Abstract
Background:
Vitamin D deficiency is associated with HF events and in animal models vitamin D down-regulates RAAS hormones.
Methods:
Patients with NYHA II-IV HF and a 25OHD level ≤ 37.5 ng/mL received weekly vitamin D3 50,000 IU (n=31) or placebo (n=33) for 6 months. Serum aldosterone, renin, echocardiography and health status were collected at baseline and 6 months.
Results:
Mean age of participants 65.9±10.4 years, women 48%, AA 64%, mean EF 37.6±13.9, NYHA class III 36 %, II 64%. The vitamin D group increased serum 25OHD (19.1± 9.3 to 61.7±20.3 ng/ml) and not in the placebo group (17.8±9.0 to 17.4±9.8 ng/ml). Aldosterone decreased in the vitamin D group (10.0±11.9 to 6.2±11.6 ng/dl) and not in the placebo group 8.9±8.6 to 9.0±12.4 ng/dl) (p=.02). There was no difference between groups in renin, echocardiographic measures or health status from baseline to 6 months. Modeling indicated that variables which predicted change in aldosterone included receiving vitamin D, increasing age, AA race, and lower GFR.
Conclusions:
Vitamin D3 repletion decreases aldosterone in patients with HF and low serum vitamin D. Vitamin D may be an important adjunct to standard HF therapy. Further will assess if vitamin D provides long-term benefit for patients with HF.
INTRODUCTION
Vitamin D has the potential to improve the symptoms of HF and modulate the disease. Vitamin D deficiency has been associated with worse cardiovascular outcomes for patients with and without HF.(1-3) Vitamin D supplementation can reduce blood pressure and improve skeletal muscle function and strength.(4, 5) Animal studies suggest that active vitamin D downregulates the renin-angiotensin-aldosterone system (RAAS), reduces retention of salt and water and reduces myocardial hypertrophy.(6, 7) However, there are few trials of vitamin D therapy in patients with HF and to date trials show mixed benefit on physical performance outcomes and inflammation. (8-10)
Our recent pilot trial of vitamin D3 for 6 months did not improve aerobic capacity or skeletal muscle strength in patients with HF who were 50 years or older.(8) Here we present secondary data analysis from the randomized trial of high dose vitamin D3 in patients with HF and its effect on the RAAS. We hypothesized that patients with HF who were treated with vitamin D plus oral calcium for 6 months would decrease serum concentrations of hormones and biomarkers (renin, aldosterone, CRP and NT-proBNP), decrease ventricular mass, improve diastolic function, and improve health status compared to those who took placebo with calcium.
METHODS
A description of the methods and the primary trial results have been published previously.(8) Briefly, this was a randomized controlled double blind placebo controlled trial of vitamin D3 50,000 IU vs. placebo weekly for 6 months in patients with HF. Both groups received calcium citrate 800mg daily. The trial was approved by the institutional review board at University Hospitals, Case Medical Center. Eligible subjects underwent informed consent and randomly assigned 1:1 to receive vitamin D3, 50,000 IU or matching placebo.
Randomization and Allocation
Patients were randomized in a permuted block scheme according to race, age and sex. Group assignment remained concealed from study staff, participants, and investigators until data collection was complete.
Patients
Patients aged ≥ 50 years and New York Heart Association Class (NYHA) II-IV regardless of ejection fraction were recruited from academic HF and general cardiology practices. Patients were required to be on maximal tolerated doses of evidenced-based HF medications as per the primary cardiologist. A serum 25 hydroxyvitamin D (25OHD) concentration required was ≤37.5 ng/ml was required. Exclusion criteria included primary hyperparathyroidism, sarcoidosis, hypercalcemia, nephrolithiasis, osteoporosis, creatinine of > 2.5mg/dl, daily intake of vitamin D > 400 international units (IU), corticosteroids, parathyroid hormone (PTH), androgen or estrogen use, current illicit drug use or ≥ 3 alcoholic drinks daily, advanced cancer, or myocardial infarction in preceding 6 months. Also excluded was use of medications known to lower serum 25OHD or the bioavailability of oral vitamin D including: ketoconazole, colestipol, cholestyramine, mineral oil, phenobarbitol, and phenytoin. Patients were screened first by medical history and second by serum 25OHD concentrations.
Measures
All blood samples were obtained from patients in the upright position. Blood was stored at 2-8°C.
Serum Analysis
Serum 25OHD was measured by chemi-illuminescence immunoassay (ARUP Salt Lake City, Utah) with an intra-assay CV of 3 and 6% and a between assay variability of 6 to 11%.
Parathyroid Hormone was measured by chemiluminometric technology (Siemens Dimension Vista Systems, Newark, DE) by University Hospitals clinical laboratory).
Aldosterone was measured in duplicate to assure accuracy; two measures were averaged for final result. If the two measures were >20% difference the test was rerun. The Siemens solid-phase Coat-A-Count radio immunoassay kits (Siemens Healthcare Diagnostics, Malvern, PA 19355) were used and had an intra-assay CV of 3.5% and an inter-assay variability of 6.9%.
N-Terminal Pro-Brain Natriuretic Peptide (NT-proBNP) was measured in duplicate using a sandwich immunoassay with electrochemiluminesence detection (Meso Scale Discovery, Gaithersburg, MD 20877) with an intra-assay CV of 5.4% and an inter-assay variability of 7.8%.
