SUMMARY
To explain how micron-sized cellular structures generate and respond to forces we need to characterize their micromechanical properties. Here we provide a protocol to build and use a dual force-calibrated microneedle-based set-up to quantitatively analyze the micromechanics of a metaphase spindle assembled in Xenopus laevis egg extracts. This cell-free extract system allows for controlled biochemical perturbations of spindle components. We describe how the microneedles are prepared and how they can be used to apply and measure forces. A multi-mode imaging system allows tracking of microtubules, chromosomes and needle tips. This set-up can be used to analyze the viscoelastic properties of the spindle on time-scales ranging from minutes to sub-seconds. A typical experiment, along with data analysis, is also detailed. We anticipate that our protocol can be readily extended to analyze the micromechanics of other cellular structures assembled in cell-free extracts. The entire procedure can take 3-4 days.
Keywords: metaphase spindle, microneedle, cell-free extracts, force spectroscopy, viscoelastic property, Xenopus, cell cycle, mitosis, microtubule
INTRODUCTION
Force plays a fundamental role in regulating the assembly and function of dynamic cellular architectures, such as the metaphase spindle. How these complex structures generate and respond to forces depends on their mechanical properties. In the last few decades, a wide range of biophysical techniques has been developed to examine the mechanics of the single molecule components (e.g., motor proteins) that play key roles in the assembly of these structures 1-3. Typically, these methods are used in aqueous solutions and involve purified components. Currently, analysis of the mechanics of micron-sized multi-component cellular structures remains difficult. One major limitation is our inability to isolate and manipulate these structures without loss of structural and functional stability.
The metaphase spindle is an anisotropic and dynamic microtubule-based structure that is assembled to segregate chromosomes during cell division 4. This micron-size structure is subjected to forces that act in various directions and over a wide range of time-scales, including those associated with the stretching of sister kinetochores 5, chromosome motion 6, and spindle positioning 7. Studies of cytoskeletal networks reconstituted from purified components 8 have provided valuable insights into how the metaphase spindle may respond to different forces. However, the structures analyzed in these studies are typically comprised of randomly arranged and non-dynamic polymers, such as a solution of chemically stabilized microtubules 9, and likely possess different characteristics from the native metaphase spindle. To understand how forces regulate the assembly and function of the metaphase spindle it is necessary to analyze its micromechanical properties in a context that is as close to physiological as possible, so that the complex and dynamic architecture of the structure is preserved.
Cell-free Xenopus egg extracts have been widely used to study metaphase spindle assembly and function 10,11. This in vitro system has facilitated the identification of a variety of spindle components and the analysis of their roles in spindle assembly and chromosome segregation. There are several advantages of this system, including the ability to directly add controlled amounts of chemical inhibitors 12,13, antibodies 14 and recombinant proteins 15,16. For the analysis of the mechanical properties of the metaphase spindle, this egg extract system has at least two important features: (1) The absence of a cell membrane allows probes to directly access the spindle; and (2) the spindle can be arrested at metaphase and remains stable over several minutes, a feature that could allow sufficient time for mechanical manipulations and data collection for a single spindle 17.
To probe the mechanical properties of the metaphase spindle we designed a dual force-calibrated microneedle-based set-up 18. Force-calibrated microneedles have been used to study the mechanical properties of a wide-range of biological structures that are micron-sized and are known to generate and/or respond to forces in the range of nanoNewtons. In particular, Bruce Nicklas's seminal work on the analysis of force-based regulation of chromosome segregation involved the use of microneedles to mechanically manipulate a single chromosome in a mitotic newt lung cell 19. Microneedles have also been used to analyze the micromechanics of muscle myofibrils 20, the sensory hair bundle 21, and the single sperm flagellum 22. Microneedles provide advantages over other approaches, such as set-ups based on optical tweezers 23, which typically have a lower force range (up to ~0.1 nanoNewton forces) and may non-specifically trap optically dense particles (e.g., vesicles) that are common in the egg extracts. Other approaches, such as magnetic tweezers 24 and atomic force microscopy (AFM) 25, can be used to apply higher forces (>1 nN), but typically require the object analyzed to be firmly immobilized, something that cannot currently be achieved for the dynamic metaphase spindle.
Here, we describe an optimized protocol to prepare micron-sized, force-calibrated glass needles, and use them to analyze the micromechanics of a metaphase spindle assembled in Xenopus egg extracts. An overview of our experimental system, materials used, and a step-by-step procedure are provided below. While this protocol has been developed for the metaphase spindle it may be readily extended to other biological structures assembled in cell-free extracts, and this is also discussed.
Experimental Design
Microscope setup
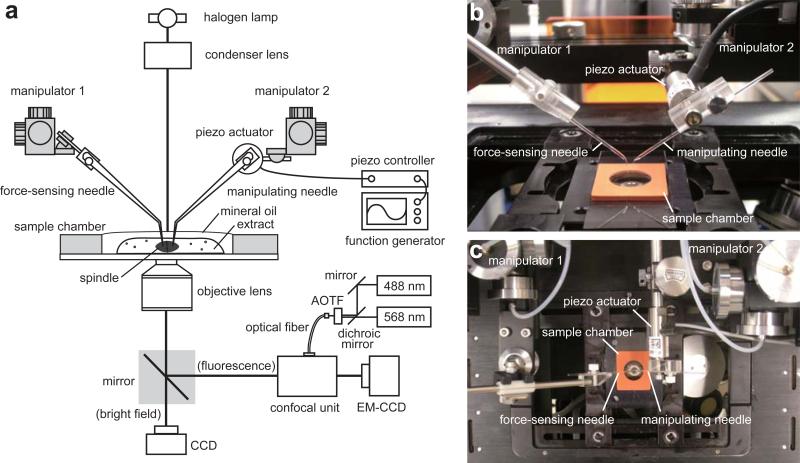
Figure 1a shows a schematic for the inverted microscope used in our analysis. This set-up allows for automated time-lapse data acquisition with high-resolution confocal fluorescence and bright field imaging, along with a microneedle-based mechanical manipulation. For confocal fluorescence imaging, a high-sensitivity CCD camera (e.g., EM-CCD) and a spinning-disk confocal unit are mounted on the side-port of the microscope. The confocal unit is equipped with two excitation lasers (e.g., 488 nm and 568 nm) such that the dye-labeled chromosomes and spindle microtubules can be visualized. The two laser beams are merged using a dichroic mirror and introduced into one end of a single-mode optical fiber, whose other end is attached to the confocal unit. The confocal unit is equipped with a dual-pass fluorescence filter set to allow for two-color imaging. An acousto-optic tunable filter (AOTF) can be placed in the laser light path to control excitation of fluorophores. The laser power should be attenuated so that photo-damage and photo-bleaching of the samples are minimal, while ensuring that the image quality is suitable for analysis. For tracking the motions of the microneedle tips, the images are obtained under bright field illumination and recorded by another CCD camera attached to the base-port of the microscope. Two micromanipulators are mounted on the microscope, one of which holds a piezo actuator. A function generator and a closed-loop piezo controller are attached to the actuator to control its motion. For automated time-lapse image acquisition, the microscope is equipped with motorized control over the filter turret, illumination shutters, and port changeover. The microscope, the AOTF and the cameras are connected to a personal computer (PC) equipped with Image acquisition software.
