Abstract
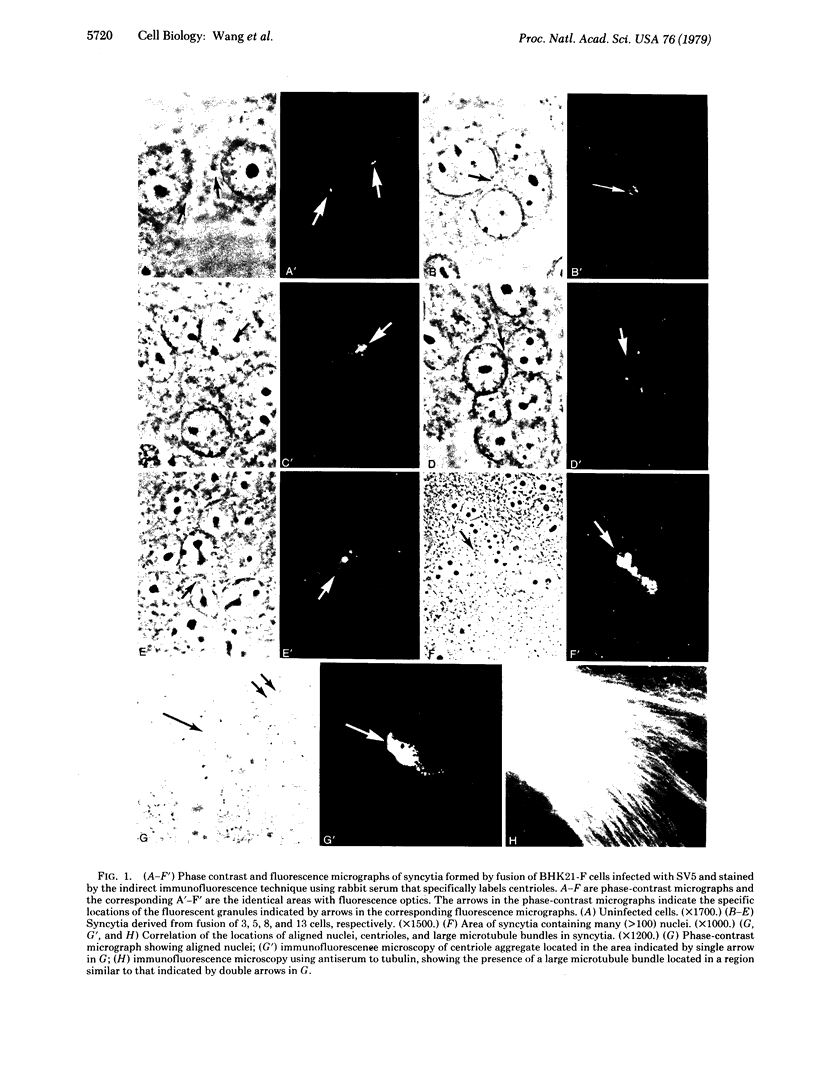
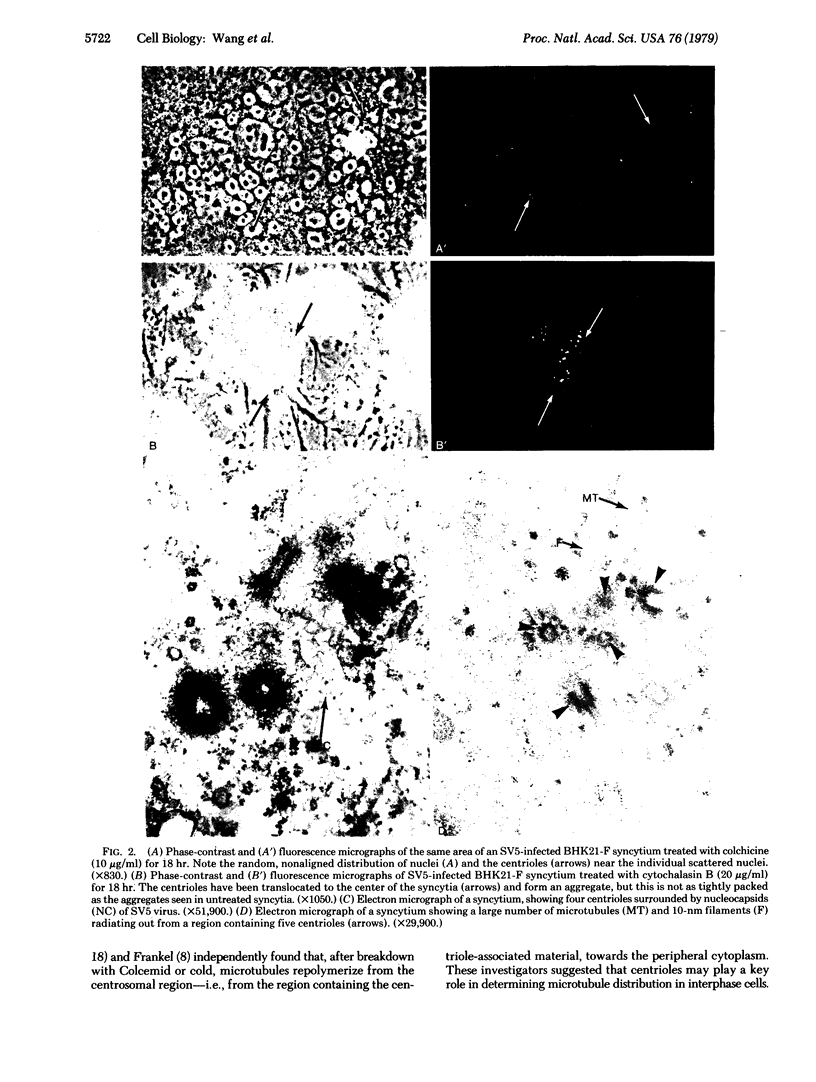
Previous reports from this laboratory have provided evidence suggesting that microtubules and 10-nm filaments serve both cytoskeletal and force-generating functions in the intracellular movement and positioning of nuclei in syncytia. It has been found that, during the process of cell fusion and nuclear migration in syncytia induced by the paramyxovirus simian virus 5, centrioles are transported in the cytoplasm and form large aggregates. These aggregates are usually found in regions adjacent to rows of aligned nuclei and large bundles of microtubules and 10-nm filaments. Colchicine prevents the translocation and aggregation of centrioles, but cytochalasin B has little effect on this process. These results suggest that the same cytoskeletal elements that are involved in nuclear migration and positioning--i.e., microtubules and 10-nm filaments--are also involved in the transport of centrioles. The possibility that aggregates of centrioles may serve as centers for the organization of microtubules and 10-nm filaments into the large bundles observed in the syncytia is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brinkley B. R., Fuller E. M., Highfield D. P. Cytoplasmic microtubules in normal and transformed cells in culture: analysis by tubulin antibody immunofluorescence. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4981–4985. doi: 10.1073/pnas.72.12.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOPPIN P. W. MULTIPLICATION OF A MYXOVIRUS (SV5) WITH MINIMAL CYTOPATHIC EFFECTS AND WITHOUT INTERFERENCE. Virology. 1964 Jun;23:224–233. doi: 10.1016/0042-6822(64)90286-7. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Holmes K. V., Dales S., Choppin P. W. An electron microscopic study of moderate and virulent virus-cell interactions of the parainfluenza virus SV5. Virology. 1966 Nov;30(3):411–426. doi: 10.1016/0042-6822(66)90119-x. [DOI] [PubMed] [Google Scholar]