Galectin-3 was measured in duplicate using enzyme linked immunosorbent assay (ELISA) kits (BG Medicine Inc, 610N Lincoln Street, Waltham, MA 02451) with an intra-assay CV of 3.3% and a between assay variability of 3.7%.
PRA was measured using Gammacoat radioimmunoassay (ARUP Salt Lake City, Utah) with an intra-assay CV of 5.5-9.2% and a between assay variability of 7.1-12.8%.
High Sensitivity C-Reactive Protein was measured by immunonephelometry using the Siemens Dimension Vista analyzer (Siemens Healthcare Diagnostics, Malvern, PA 19355) with a between assay variability of 5.0%.
Glomerular filtration rate (GFR) was calculated using the chronic kidney disease (CKD)-Epi equation.(11)
Urine Analysis
Spot urine calcium and creatinine was measured in the University Hospital clinical laboratory to evaluate safety of high dose vitamin D. An increase in urinary calcium can indicate impending risk of hypercalcemia.
Echocardiogram
A subset of participants (n=34) was evaluated at baseline and 6 months with transthoracic two-dimensional and Doppler echocardiography. The subset was inclusive of all those who enrolled after the first year of the trial. Transthoracic echocardiograms were performed using commercially available ultrasound systems (Vivid 7, GE Healthcare, Wauwatosa, WI or iE33, Philips Medical Systems, Andover MA). Participants were studied by one of three senior sonographers during quiet respiration in the left lateral decubitus position. Standard M-mode and two-dimensional echocardiography, pulsed-wave spectral and Doppler tissue imaging echocardiographic parameters were obtained from parasternal and apical windows. M-mode imaging was used to derive LV dimensions, wall thicknesses, mass, and shortening fraction. Two-dimensional echocardiography was used to calculate LV ejection fraction from the 4- and 2- chamber acoustic windows using Simpson’s rule. Spectral Doppler-derived LV diastolic inflow (E and A waves, deceleration time) was recorded in the apical four-chamber view and early diastolic tissue Doppler velocities (e’) were measured at the septal and lateral corners of the mitral annulus. The ratios of E/A and E/e’ and deceleration time were measures of diastolic LV function. Images were stored digitally and analyzed randomly offline (Heartlab, Agfa, Hackensack NJ) by a single investigator blinded to all clinical data. Three representative beats were averaged. All measurements were performed in accordance with American Society of Echocardiography guidelines.(12, 13)
Kansas City Cardiomyopathy Questionnaire (KCCQ)(14)
The KCCQ was measured at baseline and 6-months. It is a 23-item self-administered instrument that gives an overall summary score but is also divided into four subscales (domains) including: symptoms (frequency, severity and recent change over time), physical limitations, social functioning, and quality of life. The questionnaire takes approximately 8-10 minutes to complete. Patients were assisted with answering the questions to assure comprehension. Scores range from 0-100; higher scores reflect better health status.
Statistical Analyses
Baseline demographics were summarized by means and standard deviations for continuous variables and frequency and proportions for categorical variables within study groups. The treatment effect (vitamin D versus placebo) was evaluated for each endpoint using an ANCOVA model that adjusts for baseline endpoint. Two multivariable models were constructed with the 6 month change in aldosterone as the endpoint. The larger model includes the covariates deemed to be of the most clinically relevant, and the smaller model includes a further reduced subset of covariates. All statistical analyses were performed using R (Vienna, Austria).
Results
Recruitment, Retention, and Adherence
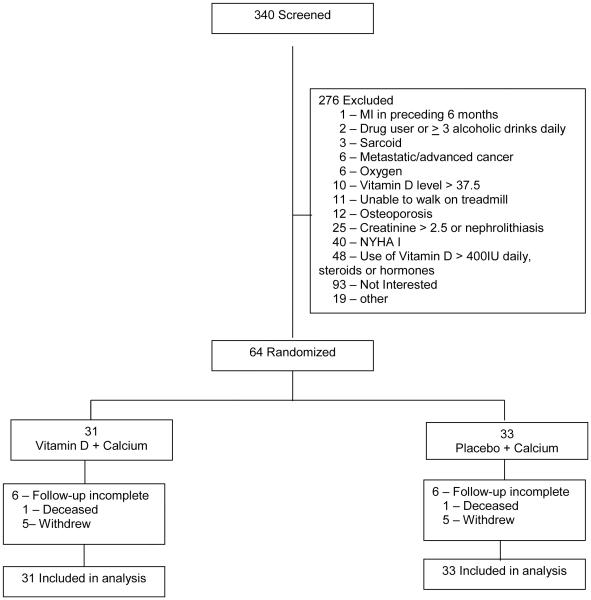
Patients were recruited from May 2007 to April 2011. Three hundred forty patients were screened for study inclusion and 276 did not meet entry criteria.(Figure 1) Sixty-four were randomized and their data included in the analysis. Based on monthly pill counts, adherence in the vitamin D group was 100% and 99.5% in the placebo group. The vitamin D group had 90.7% and placebo group 90.8% adherence to the calcium pills.
Figure 1.