Figure 1.
Experimental set-up for analysis of metaphase spindle micromechanics. (a) Schematic of the system. A pair of microneedles is held by micromanipulators mounted on an inverted microscope. The CSF-arrested extract containing metaphase spindles is placed in a sample chamber. Confocal fluorescence imaging is used for visualizing chromosomes and spindle microtubules. Brigit field imaging is used for tracking motion of the microneedle tips. Photographs of a side view (b) and a top view (c) of the set-up around the sample chamber are presented.
Microneedle-based mechanical manipulation
We use two microneedles to analyze spindle micromechanics (Figure 1b and c). One microneedle, which is used to apply forces (referred to as the manipulating microneedle), is stiffer than the other and is attached to a piezo actuator, such that its motion can be precisely controlled. The other needle, which is used to measure forces (referred to as the force-sensing microneedle), is more flexible and behaves like a Hookean spring. The force at the tip of this needle can be estimated by multiplying its pre-calibrated stiffness by the tip's deflection. The tips of the manipulating and force-sensing microneedles are inserted into a single metaphase spindle assembled in Xenopus egg extracts. Neither heat nor strong background light is produced in this method; therefore, long-term and high spatiotemporal resolution imaging is possible, while limiting damage to the samples. Our set-up also incorporates a low-adsorptive coating on the coverslip and the microneedles, allowing for the measurement of the native micromechanical properties of the spindle without immobilizing the structure.
Viscoelastic analysis
To measure the viscoelastic properties of a material, the relationship between applied force (or stress) and deformation of the material (or strain) needs to be determined over a range of time-scales. For the viscoelastic analysis of the metaphase spindle on biologically relevant time-scales, we employ a method called sinusoidal analysis (also referred as ‘dynamic measurement’ or ‘oscillatory measurement’) 26. In this method, a sinusoidally varying force is applied to the sample at a fixed frequency, and the resultant deformation is measured. The analysis is carried out at different frequencies of the sinusoidal function. While other measurement techniques, such as ‘steady-shear’ measurements, can be used, the sinusoidal analysis most readily allows the determination of relevant parameters, such as rigidity and how viscous or elastic the material is at a particular time-scale 26. For a proper analysis, a few spindles are analyzed in one egg extract preparation. It is critical that this is repeated for at least three different independent extract preparations. The mechanical properties of the cytoplasm can be obtained using the same analysis without any spindles and can be used as a control.
Extension and application
This protocol has been developed to quantitatively analyze the mechanical properties of the metaphase spindle without immobilization of the structure. Capturing specific structures within the spindle, such as chromosomes or the spindle pole, may be achieved by using antibodies or tagged proteins. These extensions would allow experiments that could shed new light on the mechanics of spindle assembly and chromosome segregation.
To apply this protocol to other biological structures that can be assembled in cell-free extracts, such as centrosomes and nuclei 27-31, it is important to ensure that the frequency and amplitude of force application is properly selected. Irreversible damage of the structure has to be avoided and the time-scales analyzed should match the underlying molecular processes. To improve spatial and temporal resolution of our system for analysis of the viscoelastic properties at faster time-scales, the needle positions will need to be tracked using faster sampling-rate cameras or even a photodiode. It may also be important to increase the natural frequency of the actuator system (e.g., using a stiffer microneedle and a higher capacitance actuator) such that the motion of the microneedle tip remains fully under the control of the applied voltage delivered from the function generator.
MATERIALS
REAGENTS
DNA dye (SYTOX Green, Invitrogen, cat. no. S7020)
Dye-labeled tubulin (X-rhodamine-labeled, 5-10 mg ml−1, stored at −80 °C), prepared as described in 32. A detailed protocol can also be found at Mitchison Lab's website (http://mitchison.med.harvard.edu/protocols.html).
Sperm nuclei (prepared from male Xenopus laevis, 2-3 × 104 μl−1, stored at −80 °C), prepared as described in 10.
Silicone-coating solution (Sigma, cat. no. SL-2)
Mineral oil (Sigma, cat. no. M8410)
Agarose (SeaKem, cat. no. 50004)
Glass cleaning solution (chromic-sulfuric acid, Fisher, cat. no. SC88)
Vaseline (Sigma, cat. no. 16415)
Acetone (reagent grade)
REAGENTS SETUP
25× Calcium solution 10 mM CaCl2, 10 mM Hepes (pH 7.7 adjusted by KOH), 1 mM MgCl2, 100 mM KCl, 150 mM Sucrose, 10 μg/ml Cytochalasine D (Sigma, cat. no. C-8273). Store in aliquots at −20 °C. 100× SYTOX dye solution 1 μM SYTOX Green in H2O. Store in aliquots at −20 °C. 20× rhodamine-labeled tubulin ~0.5 mg ml−1 in CSF-extract (adjust the stock concentration as appropriate). Store in aliquots at −80 °C.
Alkaline ethanol solution 5%(w/v) sodium hydroxide, 80%(v/v) ethanol. Prepare fresh.
EQUIPMENT
Glass rod (Narishige, cat. no. G-1000)
Glass capillary puller (Narishige, cat. no. PC-10)
Microforge (World Precision Instruments, cat. no. MF-200) (an instrument designed to fabricate the tips of glass capillaries and microneedles)
Platinum heating filament (World Precision Instruments, cat. no. MF200-H4)
Three-axis micromanipulators (Narishige, cat. no. MHW-3 or equivalent) ×2
▲ CRITICAL The micromanipulators should allow for both coarse and fine movement control. Also, they should be equipped with low-drift or drift-free mechanisms so as to minimize drifting of the microneedles during the measurement, which is particularly important for obtaining data on minute time-scales.
Capillary holder ×2
Platinum wire (Omega Engineering, cat. no. SPPL-001); diameter ~25 μm
Forceps
Micro scissors
Gas microburner
Weighing scale
Piezo actuator (Physics Instruments, cat. no. P-841.10)
Piezo controller (Physics Instruments, cat. no. E-665)
▲CRITICAL Other combinations of piezo actuators and their controllers can also be used, but they should be equipped with a closed-loop circuit by which the piezo actuator can be operated with high linearity and repeatability in response to the applied voltage. Also, it is necessary to check the maximum driving frequency of the piezo system, above which the motion of the actuator may be attenuated.
