google Scholar]
- 64.Reilly CC, Ward K, Jolley CJ, Lunt AC, Steier J, Elston C, Polkey MI, Rafferty GF, Moxham J. Neural respiratory drive, pulmonary mechanics and breathlessness in patients with cystic fibrosis. Thorax. 2011;66:240–246. doi: 10.1136/thx.2010.142646. [DOI] [PubMed] [Google Scholar]
- 65.Luo YM, Li RF, Jolley C, Wu HD, Steier J, Moxham J, Zhong NS. Neural respiratory drive in patients with COPD during exercise tests. Respiration. 2011;81:294–301. doi: 10.1159/000317136. [DOI] [PubMed] [Google Scholar]
- 66.Chiti L, Biondi G, Morelot-Panzini C, Raux M, Similowski T, Hug F. Scalene muscle activity during progressive inspiratory loading under pressure support ventilation in normal humans. Respir Physiol Neurobiol. 2008;164:441–448. doi: 10.1016/j.resp.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 67.Hug F, Raux M, Morelot-Panzini C, Similowski T. Surface EMG to assess and quantify upper airway dilators activity during non-invasive ventilation. Respir Physiol Neurobiol. 2011;178:341–345. doi: 10.1016/j.resp.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 68.Ward ME, Corbeil C, Gibbons W, Newman S, Macklem PT. Optimization of respiratory muscle relaxation during mechanical ventilation. Anesthesiology. 1988;69:29–35. doi: 10.1097/00000542-198807000-00005. [DOI] [PubMed] [Google Scholar]
- 69.Parthasarathy S, Jubran A, Laghi F, Tobin MJ. Sternomastoid, rib cage, and expiratory muscle activity during weaning failure. J Appl Physiol. 2007;103:140–147. doi: 10.1152/japplphysiol.00904.2006. [DOI] [PubMed] [Google Scholar]
- 70.Schmidt M, Chiti L, Hug F, Demoule A, Similowski T. Surface electromyogram of inspiratory muscles: a possible routine monitoring tool in the intensive care unit. Br J Anaesth. 2011;106:913–914. doi: 10.1093/bja/aer141. [DOI] [PubMed] [Google Scholar]
- 71.Raux M, Straus C, Redolfi S, Morelot-Panzini C, Couturier A, Hug F, Similowski T. Electroencephalographic evidence for pre-motor cortex activation during inspiratory loading in humans. J Physiol. 2007;578:569–578. doi: 10.1113/jphysiol.2006.120246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Raux M, Ray P, Prella M, Duguet A, Demoule A, Similowski T. Cerebral cortex activation during experimentally induced ventilator fighting in normal humans receiving noninvasive mechanical ventilation. Anesthesiology. 2007;107:746–755. doi: 10.1097/01.anes.0000287005.58761.e8. [DOI] [PubMed] [Google Scholar]