Flow Diagram
Patient Characteristics
Mean age for the participants in intervention group was 65.8 ± 10.6, 51.6% women and 61.3% African American. For the placebo group mean age was 66.0 ± 10.4, 45.5% women and 66.7% African American. The majority of patients were non-ischemic and mean ejection fraction (EF) 37.6 ± 13.9 % and NYHA class III 36 %, II 64%.(Table 1)
Table 1.
Baseline Demographics
| Characteristic | n, if different |
Vitamin D (n=31) |
Placebo (n=33) |
|---|---|---|---|
| Age (years), mean (SD)* | 65.8 ± 10.6 | 66.0 ± 10.4 | |
| Women n, (%) | 16 (51.6) | 15 (45.5) | |
| African American n, (%) | 19 (61.3) | 22 (66.7) | |
| BMI† (kg/m2), mean (SD) | 30, 33 | 34.8 ± 7.2 | 31.3 ± 6.9 |
| Ischemic Etiology n, (%) | 8 (25.8) | 10 (30.3) | |
| Ejection fraction mean (SD) | 39.2 ± 13.2 | 36.1 ± 14.5 | |
| NYHA Class | |||
| II n, (%) | 17 (55) | 24 (73) | |
| III n, (%) | 14 (45) | 9 (27) | |
| Hypertension n, (%) | 26 (83.9) | 28 (84.8) | |
| Hyperlipidemia n, (%) | 26 (83.9) | 25 (75.8) | |
| Diabetes n, (%) | 16 (51.6) | 14 (42.4) | |
| Pulmonary disease‡ n, (%) | 16 (51.6) | 16 (48.5) | |
| Depression n, (%) | 10 (32.3) | 10 (30.3) | |
| Labs | |||
| 25OHD (ng/ml) | 19.1 ± 9.3 | 17.8 ± 9.0 | |
| PTH (pg/ml) | 30, 33 | 62.3 ± 44.3 | 72.8 ± 40.2 |
| Plasma Renin Activity (ng/ml/hr) | 29, 28 | 7.6 ± 13.4 | 6.7 ± 8.6 |
| Aldosterone (ng/dl) | 31, 31 | 10.0 ± 11.9 | 8.9 ± 8.6 |
| CRP (mg/dl) | 25, 26 | 5.8 ± 10.2 | 8.5 ± 27.2 |
| NT-ProBNP (pg/ml) | 31, 31 | 2570 ± 3800 | 4880 ± 6390 |
| Galectin-3 (ng/ml) | 31, 31 | 17.9 ± 6.1 | 19.7 ± 6.4 |
| Calcium | 9.3 ± 0.5 | 9.2 ± 0.4 | |
| GFR | 31, 32 | 65.5 ± 24.2 | 61.2 ± 19.9 |
| Medications§ | |||
| ACE Inhibitor, n (%) | 20 (64.5) | 22 (66.7) | |
| Enalapril EQ|| dose (mg) | 40, 32.8 (10,80) |
40, 30.2 (5, 80) |
|
| Angiotensin Inhibitor Blocker, n (%) | 8 (25.8) | 9 (27.3) | |
| Valsartan EQ dose (mg) | 240, 215.0 (40, 320) |
240, 202.2 (40, 320) |
|
| Beta Blocker, n (%) | 29 (93.5) | 28 (84.8) | |
| Metoprolol EQ dose (mg) | 150, 130.4 (25, 200) |
175, 139.3 (25, 200) |
|
| Loop Diuretic, n (%) | 22 (71.0) | 26 (78.8) | |
| Furosemide EQ dose (mg) | 40, 62.4 (6, 200) |
40, 70.0 (10, 400) |
|
| Aldosterone Antagonist, n (%) | 7 (23) | 13 (39) |
Standard Deviation
Body Mass Index
Includes COPD, emphysema, asthma, sleep apnea
Doses are given in mean daily dose and converted to a standard medication.
Equivalency dose
Effects on Serum 25OHD, PTH and Calcium
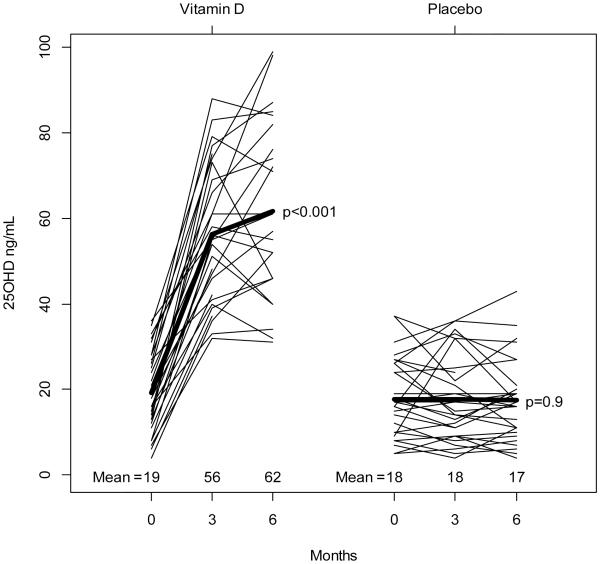
At 6 months, the serum 25OHD increased by 42.3 ± 16.4 ng/ml in the vitamin D group and decreased by −0.2 ± 6.6 ng/ml in the placebo group (p=.001). PTH decreased by −23.1± 40.0 pg/ml in the vitamin D group and by −3.1 ± 38.1 pg/ml in the placebo group (p=.01). (Figures 2 and 3) Calcium concentrations had no significant change in either group.