Function generator (Hewlett-Packard, 33120A)
Rubber gasket (22 × 25 × 1 mm, 13 mm aperture, Grace Bio-labs, cat. no. 664113 or similar)
Coverslips: 24 × 60 mm, No. 1
Slide staining box
Conical tubes: 15 ml and 50 ml
Beaker: 250 ml
Sample tubes: 1.5 ml
Water bath (at 18 °C)
Thermostat
Thermometer
Microneedle storage box, e.g., pipette tip box with a Styrofoam block. A double-stick tape on the upper surface of the block can be used to hold microneedles.
Humidified chamber, e.g., pipette tip box containing a coverslip rack and moist kimwipes. The rack should hold coverslips vertically.
Inverted fluorescence microscope (Nikon TE2000 or comparable)
Spinning disk confocal scanner unit (CSU-10, Yokogawa)
Oil-immersion objective lens (100×, PlanApo, ~1.4 NA)
EM-CCD camera (Photometrics Cascade 512B or comparable)
CCD camera (Photometrics CoolSNAP or comparable)
488 nm laser (Model LS300A, Dynamic Laser)
568 nm laser (Model LS300K, Dynamic Laser)
Dichroic mirror (520DCLP, Chroma), used for merging two laser lines
Dual pass fluorescence filters (Z488/568, Chroma), placed in the confocal scanner unit
Single-mode optical fiber
AOTF device (NEOS Technologies), used for switching the excitation lasers
Image acquisition software (Metamorph, Molecular Devices)
Image processing software (Image J)
Data analysis software (Origin, Origin Lab Corporation)
EQUIPMENT SETUP
Microscope for microneedle calibration
Attach 10× and 40× dry objectives to an inverted microscope, equipped with bright field illumination. The focusing knob should be equipped with a micrometer scale in order to precisely read the objective's height. To hold glass microneedles, mount micromanipulators onto the microscope.
PROCEDURE
Preparation of a reference needle
Heat the middle of a glass rod using a gas microburner and pull one of its ends into a long and thin fiber. Break it in the middle of the fiber. Bend the tip of the fiber into an L shape by heating with a microburner. The fiber should measure 10-20 mm in length and ~100 μm in diameter (Figure 2a).
Hold the shaft of the glass rod and mount it horizontally on an inverted microscope, with the bent tip facing upward. Adjust the position of the glass rod so that its tip is placed within the field of view of the microscope.
Cut a piece of platinum wire into various lengths (~1-20 mm). Estimate the weight of the individual pieces based on its average mass per unit length. Use forceps to bend each piece of the wire into a horseshoe shape.
Hang the horseshoe-shaped piece of wire at the tip of the fiber of the glass rod mounted on the microscope (Figure 2b). Using a low resolution objective (10×), measure the vertical displacement of the tip caused by bending. Record the weight and the resultant displacement of the needle tip.
Repeat step 4 for the different pieces of wire.
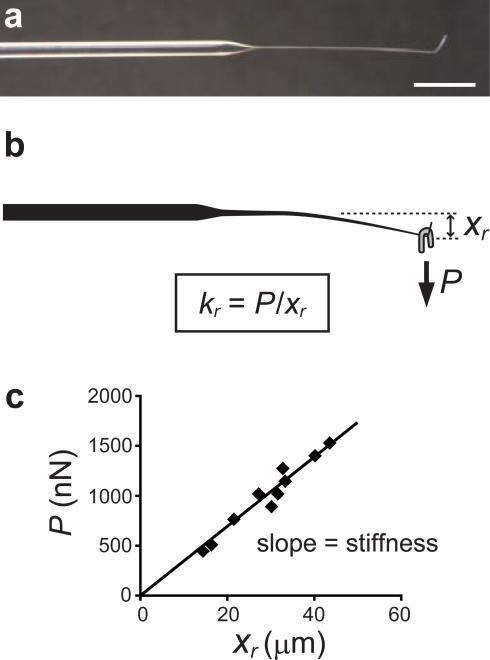
Plot the weight (P, y-axis) and the displacement (xr, x-axis) in a scatter graph (Figure 2c). Determine the stiffness (= kr, N m−1) by calculating the slope using a linear regression analysis. This needle is used as a reference (hereafter, the reference needle) to calibrate the stiffness of other needles that are used in actual experiments.
Figure 2.
Calibration of the reference needle. (a) Image of the reference needle. Scale bar, 5 mm. (b) A horse-shoe shaped weight (gray) is hung on the L-shaped tip of the reference needle to apply a load (P, nN). This results in the vertical displacement of the needle tip (xr, μm). The stiffness of the reference needle (kr) is determined by the slope of the load-displacement relationship obtained using several different weights. An example of a calibration data set is shown in (c) (see also Table 1 for description of the variables used.).
? TROUBLESHOOTING
PAUSE POINT: The reference needle can be stored for several days in a dust-free box.
Preparation of microneedles
-
7
Heat and pull a glass rod using a capillary puller such that the taper is ~5 mm long and the diameter of the tip is ~10 μm.
-
8
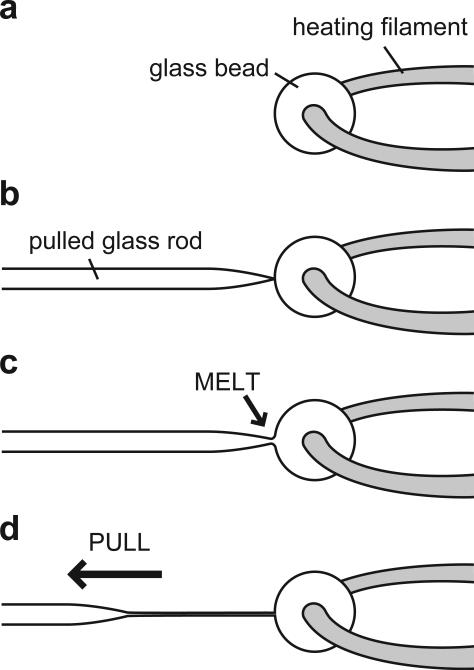
To further process the needle in order to prepare a longer and thinner fiber, two additional steps are required: (i) Take another glass rod and place it in the pipette holder of the microforge. Contact its tip with the platinum heating filament and heat to melt the tip of the glass rod. Continue heating until a glass bead forms around the midpoint of the filament. Then remove the glass rod, leaving a glass bead (~100 μm) on the filament (Figure 3a). (ii) Place the pulled glass rod (prepared in Step 7) in the pipette holder and touch the glass bead with its tip (Figure 3b). Raise the temperature of the filament until the color of the glass bead turns orange. As soon as the tip and the glass bead melt and fuse together (Figure 3c), pull the glass rod quickly (~1-10 cm in ~100 ms) away from the glass bead such that the fused part is stretched to form a long and thin fiber (Figure 3d). The fiber should measure 50-500 μm in length and 1-2 μm in diameter (Figure 4a).