- Connolly J. A., Kalnins V. I. Visualization of centrioles and basal bodies by fluorescent staining with nonimmune rabbit sera. J Cell Biol. 1978 Nov;79(2 Pt 1):526–532. doi: 10.1083/jcb.79.2.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel F. R. Organization and energy-dependent growth of microtubules in cells. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2798–2802. doi: 10.1073/pnas.73.8.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould R. R., Borisy G. G. The pericentriolar material in Chinese hamster ovary cells nucleates microtubule formation. J Cell Biol. 1977 Jun;73(3):601–615. doi: 10.1083/jcb.73.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K. V., Choppin P. W. On the role of microtubules in movement and alignment of nuclei in virus-induced syncytia. J Cell Biol. 1968 Dec;39(3):526–543. doi: 10.1083/jcb.39.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K. V., Choppin P. W. On the role of the response of the cell membrane in determining virus virulence. Contrasting effects of the parainfluenza virus SV5 in two cell types. J Exp Med. 1966 Sep 1;124(3):501–520. doi: 10.1084/jem.124.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke M., Krishnan N. Hot alcoholic phosphotungstic acid and uranyl acetate as routine stains for thick and thin sections. J Cell Biol. 1971 Aug;50(2):550–557. doi: 10.1083/jcb.50.2.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill M., Brinkley B. R. Human chromosomes and centrioles as nucleating sites for the in vitro assembly of microtubules from bovine brain tubulin. J Cell Biol. 1975 Oct;67(1):189–199. doi: 10.1083/jcb.67.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Weber K. Cytoplasmic microtubules in tissue culture cells appear to grow from an organizing structure towards the plasma membrane. Proc Natl Acad Sci U S A. 1976 Mar;73(3):867–871. doi: 10.1073/pnas.73.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E., Cross R. K., Choppin P. W. Involvement of microtubules and 10-nm filaments in the movement and positioning of nuclei in syncytia. J Cell Biol. 1979 Nov;83(2 Pt 1):320–337. doi: 10.1083/jcb.83.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E., Goldman R. D. Functions of cytoplasmic fibers in intracellular movements in BHK-21 cells. J Cell Biol. 1978 Dec;79(3):708–726. doi: 10.1083/jcb.79.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Pollack R., Bibring T. Antibody against tuberlin: the specific visualization of cytoplasmic microtubules in tissue culture cells. Proc Natl Acad Sci U S A. 1975 Feb;72(2):459–463. doi: 10.1073/pnas.72.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]