Figure 2.
Change in Serum Concentrations of 25OHD
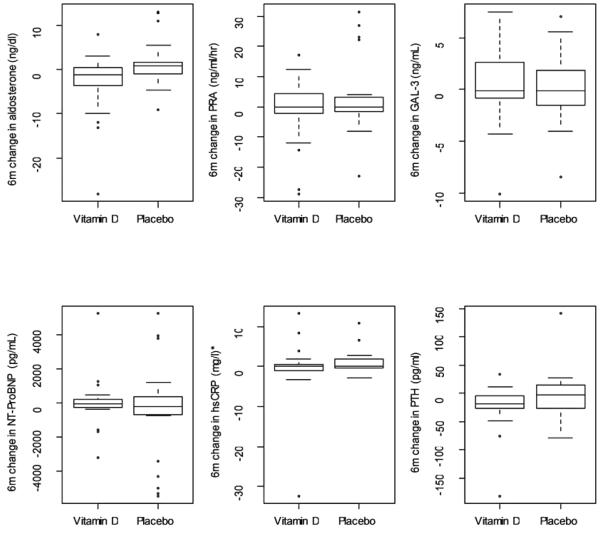
Figure 3.
Changes in Biomarkers, Vitamin D vs. Placebo
*One CRP outlier suppressed in placebo group to compress the y-axis.
6m-6 month
PRA –plasma renin activity
GAL-3 – Galactin 3
hsCRP- high sensitivity c-reactive protein
PTH – parathyroid hormone
Effect on Hormones and Biomarkers
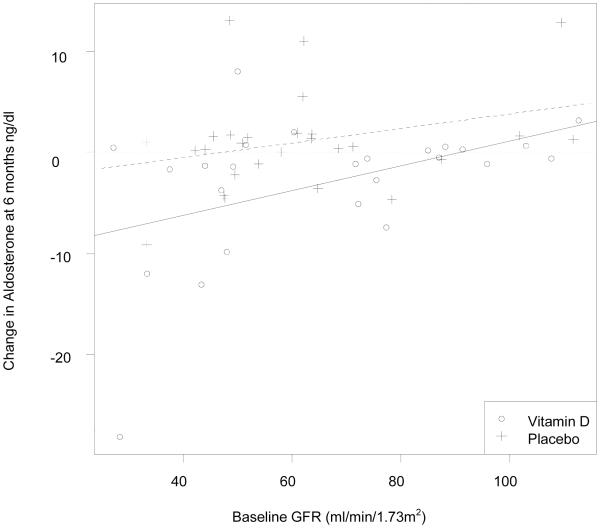
Serum aldosterone changed from 10.0 ± 11.9 ng/dl to 6.3 ± 7.0 ng/dl (37% reduction) in the vitamin D group and from 8.9 ± 8.6 ng/dl to 9.0 ± 12.4 ng/dl in the placebo group. The ANCOVA model controlling for baseline aldosterone and group produced a treatment effect of −3.7 (p=.02). Change in PRA in the vitamin D group 7.6 ± 13.4 ng/ml/hr to 6.3 ± 7.0ng/ml/hr and in the placebo group 6.7 ± 8.6 ng/ml to 9.2 ± 8.ng/ml/hr (p=.2 for change between groups). There was no significant change between baseline and 6 month serum hsCRP, NT-proBNP, or galectin-3. (Figure 3) Those patients with the lowest glomerular filtration rate tended to have the highest aldosterone at baseline (correlation = −0.36, p=0.004) and the largest decrease in aldosterone concentrations. (Figure 4) Univariate and multiple regression models with change in aldosterone as the dependent variable are shown in Table 2. The first model shows the relationship between demographics and the change in aldosterone. The second model adds clinical variables. There was no relationship between the change in aldosterone and medications (loop diuretics, ACE-I, ARB, aldosterone antagonist or BB).
Figure 4.
Change in Aldosterone According to Baseline GFR
Table 2.
Univariate and multivariable modeling for change in aldosterone at 6 months
| Covariate effect | Univariate Effect size (CI) p-value |
ultivariable Model 1 Effect size (CI) p-value |
Multivariable Model 2 Effect size (CI) p-value |
|---|---|---|---|
| Group=Vitamin D | −3.7 (−0.6, −6.8) 0.02 |
−4.4 (−7.3, −1.5) 0.003 |
−4.2 (−7.0, −1.3) 0.005 |
| 5 years of age | −0.7 (−1.5, 0.1) 0.07 |
−1.1 (−1.8, −0.3) 0.006 |
−1.0 (−1.7, −0.3) 0.008 |
| Female | −1.4 (−4.7, 1.8) 0.38 |
−0.1 (−3.5, 3.3) 0.943 |
1.0 (−2.4, 4.3) 0.56 |
| African American | −1.9 (−5.3, 1.5) 0.27 |
−2.6 (−6.6, 1.4) 0.194 |
−4.7 (−9.2, −0.2) 0.04 |
| 5 units of baseline 250HD | 0.1 (0.0, 0.3) 0.09 |
0.8 (0.0, 1.7) 0.049 |
0.5 (−0.3, 1.4) 0.22 |
| 10 units of baseline PTH | 0.0 (−0.4, 0.4) 0.85 |
-- | 0.2 (−0.2, 0.6) 0.25 |
| HF type=ischemic | −3.0 (−6.5, 0.5) 0.09 |
-- | −2.1 (−5.6, 1.4) 0.23 |
| NYHA=3 | −2.9 (−6.3, 0.5) 0.1 |
-- | −2.2 (−5.3, 0.9) 0.16 |
| 10 units of baseline GFR | 0.6 (−0.2, 1.3) 0.14 |
− | 0.9 (0.1, 1.7) 0.04 |
|
| |||
| Multivariable R2 | -- | 0.43 | 0.53 |
In each model, baseline aldosterone is included.