Figure 3.
Fabrication of the microneedles. (a) A glass bead is attached to the heating filament of a microforge. (b) The tip of a pulled glass rod is touched to the glass bead. (c) The tip and the bead melt and fuse together as the temperature of the filament increases. (d) A long and thin fiber can be prepared by quickly moving the glass rod away from the glass bead.
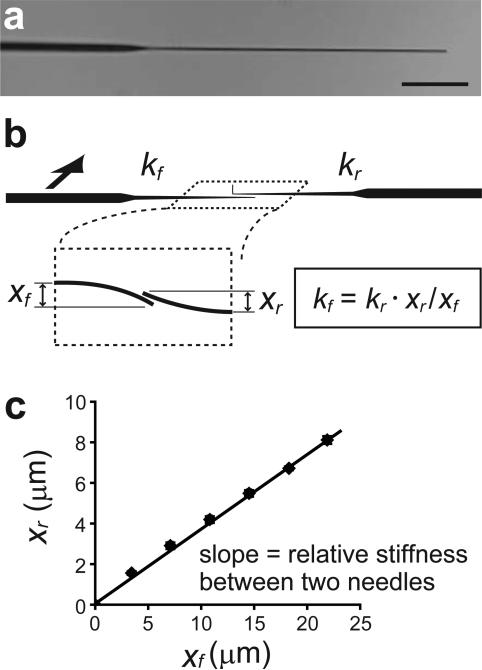
Figure 4.
Calibration of the microneedles. (a) Image of a force-sensing microneedle. Scale bar, 50 μm. To calibrate the stiffness of a force-sensing microneedle (kf), the tips of the reference needle and the force-sensing microneedle are pushed against each other (b). The stiffness kf is determined based on the pre-calibrated stiffness of the reference needle (kr) and the relative displacement of the tips of the reference needle (xr) and the force-sensing microneedle (xf). An example of the calibration result is shown in (c)(see also Table 1 for description of the variables used.).
CRITICAL STEP: The glass fiber's length and diameter determine stiffness. Changing the speed and timing of pulling, or the temperature, can be used to control the microneedle's length and diameter. In general, faster pulling and higher temperature yield a longer and thinner fiber.
-
9
Cut the tip of the fiber to a desired length using precision scissors.
Calibration of microneedles
-
10
Hold the pre-calibrated reference needle (prepared in Steps 1-6) and the microneedle (prepared in Steps 7-9) using two micromanipulators mounted on an inverted microscope. Make the tips of the two needles face toward each other and align them in parallel (Figure 4b).
-
11
Bring the tips of the two needles into contact. Using the micromanipulator, push one of the needles against the other (inset, Figure 4b). Measure the bending displacements of the two needle tips, and then move the needle back to the original position. Use a 40× objective to measure the displacements. Repeat the measurement at 5-10 different equilibrium points. The bending should be measured in the direction(s) that will be used in the actual mechanical measurement in subsequent steps.
?TROUBLESHOOTING
-
12
Determine the stiffness of the microneedle (= kf) according to kf = kr·xr/xf, where kr is the pre-calibrated stiffness of the reference needle, and xr/xf is the relative bending displacement of the tips of the reference needle (xr) and the microneedle (xf) at equilibrium points (Figure 4c). Prepare several microneedles with various kf values. The manipulating microneedles should be >100 times stiffer than the force-sensing microneedles.
CRITICAL STEP: The stiffness of microneedles can be adjusted by changing their diameter and length. The bending stiffness of a rod clamped at one end is proportional to the fourth power of its diameter and inversely proportional to the third power of its length.
?TROUBLESHOOTING
PAUSE POINT: The force-calibrated microneedles can be stored in a dust-free box.
Preparation of agarose-coated coverslips
-
13
Dispense alkaline ethanol solution into a slide staining box. Place 24 × 60 mm coverslips in the staining box and sonicate for 20 min.
-
14
Discard the solution. Wash the coverslips 5-6 times with deionized water. Immerse them in deionized water and sonicate for 5-10 min.
-
15
Repeat Step 14 three times.
-
16
Transfer the coverslips to a coverslip holder. Dry the coverslips for about 10 min at a clean bench.
CRITICAL STEP: Immediately proceed to Step 17 after drying.
-
17
Prepare 1 % (w/v) agarose solution in a 15 ml conical tube. Weigh the tube containing the agarose solution.
-
18
Place the conical tube in a water-filled 250 ml beaker, and heat. Maintain gentle boiling (100 °C) and flick the tube once every few minutes until all the agarose is dissolved. Do not turn the tube upside down as the hot agarose solution may leak from the tube top. Small bubbles may appear at first, but the solution should become transparent in 2-3 min. Once all the agarose is dissolved, lower the temperature of the solution to ~80 °C. At the same time, prepare warm (~80 °C) deionized water.
-
19
Weigh the tube again. Add warm deionized water to recover the initial weight. Mix thoroughly.
-
20
In a 50 ml conical tube, dispense 5 ml of the 1% (w/v) agarose solution and 45 ml of warm deionized water such that the final concentration of agarose is 0.1%. Mix thoroughly. Keep the solution at ~80 °C.
-
21
Hold one edge of the washed coverslips with forceps, and dip it into the warm agarose solution for a few seconds. Gently remove the coverslip from the solution. The surface of a properly coated coverslip typically has a rainbow-colored reflection.
?TROUBLESHOOTING
-
22
Place the coverslips in a rack and incubate them at a clean bench for 10-20 min at room temperature (~23 °C). Keep the coverslips vertical until the agarose has solidified.
?TROUBLESHOOTING
PAUSE POINT: The agarose-coated coverslips can be stored in a humidified chamber at 4 °C for 2-3 days.
Spindle assembly
-
23
Prepare cytostatic factor (CSF) extract in a sample tube and keep on ice. A detailed protocol for preparing these extracts can be found in ref. 10 and 11.
-
24
Pipette 50 μl (= 1 volume) of the CSF extract into a 1.5 ml sample tube. Add sperm nuclei at the final concentration of ~600 nuclei per μl. Mix gently by tapping the tube.
-
25
Add 1/25 volume of 25× calcium solution into the reaction and mix well.
-
26
Incubate the reaction in a water bath at 18 °C to drive the extract into interphase.
-
27
At 80 min, add 1 volume of fresh CSF extract, 1/100 volume of 100× DNA dye and 1/20 volume of 20× rhodamine-labeled tubulin to the reaction. Mix gently by tapping the tube. Incubate at 18 °C to drive the extract back into metaphase.