PTH –parathyroid hormone
NYHA –New York Heart Association classification
GFR –glomerular filtration rate
Effect on Urinary Calcium/Creatinine Ratio
The spot urine calcium to creatinine ratio at baseline in the vitamin D group was 104.1 ± 60.0 mg/dl and in the placebo group was 87.4 ± 49.3 mg/dl (p=0.3). The 3 month change in spot urine calcium to creatinine in the vitamin D group 27.9 ± 48.0 mg/dl and in the placebo group 17 ± 58.2 mg/dl (p=0.2).
Effect on Patient Symptoms, Echocardiography and Health Status
There was no change in any echocardiographic variables within or between groups (Table 3). Health status, measured by the KCCQ, showed no statistical difference between groups, however there was a clinically relevant change (≥5 point) in all domains of the KCCQ (15) (Table 3). Outlier sensitivity analysis did not change results. There was no difference between groups in blood pressure or in those who reported worsening heart failure symptoms during the course of the study or in the number of patients who were hospitalized and/or received in-office IV diuretics.
Table 3.
Change in Echocardiography and Health Status by Group
| 6 month change in endpoint | ANCOVA model | ||||
|---|---|---|---|---|---|
| Echocardiogram | N* | Vitamin D | Placebo | Vitamin D – Placebo |
p-value |
|
| |||||
| Ejection fraction | 19, 15 | 0.8 ± 4.5 | 2.3 ± 3.5 | −0.8 | 0.56 |
| LV septal wall thickness | 19, 15 | 0.0 ± 0.2 | 0.0 ± 0.1 | 0.0 | 0.49 |
| LV posterior wall thickness | 19, 15 | 0.0 ± 0.2 | 0.0 ± 0.1 | 0.1 | 0.25 |
| LV mass | 19, 15 | −11 ± 40 | −17 ± 47 | −2 | 0.89 |
| LV mass index | 19, 15 | −5 ± 19 | −8 ± 21 | −1 | 0.92 |
| e/e’ septal mitral annulus | 19, 14 | −1.0 ± 5.2 | −4.1 ± 8.0 | 1.1 | 0.61 |
| Quantitative EF 4 chamber view |
17, 14 | −1.0 ± 13.8 | 10.4 ± 13.7 | −3.7 | 0.33 |
| Quantitative EF 2 chamber view |
18, 14 | 1.1 ± 17.1 | 4.9 ± 11.4 | 0.9 | 0.84 |
|
| |||||
| KCCQ | |||||
|
| |||||
| Overall Summary Score | 25, 27 | 8.9 ± 20.4 | 2.2 ± 13.0 | 6.2 | 0.16 |
| Clinical Summary Score | 25, 27 | 8.4 ± 17.0 | 0.3 ± 12.5 | 7.0 | 0.09 |
| Physical limitations | 25, 27 | 7.1 ± 18.7 | 1.6 ± 14.1 | 5.1 | 0.25 |
| Total symptoms | 25, 27 | 9.7 ± 22.8 | −1.0 ± 16.7 | 7.5 | 0.15 |
| Quality of life | 25, 27 | 9.0 ± 28.1 | 4.6 ± 17.5 | 6.4 | 0.27 |
| Social limitations | 24, 27 | 9.9 ± 33.0 | 3.7 ± 19.9 | 5.6 | 0.43 |
A subset of participants was evaluated by echocardiography.
EF- ejection fraction
LV – left ventricular
KCCQ –Kansas City Cardiomyopathy Questionnaire
Discussion
This secondary data analysis of a RCT of vitamin D3 in patients with HF indicates that repletion with vitamin D3 may cause a decrease in serum aldosterone concentration in vitamin D deficient patients with HF. Clinical effect was only demonstrated in an increase in the KCCQ scores (>5 point change) in the vitamin D group albeit not a statistically significant increase. No other measures showed a clinical change including echocardiographic changes in cardiac structure/remodeling, hsCRP, NT-proBNP, patient symptoms, or blood pressure. However, in other studies, the KCCQ has proven to be a sensitive measure of clinical change (better than NYHA or the 6 minute walk) for both those patients who are worsening and improving.(15, 16) The vitamin D3 dose of 50,000 IU did not cause hypercalcemia or hypercalciuria.
This is the first study in patients with HF to demonstrate a reduction in aldosterone with oral vitamin D3. Two previous studies investigate the effects of vitamin D in patients with HF. Witham et. al. studied 105 Caucasian patients and found no significant change in aldosterone; however the serum 25OHD did not reach as high a concentration in the treatment group as in our study.(9) A second study by Schroten et. al., was an open label study which showed no change in aldosterone, but a significant decrease in PRA (17).(discussed below) Another small study of patients with essential hypertension (not HF) showed a reduction in aldosterone concentrations of 30% with oral vitamin D3.(18)
Aldosterone is important to the pathogenesis of heart failure contributing to myocardial fibrosis while blockade of aldosterone improves outcomes.(19, 20) Much of the focus in heart failure therapeutics has been in RAAS blockade, however ACE inhibitors, diuretics and aldosterone antagonists can all contribute to persistently elevated aldosterone concentrations; i.e. aldosterone escape.(21, 22) Higher serum aldosterone concentrations are associated with worse cardiovascular outcomes especially those with lower GFR.(23) A retrospective study of vitamin D treatment reduced CV events in patients with CKD.(24) However, the clinical benefit of reducing aldosterone concentrations is unclear in the context modern HF therapy. The amount of decrease in aldosterone in our trial was comparable to that seen in the Valsartan Heart Failure Trial (Val-Heft) in which aldosterone declined by 34.6 pg/ml (3.46 ng/dl), 31.9 pg/ml (3.19 ng/dl) at 4 and 12 months respectively in the treatment group.(25) However, despite a decline in aldosterone in the Val-Heft Trial, there was no relationship to improved outcomes.
In the complete linear regression model, age, treatment with vitamin D, being African American, and baseline GFR most strongly predict the change in aldosterone. In a study of hypertensive African Americans vitamin D treatment led to a modest reduction in blood pressure.(4) The inverse relationship between GFR and aldosterone is consistent with limited animal and human data in CKD.(23, 26) Those with CKD are known to have activated RAAS. Explanation as to why there was a more robust suppression of aldosterone for patients with lower GFR (as seen in Figure 4) by vitamin D is theoretical. Patients with CKD (GFR<60) may have low incident 1,25(OH)2D levels despite adequate 25OHD levels.(27) In CKD, 1,25(OH)2D production is dependent on the availability of 25OHD in the circulation.(28) We therefore speculate that treatment with high dose vitamin D increased 1,25(OH)2D levels, resulting in more pronounced suppression of the RAAS as seen in animal models.
Although the PRA did not significantly decrease in the vitamin D group in comparison with controls, a renin mediated mechanism was reported in mouse models. These studies support that vitamin D acts through downregulation of RAAS. Vitamin D receptor knockout mice have hypertension, cardiac hypertrophy and blood pressure which normalizes by administration of captopril.(29) In wild type mice, blockade of 1,25(OH)2D increases renin expression and with injection of 1,25(OH)2D renin is suppressed.(7) Schroten et. al. reported a decrease in renin in human HF patients given vitamin D.(17) However, there are a number of design differences between our study and Schroten et. al. which may explain the differences in our results. First the study subjects from the Schroten trial were > 90% male, all Caucasian, and all had systolic failure. This is in contrast to our study which is half woman, over half African American and mixed systolic and preserved systolic failure. In addition the study period for the Schroten study was much shorter, only 6 weeks with lower vitamin D dosing (2000 IU daily). However, our trial is limited by both its small size and the number of variables examined.
There are also studies in which high PTH levels are associated with HF.(30) In our study the decrease in PTH was not independently associated with the change in aldosterone. However, it is impossible to examine the change in PTH separately from the change in 25OHD since they occurred simultaneously and are interrelated. There is building evidence from both animals and humans that PTH may have an important role in cardiovascular disease and stimulate aldosterone secretions.(31)
Alternatively, there is possibility that vitamin D could act through a renin independent mechanism. In vitro study shows that the active form of vitamin D, 1,25(OH)2D, has a direct effect on adrenal cortical cells by down-regulating the enzymes in the steroidogenesis pathway. A non-significant decrease in aldosterone was seen with exposure to active vitamin D and significant decreases in other androgenic hormones.(32) This may be an alternative explanation if a lowering of aldosterone is not mediated through renin and requires more study.
Nutritional vitamin D is easily attainable and inexpensive. RAAS hormones are a major contributor to retention of sodium and water in HF pathophysiology and drugs which block RAAS control symptoms and prolong life. If vitamin D truly down-regulates aldosterone, it may be an important therapeutic addition to standard HF therapies, especially in patients with CKD. HF patients who do not tolerate RAAS blockade or who have aldosterone escape may also be a group of patients who would benefit. It is unknown if vitamin D treatment will result in improved symptom control although the improvement in KCCQ scores in the treatment group indicate this warrants further study.
Limitations
This is a secondary analysis from a small RCT and results should be viewed in this context. Recruitment required that we screened over 300 patients to enroll 64, however this is not atypical vitamin D studies. The study is also limited by the testing of multiple endpoints which should be taken into account when assessing the strength of evidence presented in this manuscript. The results from this trial do not provide any information regarding how vitamin D lowers aldosterone and therefore the mechanism is theoretical. It is possible that PTH is the hormone that interacts and lowers serum aldosterone but since PTH and 25OHD change simultaneously it impossible to tell which has the direct effect. Other unmeasured factors could have effected renin and aldosterone concentrations such as dietary sodium content and serum potassium. Measurement of 1,25(OH)2D levels were not performed and would have enhanced the understanding of our results. Only a subset of patients received echocardiographic measures should be studied in a larger sample.