-
28
At 45-60 min, metaphase spindles should have formed. The spindles should be robust for 1-2 hours when the extract is of good quality.
Force measurement and live imaging
-
29
Clean the microneedle tips (prepared in Steps 7-12) by soaking them in a glass cleaning solution. Wash them in deionized water twice and then in acetone once. Dry the surface in air.
-
30
Immerse the tips of the microneedles in a silicone-coating solution for 30-60 s. Remove the microneedles from the solution and rinse with deionized water.
-
31
Load the microneedles onto micromanipulators mounted on an inverted fluorescence microscope (Figure 1). The force-sensing microneedle can be directly attached to a micromanipulator. The manipulating microneedle should be attached via a piezo actuator to control its motion. Apply an offset voltage to the piezo actuator so that the displacement of the actuator is in the middle of its entire traveling distance. This allows the actuator to move in both directions in the subsequent steps. Adjust the orientation of the microneedles so that the tip is perpendicular to the imaging plane of the microscope. If necessary, bend the needle shaft in the middle using a microburner to obtain the best angle (see Figure 1b).
CAUTION: Tilting and shearing forces must be avoided when attaching the manipulating microneedle to the piezo actuator because they will damage the actuator.
-
32
Assemble a sample chamber using the agarose-coated coverslip (prepared in Step 13-22) placed on a rubber gasket. Apply Vaseline on one side of the rubber gasket and press firmly to attach it to the coverslip to prevent samples from leaking.
-
33
Place the sample chamber on the microscope stage.
-
34
Using the coarse-movement knobs of the micromanipulators, move the tips of the microneedles close to the coverslip surface. The Bertrand lens of the microscope can be used to observe the microneedle tips located far from the objective's focal plane. Adjust x, y, and z positions of the microneedle tips to bring them to the center of the field of view using the fine-movement knobs of the micromanipulators. In subsequent steps, a metaphase spindle in the sample chamber is located by moving the microscope stage. Put a mark on the z-direction knob of the micromanipulators to record the height of the microneedle tips and move the microneedles upward while holding the x and y positions. This enables the samples to be moved in the chamber, while allowing the microneedles to be returned to the recorded position once the spindle has found. This step prevents breakage of the microneedle tips.
CRITICAL STEP: It is also important to check that the two microneedle tips can be brought close to each other (at least ~5 μm in x-y plane) and moved freely without any contact.
-
35
Spread 2-5 μL of the extract containing metaphase spindles (prepared in Steps 23-28) onto the coverslip surface.
-
36
Layer ~100 μL of mineral oil gently onto the extract to prevent the sample from drying.
-
37
Incubate the sample for several minutes to allow the sample fluid to settle. It typically takes 1-5 min, but may vary sample to sample. Prepare a new chamber if the sample does not settle.
-
38
Image chromosomes using the fluorescence signal of the DNA dye and search for a metaphase spindle floating in the chamber. Once a spindle is found, switch to the tubulin-dye channel to check its size and morphology. Select a spindle with a representative shape and size (e.g., 30-50 μm in length with bipolar structure and chromosomes aligned at the metaphase plate).
-
39
Switch to the bright field channel and carefully move the manipulating microneedle down to the position recorded in Step 34. Switch back to the tubulin-dye channel. By steering the tip of the manipulating microneedle, gently push the side of the spindle to adjust its position and orientation.
CRITICAL STEP: Confirm that the spindle can be moved freely in the chamber without sticking to the coverslip surface.
-
40
Place the tip of the manipulating microneedle on top of the spindle. Insert the tip into the spindle, moving the tip no closer than 1-2 μm from the coverslip surface. The distance of the tip from the coverslip surface can be checked using confocal images of spindle microtubules. Also, ensure that the tip is not contacting any chromosomes in the spindle.
-
41
As in Steps 39-40, move the force-sensing microneedle down to the chamber and insert its tip into another part of the spindle. For measurements along the pole-to-pole axis of the spindle, insert the manipulating and the force-sensing microneedles into each half of the bipolar spindle. For measurements perpendicular to the pole-to-pole axis of the spindle, insert both needles in one half of the spindle (Figure 5a and b).
Figure 5.
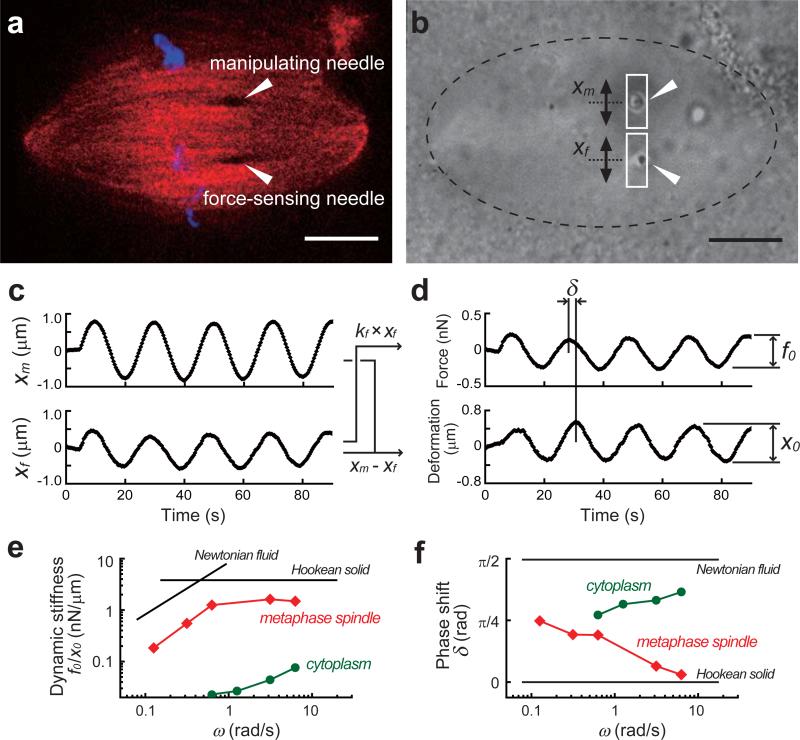
An example of an experiment and analysis. (a) A metaphase spindle is visualized by confocal imaging of chromosomes (SYTOX-dye, blue) and spindle microtubules (X-rhodamine tubulin, red). The positions of the microneedle tips (dark spots in the structure) are indicated (arrow heads). (b) Bright field images are used to track the motion of the two microneedle tips (arrow heads). The manipulating microneedle can be moved via the piezo actuator along the spindle's short axis. A rectangular ROI is drawn around each microneedle tip and analyzed. Scale bars, 10 μm. (c) The motions of the manipulating microneedle tip (xm) and the force-sensing microneedle tip (xf) are measured. (d) Force is determined by multiplying the displacement of the force-sensing microneedle tip by its pre-calibrated stiffness (i.e., kf × xf). The distance between the two microneedle tips (i.e., xm – xf) yields deformation. The amplitudes of the force and deformation (f0 and x0) and the phase shift (δ) between the two responses can be obtained by fitting the responses to sinusoidal functions. (e, f) An example of the frequency-dependent change in the viscoelastic properties of the metaphase spindle (red squares) and the cytoplasm (green circles). The dynamics stiffness (f0/x0) and the phase shift (δ) are determined at 4-5 different frequencies. The graphs also show typical responses of a perfect elastic solid (Hookean solid) and a linear viscous fluid (Newtonian fluid). Note that the absolute value in (e) depends on the material (see also Table 1 for a description of the variables used).