Conclusion
A robust change in serum 25OHD with a decrease in PTH resulted in a modest decrease in serum aldosterone in patients with HF without a statistically significant clinical benefit. Vitamin D3 as an adjunct to standard HF therapy requires further study.
Acknowledgements
We thank Marianne Vest, Anna Liner and Jill Bradisse for their work in conduction the trial. We also thank BG Medicine for providing kits for Galectin-3 measurement.
Funding Sources
Dr. Boxer and this work are supported by the KL2RR024990 and in part by the American Heart Association Scientist Development Grant 0635055N and the Joan C. Edwards Fund, Cleveland, OH. This publication was made possible by the Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Science component of the NIH and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
There are no relevant disclosures from any of the authors.
ClinicalTrials.gov Identifier: NCT01125436
References
- 1.Liu LC, Voors AA, van Veldhuisen DJ, van der Veer E, Belonje AM, Szymanski MK. Vitamin D status and outcomes in heart failure patients. Eur J Heart Fail. 2011;13(6):619–25. doi: 10.1093/eurjhf/hfr032. Epub 2011/05/06. [DOI] [PubMed] [Google Scholar]
- 2.Ginde AA, Scragg R, Schwartz RS, Camargo CA., Jr. Prospective study of serum 25-hydroxyvitamin D level, cardiovascular disease mortality, and all-cause mortality in older U.S. adults. J Am Geriatr Soc. 2009;57(9):1595–603. doi: 10.1111/j.1532-5415.2009.02359.x. Epub 2009/06/25. [DOI] [PubMed] [Google Scholar]
- 3.Lavie CJ, Dinicolantonio JJ, Milani RV, O'Keefe JH. Vitamin D and cardiovascular health. Circulation. 2013;128(22):2404–6. doi: 10.1161/CIRCULATIONAHA.113.002902. Epub 2013/11/28. [DOI] [PubMed] [Google Scholar]
- 4.Forman JP, Scott JB, Ng K, Drake BF, Suarez EG, Hayden DL. Effect of vitamin D supplementation on blood pressure in blacks. Hypertension. 2013;61(4):779–85. doi: 10.1161/HYPERTENSIONAHA.111.00659. Epub 2013/03/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 6.Li YC. Vitamin D regulation of the renin-angiotensin system. J Cell Biochem. 2003;88(2):327–31. doi: 10.1002/jcb.10343. [DOI] [PubMed] [Google Scholar]
- 7.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110(2):229–38. doi: 10.1172/JCI15219. Epub 2002/07/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boxer RS, Kenny AM, Schmotzer BJ, Vest M, Fiutem JJ, Pina IL. A Randomized Controlled Trial of High-Dose Vitamin D3 in Patients With Heart Failure. J Am Coll Cardiol HF. 2013;1(1):84–90. doi: 10.1016/j.jchf.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Witham MD, Crighton LJ, Gillespie ND, Struthers AD, McMurdo ME. The effects of vitamin D supplementation on physical function and quality of life in older patients with heart failure: a randomized controlled trial. Circ Heart Fail. 2010;3(2):195–201. doi: 10.1161/CIRCHEARTFAILURE.109.907899. [DOI] [PubMed] [Google Scholar]
- 10.Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83(4):754–9. doi: 10.1093/ajcn/83.4.754. Epub 2006/04/08. [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI. new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. Epub 2009/05/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–63. doi: 10.1016/j.echo.2005.10.005. Epub 2005/12/27. [DOI] [PubMed] [Google Scholar]
- 13.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22(2):107–33. doi: 10.1016/j.echo.2008.11.023. Epub 2009/02/04. [DOI] [PubMed] [Google Scholar]
- 14.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35(5):1245–55. doi: 10.1016/s0735-1097(00)00531-3. Epub 2000/04/12. [DOI] [PubMed] [Google Scholar]
- 15.Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005;150(4):707–15. doi: 10.1016/j.ahj.2004.12.010. Epub 2005/10/08. [DOI] [PubMed] [Google Scholar]
- 16.Rumsfeld JS, Alexander KP, Goff DC, Jr., Graham MM, Ho PM, Masoudi FA. Cardiovascular Health: The Importance of Measuring Patient-Reported Health Status: A Scientific Statement From the American Heart Association. Circulation. 2013;36(4):216–20. doi: 10.1161/CIR.0b013e3182949a2e. Epub 2013/05/08. [DOI] [PubMed] [Google Scholar]
- 17.Schroten NF, Ruifrok WP, Kleijn L, Dokter MM, Sillje HH, Lambers Heerspink HJ. Short-term vitamin D3 supplementation lowers plasma renin activity in patients with stable chronic heart failure: an open-label, blinded end point, randomized prospective trial (VitD-CHF trial) Am Heart J. 2013;166(2):357–64. doi: 10.1016/j.ahj.2013.05.009. e2. Epub 2013/07/31. [DOI] [PubMed] [Google Scholar]