<CRITICAL STEP> Be extremely cautious not to break the calibrated tips of the microneedles!
-
42
Set the waveform of the function generator to ‘sinusoidal function’. Set the amplitude and the frequency of the sinusoidal function that will be applied to the piezo actuator in subsequent steps. Select the output mode to ‘trigger’ so that the voltage is delivered to the actuator just after the trigger button is pressed. Also set the number of cycles so that the sinusoidal function is repeated for a limited number of periods. Typically 2-10 cycles are used and can be adjusted depending on the frequency.
-
43
Switch to the bright field channel and start the image acquisition. The sampling rate of the recording should be adjusted depending on the typical speed of the microneedle motion in the measurement. For the analysis described in Steps 48-59, the sampling rate should be at least 10-20 times faster than the frequency of the applied voltage. The sampling rate may be increased by narrowing the region of image acquisition of the camera or by reducing the exposure time.
-
44
Press the trigger button in the function generator to start applying voltage to the piezo actuator. The piezo controller should be used in the closed-loop mode so that the piezo actuator moves linearly in response to the applied voltage and without hysteresis. The force developed in the spindle can be measured by the displacement of the tip of the force-sensing microneedle. Deformation in the structure in response to the applied force can be monitored by the change in distance between the two microneedle tips. Details of the analyses are provided in the Data Analysis section. Deformations arising in the microtubule network of the spindle can also be observed by confocal fluorescence imaging. For the fluorescence imaging, maximize the camera sensitivity and minimize the exposure time to obtain sufficient image quality with minimum photo-toxicity and photo-bleaching.
?TROUBLESHOOTING
CRITICAL STEP: The motion of the manipulating microneedle tip should correspond to that of the piezo actuator only when the natural frequency of the microneedle is well above the driving frequency of the actuator. Therefore, it is important to confirm before the actual experiments that the motion of the manipulating microneedle tip is under the control of the piezo actuator over the frequency ranges examined (e.g., 0.02-2.0 Hz in this protocol).
-
45
Stop the recording. Save the recording as an image stack file in TIFF format.
-
46
Change the frequency of the sinusoidal function and repeat Steps 42-45 to obtain a data set from the single metaphase spindle. A typical experiment lasts 5-8 min 18.
CAUTION: The deterioration of the sample should be monitored by measuring a reference mechanical response of the spindle between measurements. A sinusoidal application of force at given amplitude (~0.2 nN) and frequency (~0.5 Hz) can be used without significantly altering the mechanical properties of the spindle 18. Typically, the sample remains stable for at least 10-20 min in the chamber (>70% of the initial reference response is maintained). If a significant deterioration is observed during the measurement, the data should be discarded and another set of measurements should be performed with a new spindle and using a smaller amplitude of oscillation.
?TROUBLESHOOTING
-
47
After the measurement is completed, detach the microneedles from the micromanipulators. Wash the tips of the microneedles by soaking them in a glass cleaning solution for 5 min. Wash them in deionized water twice and then in acetone once. Put them back into a dust-free box for storage and reuse.
Data analysis
-
48
Install Image J (http://rsbweb.nih.gov/ij/) and Origin to a PC. The protocol provided below is performed using Image J and Origin, but other software with equivalent functions can also be used.
-
49
Open Image J. Open one of the image stack files obtained from the micromanipulation experiments (Steps 45).
-
50
In the first frame of the image stack, draw a rectangular region of interest (ROI) around the manipulating microneedle tip (white rectangle in Figure 5b). The height of the ROI should have a length larger than the entire motion of the microneedle tip (typically 2-5 μm long) and the width should fit the size of the microneedle tip (i.e., 1-2 μm). Extract the intensity profile in the ROI from each image as text files.
CAUTION: The motion of the microneedle tip should be parallel to the long axis of the ROI. If they are not aligned with each other, rotate the entire image stack using the ‘Rotate arbitrary’ function under the ‘Image’ tab in Image J.
-
51
Open the intensity profile of the first frame (obtained in Step 50) in Origin, and calculate the position of the center of mass of the microneedle tip using the built-in Gaussian curve fitting function.
-
52
By repeating Steps 51 for all of the image frames, obtain time courses of the displacement of the tip of the manipulating microneedle (xm) (upper panel, Figure 5c). The centroid tracking of the microneedle tips can also be performed using ‘Track Objects’ module in MetaMorph or other free software (e.g., Video Spot Tracker, http://cismm.cs.unc.edu/downloads/).
-
53
Perform the same analysis (Steps 50-52) for the force-sensing microneedle tip and obtain the time course of its displacement (xf) (lower panel, Figure 5c).
-
54
At each time point, estimate the applied force by multiplying the displacement of the tip of the force-sensing microneedle (xf) by its pre-calibrated stiffness (kf). This yields the time course of force response (top panel in Figure 5d).
-
55
At each time point, estimate the deformation in the spindle by calculating the distance between the two microneedle tips (xm - xf). This yields the time course of deformation response (bottom panel in Figure 5d).
-
56
Using a nonlinear least-squares fitting tool in Origin, fit the time course of the force response (obtained in Step 54) to a sinusoidal function: f(t) = f0•sin(ωt + θ1) + a1t + b1. Here, a1 and b1 are used as parameters to correct for drift and offset, respectively. Set the parameter ω (angular frequency, = 2πF) as an invariable. Here, F is the input frequency used in the experiment (in Step 44). Set the other parameters (f0, θ1, a1, and b1) as free variables. Put appropriate initial values for the variables and perform the fitting. Repeat the fitting until the χ2 value converges. In the same way, fit the time course of the deformation response (obtained in Step 55) to another sinusoidal function: x(t) = x0•sin(ωt + θ2) + a2t + b2. Acquire the values for the amplitudes of the two sinusoids, f0 and x0, and the oscillation phases, θ1 and θ2.
CRITICAL STEP: The free parameters should be determined independently of the initial values. Also, the drift and offset (i.e., ai and bi) should be below a threshold (typically <5% of the amplitudes). Only the data that satisfy these criteria can be used for the subsequent analysis.
-
57
Calculate the ratio of the amplitude of force (f0) to that of deformation (x0), i.e., f0/x0. This represents the dynamic stiffness, which is the overall rigidity of the structure at a given frequency.
-
58
Calculate the difference in phase between the two sinusoids, i.e., (θ1 – θ2) = δ. This represents the phase shift, which is the measure of how viscous or elastic the structure is at a given frequency.
-
59
Repeat Steps 49-58 for data obtained at different frequencies. Plot the dynamic stiffness and the phase shift as a function of frequency which covers the time-scale of interest (Figure 5e and 5f). A perfect elastic solid, such as a Hookean spring, has stiffness that is independent of the frequency of force application and shows δ = 0. On the other hand, a linear viscous fluid, such as glycerol, reveals a linear increase in its stiffness with the frequency and shows δ = π/2 independent of the frequency
CRITICAL STEP: Only data from spindles exhibiting a reference mechanical response above a threshold (e.g., >70% of the initial reference value) should be used to generate the frequency-dependent profile (see CAUTION in Step 46).
TIMING
Step 1-6: Preparation and calibration of a reference needle --- 3-4 h
Step 7-9: Preparation of microneedles --- ~30 min per microneedle
Step 10-12: Calibration of microneedles --- ~1 h per microneedle
Step 13-22: Preparation of agarose-coated coverslips --- 1-2 h
Step 23: Preparation of CSF extracts --- ~2 h (5 d including priming frogs)
Step 24-28: Spindle assembly reaction --- 140 min
Step 29-34: Setting microneedles --- ~20 min
Step 35-47: Mechanical measurement in the spindle --- 10-30 min per sample
Step 48-59: Data analysis --- 4-6 h
TROUBLESHOOTING
Troubleshooting advice can be found in Table 2.
Table 2.
Troubleshooting table.
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 6 | Force-displacement relationship is not linear | Bending displacement of the needle tip is too large | Use lighter weights or prepare a stiffer needle |
| 11 | Needle tips vibrate or fluctuate during calibration | Air currents interfering with measurement | Shut off any possible sources of air flow or perform calibration in water |
| 12 | Calibration error is huge | The difference in stiffness between two needles is too large (>10-fold) | Prepare a more flexible reference needle, or prepare another needle which has an intermediate stiffness between those two needles such that the difference is less than 10-fold |
| 21 | Agarose surface looks bumpy and has uneven thickness | Agarose is concentrated when warmed The temperature of agarose solution during coating process is too low |
Keep the agarose concentration exact (0.1% final) Turn up the temperature of agarose solution to 80°C while keeping the agarose concentration |
| 22 | Agarose is patchy on coverslip surface | Hydrophilic surface has deteriorated | Reduce the time of surface drying |
| 44 | Spindle rotates and moves in chamber | Extract solution has not fully settled | Spread the extract with even thickness on the coverslip and layer the oil on top very gently |
| 46 | Spindle collapses over time | Bad extracts Photodamage |
Use fresh and high-quality extract Use minimal exposure of fluorescence light by optimizing camera sensitivity, binning, interval of time-lapse, filter set, etc |
ANTICIPATED RESULTS
Figure 5 provides an example of the frequency-dependent viscoelastic properties of the metaphase spindle analyzed using the protocol detailed here. In this measurement, two microneedles were inserted into one half of the bipolar metaphase spindle (Figure 5a and b). The manipulating microneedle was moved perpendicular to the pole-to-pole axis of the spindle with a sinusoidal function at a given frequency (upper panel, Figure 5c), resulting in the displacement of the force-sensing microneedle tip (lower panel, Figure 5c). The force and deformation responses were analyzed by tracking the motion of both microneedle tips (Figure 5d) and the two viscoelastic parameters, the dynamic stiffness and the phase shift, were determined. By changing the frequency of the sinusoidally varying force, the frequency-dependent changes in viscoelastic parameters can be obtained (red squares, Figure 5e and f). The data indicate that the structure of the spindle becomes less rigid and more viscous as the force is applied at lower frequencies (or slower time-scales). The characteristics should be distinguishable from those of the cytoplasm obtained with the same analysis without any spindles (green circles, Figure 5e and f).
Table 1.
Variables used in the text.
| Variable | Definition | SI Unit |
|---|---|---|
| P | Load applied at the reference needle tip | N |
| kr | Stiffness of the reference needle | N m−1 |
| xr | Displacement of the reference needle tip | m |
| kf | Stiffness of force-sensing microneedle | N m−1 |
| xf | Displacement of force-sensing microneedle tip | m |
| xm | Displacement of manipulating microneedle tip | N m−1 |
| f0 | Amplitude of force response in the sinusoidal analysis | N |
| x0 | Amplitude of deformation response in the sinusoidal analysis | m |
| F | Frequency of the sinusoidal function for force application | Hz |
| ω | Angular frequency of the sinusoidal function for force application (= 2πF) | rad s−1 |
| ai | Fitting parameter for drift correction | |
| bi | Fitting parameter for offset correction | |
| θ i | Oscillation phase of the force/deformation response | rad |
| δ | Phase shift between the force and deformation sinusoids | rad |
ACKNOWLEDGEMENTS
T.M.K. acknowledges support from NIH/NIGMS (GM065933). Y.S. was a recipient of the Uehara memorial foundation postdoctoral fellowship and is supported by the JSPS postdoctoral fellowship for research abroad.
Footnotes
Shimamoto Y., Maeda Y. T., Ishiwata S., Libchaber A. J., Kapoor T. M. Insights into the micromechanical properties of the metaphase spindle. Cell 145, 1062-1074 (2011) DOI: 10.1016/j.cell.2011.05.038
AUTHOR CONTRIBUTIONS
Y.S. developed the protocol. Y.S. and T.M.K. prepared the manuscript.
COMPETING INTERESTS STATEMENT
The authors declare that they have no competing financial interests.