- 18.Carrara D, Bernini M, Bacca A, Rugani I, Duranti E, Virdis A. Cholecalciferol administration blunts the systemic renin-angiotensin system in essential hypertensives with hypovitaminosis. D. J. Renin Angiotensin Aldosterone Syst. 2014;15(1):82–7. doi: 10.1177/1470320312471149. Epub 2014/01/04. [DOI] [PubMed] [Google Scholar]
- 19.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341(10):709–17. doi: 10.1056/NEJM199909023411001. Epub 1999/09/02. [DOI] [PubMed] [Google Scholar]
- 20.Lijnen P, Petrov V. Induction of cardiac fibrosis by aldosterone. J Mol Cell Cardiol. 2000;32(6):865–79. doi: 10.1006/jmcc.2000.1129. Epub 2000/07/11. [DOI] [PubMed] [Google Scholar]
- 21.Jorde UP, Vittorio T, Katz SD, Colombo PC, Latif F, Le Jemtel TH. Elevated plasma aldosterone levels despite complete inhibition of the vascular angiotensin-converting enzyme in chronic heart failure. Circulation. 2002;106(9):1055–7. doi: 10.1161/01.cir.0000030935.89559.04. Epub 2002/08/28. [DOI] [PubMed] [Google Scholar]
- 22.Rousseau MF, Gurne O, Duprez D, Van Mieghem W, Robert A, Ahn S. Beneficial neurohormonal profile of spironolactone in severe congestive heart failure: results from the RALES neurohormonal substudy. J Am Coll Cardiol. 2002;40(9):1596–601. doi: 10.1016/s0735-1097(02)02382-3. Epub 2002/11/13. [DOI] [PubMed] [Google Scholar]
- 23.Tomaschitz A, Pilz S, Ritz E, Grammer T, Drechsler C, Boehm BO. Association of plasma aldosterone with cardiovascular mortality in patients with low estimated GFR: the Ludwigshafen Risk and Cardiovascular Health (LURIC) Study. Am J Kidney Dis. 2011;57(3):403–14. doi: 10.1053/j.ajkd.2010.10.047. Epub 2010/12/28. [DOI] [PubMed] [Google Scholar]
- 24.Lishmanov A, Dorairajan S, Pak Y, Chaundhary K, Chockalingam A. Treatment of 25-OH Vitamin D Deficiency in Older Men With Chronic Kidney Disease Stages 3 and 4 Is Associated With Reduction in Cardiovascular Events. Am J Ther. 2011 doi: 10.1097/MJT.0b013e3182211b3b. Epub 2011/12/22. doi:10/1007/s11255-010-9897-2. [DOI] [PubMed] [Google Scholar]
- 25.Cohn JN, Anand IS, Latini R, Masson S, Chiang YT, Glazer R. Sustained reduction of aldosterone in response to the angiotensin receptor blocker valsartan in patients with chronic heart failure: results from the Valsartan Heart Failure Trial. Circulation. 2003;108(11):1306–9. doi: 10.1161/01.CIR.0000091234.45664.62. Epub 2003/08/27. [DOI] [PubMed] [Google Scholar]
- 26.Ponda MP, Hostetter TH. Aldosterone antagonism in chronic kidney disease. Clin J Am Soc Nephrol. 2006;1(4):668–77. doi: 10.2215/CJN.00120106. Epub 2007/08/21. [DOI] [PubMed] [Google Scholar]
- 27.Levin A, Le Barbier M, Er L, Andress D, Sigrist MK, Djurdjev O. Incident isolated 1,25(OH)(2)D(3) deficiency is more common than 25(OH)D deficiency in CKD. J Nephrol. 2012;25(2):204–10. doi: 10.5301/JN.2011.8429. Epub 2011/06/21. [DOI] [PubMed] [Google Scholar]
- 28.Halloran BP, Schaefer P, Lifschitz M, Levens M, Goldsmith RS. Plasma vitamin D metabolite concentrations in chronic renal failure: effect of oral administration of 25-hydroxyvitamin D3. J Clin Endocrinol Metab. 1984;59(6):1063–9. doi: 10.1210/jcem-59-6-1063. Epub 1984/12/01. [DOI] [PubMed] [Google Scholar]
- 29.Xiang W, Kong J, Chen S, Cao LP, Qiao G, Zheng W. Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrinol Metab. 2005;288(1):E125–32. doi: 10.1152/ajpendo.00224.2004. Epub 2004/09/16. [DOI] [PubMed] [Google Scholar]
- 30.Kestenbaum B, Katz R, de Boer I, Hoofnagle A, Sarnak MJ, Shlipak MG. Vitamin D, parathyroid hormone, and cardiovascular events among older adults. J Am Coll Cardiol. 2011;58(14):1433–41. doi: 10.1016/j.jacc.2011.03.069. Epub 2011/09/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomaschitz A, Ritz E, Pieske B, Rus-Machan J, Kienreich K, Verheyen N. Aldosterone and parathyroid hormone interactions as mediators of metabolic and cardiovascular disease. Metabolism. 2014;63(1):20–31. doi: 10.1016/j.metabol.2013.08.016. Epub 2013/10/08. [DOI] [PubMed] [Google Scholar]
- 32.Lundqvist J, Norlin M, Wikvall K. 1alpha,25-Dihydroxyvitamin D3 affects hormone production and expression of steroidogenic enzymes in human adrenocortical NCI-H295R cells. Biochim Biophys Acta. 2010;1801(9):1056–62. doi: 10.1016/j.bbalip.2010.04.009. Epub 2010/04/28. [DOI] [PubMed] [Google Scholar]