REFERENCES
- 1.Brenner MD, Zhou R, Ha T. Forcing a connection: impacts of single-molecule force spectroscopy on in vivo tension sensing. Biopolymers. 2011;95:332–44. doi: 10.1002/bip.21587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neuman KC, Nagy A. Single-molecule force spectroscopy: optical tweezers, magnetic tweezers and atomic force microscopy. Nat Methods. 2008;5:491–505. doi: 10.1038/nmeth.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veigel C, Schmidt CF. Moving into the cell: single-molecule studies of molecular motors in complex environments. Nat Rev Mol Cell Biol. 2011;12:163–76. doi: 10.1038/nrm3062. [DOI] [PubMed] [Google Scholar]
- 4.Inoue S, Salmon ED. Force generation by microtubule assembly/disassembly in mitosis and related movements. Mol Biol Cell. 1995;6:1619–40. doi: 10.1091/mbc.6.12.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waters JC, Skibbens RV, Salmon ED. Oscillating mitotic newt lung cell kinetochores are, on average, under tension and rarely push. J Cell Sci. 1996;109(Pt 12):2823–31. doi: 10.1242/jcs.109.12.2823. [DOI] [PubMed] [Google Scholar]
- 6.Nicklas RB. Chromosome velocity during mitosis as a function of chromosome size and position. J Cell Biol. 1965;25(SUPPL):119–35. doi: 10.1083/jcb.25.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grill SW, Howard J, Schaffer E, Stelzer EH, Hyman AA. The distribution of active force generators controls mitotic spindle position. Science. 2003;301:518–21. doi: 10.1126/science.1086560. [DOI] [PubMed] [Google Scholar]
- 8.Gardel ML, Kasza KE, Brangwynne CP, Liu J, Weitz DA. Mechanical response of cytoskeletal networks. Methods Cell Biol. 2008;89:487–519. doi: 10.1016/S0091-679X(08)00619-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin Y, Koenderink GH, C. MF, Weitz DA. Viscoelastic properties of microtubule networks. Macromolecules. 2007;40:771407720. [Google Scholar]
- 10.Murray AW. Cell cycle extracts. Methods Cell Biol. 1991;36:581–605. [PubMed] [Google Scholar]
- 11.Hannak E, Heald R. Investigating mitotic spindle assembly and function in vitro using Xenopus laevis egg extracts. Nat Protoc. 2006;1:2305–14. doi: 10.1038/nprot.2006.396. [DOI] [PubMed] [Google Scholar]
- 12.Kapoor TM, Mitchison TJ. Eg5 is static in bipolar spindles relative to tubulin: evidence for a static spindle matrix. J Cell Biol. 2001;154:1125–33. doi: 10.1083/jcb.200106011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyamoto DT, Perlman ZE, Burbank KS, Groen AC, Mitchison TJ. The kinesin Eg5 drives poleward microtubule flux in Xenopus laevis egg extract spindles. J Cell Biol. 2004;167:813–8. doi: 10.1083/jcb.200407126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walczak CE, Mitchison TJ, Desai A. XKCM1: a Xenopus kinesin-related protein that regulates microtubule dynamics during mitotic spindle assembly. Cell. 1996;84:37–47. doi: 10.1016/s0092-8674(00)80991-5. [DOI] [PubMed] [Google Scholar]
- 15.Houghtaling BR, Yang G, Matov A, Danuser G, Kapoor TM. Op18 reveals the contribution of nonkinetochore microtubules to the dynamic organization of the vertebrate meiotic spindle. Proc Natl Acad Sci U S A. 2009;106:15338–43. doi: 10.1073/pnas.0902317106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wittmann T, Hyman T. Recombinant p50/dynamitin as a tool to examine the role of dynactin in intracellular processes. Methods Cell Biol. 1999;61:137–43. doi: 10.1016/s0091-679x(08)61978-0. [DOI] [PubMed] [Google Scholar]
- 17.Gatlin JC, Matov A, Danuser G, Mitchison TJ, Salmon ED. Directly probing the mechanical properties of the spindle and its matrix. J Cell Biol. 2010;188:481–9. doi: 10.1083/jcb.200907110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimamoto Y, Maeda YT, Ishiwata S, Libchaber AJ, Kapoor TM. Insights into the micromechanical properties of the metaphase spindle. Cell. 2011;145:1062–74. doi: 10.1016/j.cell.2011.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicklas RB. How cells get the right chromosomes. Science. 1997;275:632–7. doi: 10.1126/science.275.5300.632. [DOI] [PubMed] [Google Scholar]
- 20.Shimamoto Y, Suzuki M, Mikhailenko SV, Yasuda K, Ishiwata S. Inter-sarcomere coordination in muscle revealed through individual sarcomere response to quick stretch. Proc Natl Acad Sci U S A. 2009;106:11954–9. doi: 10.1073/pnas.0813288106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howard J, Hudspeth AJ. Mechanical relaxation of the hair bundle mediates adaptation in mechanoelectrical transduction by the bullfrog's saccular hair cell. Proc Natl Acad Sci U S A. 1987;84:3064–8. doi: 10.1073/pnas.84.9.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okuno M, Hiramoto Y. Direct measurements of the stiffness of echinoderm sperm flagella. J Exp Biol. 1979;79:235–243. [Google Scholar]
- 23.Svoboda K, Block SM. Biological applications of optical forces. Annu Rev Biophys Biomol Struct. 1994;23:247–85. doi: 10.1146/annurev.bb.23.060194.001335. [DOI] [PubMed] [Google Scholar]
- 24.Tanase M, Biais N, Sheetz M. Magnetic tweezers in cell biology. Methods Cell Biol. 2007;83:473–93. doi: 10.1016/S0091-679X(07)83020-2. [DOI] [PubMed] [Google Scholar]
- 25.Fisher TE, Marszalek PE, Fernandez JM. Stretching single molecules into novel conformations using the atomic force microscope. Nat Struct Biol. 2000;7:719–24. doi: 10.1038/78936. [DOI] [PubMed] [Google Scholar]
- 26.Janmey PA, Georges PC, Hvidt S. Basic rheology for biologists. Methods Cell Biol. 2007;83:3–27. doi: 10.1016/S0091-679X(07)83001-9. [DOI] [PubMed] [Google Scholar]
- 27.Theriot JA, Rosenblatt J, Portnoy DA, Goldschmidt-Clermont PJ, Mitchison TJ. Involvement of profilin in the actin-based motility of L. monocytogenes in cells and in cell-free extracts. Cell. 1994;76:505–17. doi: 10.1016/0092-8674(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 28.Gaglio T, Saredi A, Compton DA. NuMA is required for the organization of microtubules into aster-like mitotic arrays. J Cell Biol. 1995;131:693–708. doi: 10.1083/jcb.131.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee K, Gallop JL, Rambani K, Kirschner MW. Self-assembly of filopodia-like structures on supported lipid bilayers. Science. 2010;329:1341–5. doi: 10.1126/science.1191710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stearns T, Kirschner M. In vitro reconstitution of centrosome assembly and function: the central role of gamma-tubulin. Cell. 1994;76:623–37. doi: 10.1016/0092-8674(94)90503-7. [DOI] [PubMed] [Google Scholar]
- 31.Levy DL, Heald R. Nuclear size is regulated by importin alpha and Ntf2 in Xenopus. Cell. 2010;143:288–98. doi: 10.1016/j.cell.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hyman A, et al. Preparation of modified tubulins. Methods Enzymol. 1991;196:478–85. doi: 10.1016/0076-6879(91)96041-o. [DOI] [PubMed] [Google Scholar]