Abstract
The purpose of this study was to accurately detect lymph-node micrometastases, i.e., metastatic cancer foci that have a size between 2.0 and 0.2 mm, in nodes excised from colorectal cancer (CRC) patients, and to determine how frequently micrometastases might be missed when standard histological examination procedures are used. A total of 311 lymph nodes were removed and examined from 90 patients with Stage I to IV CRC. The number of slices of histology sections ranged from 6 to 75 per node (average=25.5; SD= 11.1), which provided a total of 7,943 slices. Lymph nodes were examined in their entire volume at every 50-µm and 100-µm intervals for nodes smaller and larger than 5 mm respectively. The total number of thin sections examined in each node and the number of thin sections where metastatic foci were present were counted. The number of thin sections with metastatic foci and the total number of slices was determined for each node. In addition, the presence or absence of metastatic foci in the “central” slice was determined. Micrometastases were found in 12/311 (3.9%) of all lymph nodes. In the 12 lymph nodes with micrometastases, the rate of metastatic slices over all slices was 39.4% (range=6.3 to 81.3%; SD=25.8%) In the central slice of each node, micrometastases were present only in 6 of 12 lymph nodes (50%); accordingly, they were not present in the central slice for half the micrometastatic nodes. These 6 nodes represented 1.9% of the 311 nodes and 11.1% of the 54 metastatic nodes. This study suggests that a significant fraction of micrometastases can be missed by traditional singleslice sectioning; half of the micrometastases would have been overlooked in our data set of 311 nodes.
Keywords: Colorectal cancer, Lymph nodes, Micrometastases, Serial histological examination
Introduction
Detection of metastases in lymph nodes is important for appropriate management and prognosis for cancer patients. Currently, lymph nodes dissected from a cancer patient are evaluated by histological examination of a few sections of each node using hematoxylin and eosin (H&E) staining. The recommended treatment for patients who are classified as node-positive typically is adjuvant therapy in conjunction with surgery.
However, the current histological procedure does not adequately detect all metastases in lymph nodes, particularly micrometastases smaller than 2 mm. Typically, dissected nodes undergo a standard histological evaluation that involves sectioning into blocks (that are 2 to 3 mm thick), or more commonly, by bisecting the node into two approximately equal portions. The initial sections then undergo fixation, embedding in paraffin, thin sectioning of the surfaces of the thick sections, placement of thin sections (that are 3 to 4 µm thick) on microscope slides, histochemical staining using H&E, and microscopic examination of stained thin sections. This method reliably detects nodal metastases that are present in the examined thin sections, but only a limited number of thin sections are obtained from the surfaces of thick sections. Because histological examination is limited to the surfaces of the thick sections and because those thick sections can be thicker than 2 mm, micrometastatic foci residing between the exposed surfaces may escape detection. Only a few thin sections are examined from each node, and generally more than 10 to15 nodes are examined for each cancer patient. The entire lymph node volume cannot be evaluated in a practical manner by the current standard histological methods, and nodes are histologically “sampled” at 2-mm or larger intervals; therefore, overlooking micrometastases, which are smaller than 2 mm, is possible. The research described in this article seeks to assess the risk of overlooking micrometastases.
The American Joint Committee on Cancer (AJCC) guidelines define micrometastases and distinguish them from isolated tumor cells (ITCs) [1]. According to the guidelines, metastases smaller than 2 mm, but larger than 0.2 mm, are considered micrometastases. In comparison, ITCs consist of individual tumor cells or small clusters of cells that are smaller than 0.2 mm and are most-reliably identified by immunohistochemistry (IHC) or molecular methods, although they also can be detected by H&E.
The incidence of overlooked micrometastases in lymph nodes of colorectal cancer (CRC) has not been well documented. In order to assure reliable detection of micrometastases, the present study was examined thin sections made at 50-µm steps for nodes that were 5 mm or smaller or at 100 µm steps for larger nodes; thin sections were made in this manner over the full volume of each examined node. This approach enabled accurately detecting micrometastases in dissected lymph nodes and determining how frequently micrometastases might be missed when traditional methods are used.
Materials and Methods
Patients
Lymph nodes were dissected from 90 patients with histologically proven stage I to IV primary CRC between July 2007 and May 2009 at the Kuakini Medical Center in Honolulu, HI. The following distribution of stages was observed among the 90 patients: 20 stage I, 41 stage IIA, 0 stage IIB, 7 stage IIIA, 14 stage IIIB, 7 stage IIIC, and 1 stage IV. (61 patients were in metastasis-free stages I and II; 29 patients were in metastasis-containing stages III and IV.) The patients included 50 males and 40 females, with ages ranging from 41 to 95 years (average=71.1; SD=12.0).
Surgical and Histological Procedures
Formal, surgical, lymph-node dissection was performed according to the current standard of care for CRC. A minimum of 12 nodes was dissected from each patient and nodes in the surgical specimen were isolated. From the isolated nodes, 1 to 7 (average=3.5; SD=1.3) lymph nodes were randomly selected for histological examination over the entire node volume. No node-selection criteria of any kind were applied, nor were any distinctions made among Stages I to IV primary CRC in patient selection. The number of examined thin sections examined per selected node ranged from 6 to 75 (average=25.5; SD=11.1); a total of 7,943 slices were evaluated.
For sizing purposes, each lymph node was approximated by an ellipsoid; sizing the lymph node consisted of measuring the three main axes (length, width, and height) of the approximating ellipsoid.
After sizing, each lymph node was fixed in 10% neutral-buffered formalin for 24 h. Fixed nodes were cut longitudinally, approximately in half. The two half-nodes were embedded in paraffin with the flat cut surface down prior to sectioning. From the fixed two half-nodes, 3-µm thin sections were obtained using a microtome at every 50 µm for nodes smaller than 5mm or 100 µm for nodes larger than 5mm. Each section spanned the two embedded half nodes. At each step, 5 slices of 3-µm sections were obtained. For light-microscopic examination, each 3-µm thin section was placed on a microscopic slide and stained with H&E. The sections from both node halves were placed on each microscopic slide. The pair of half-node sections having the best histological quality were was used for microscopic examination. All examined microscopic slides were photographed using a digital camera (FujiFilm FinePix S9100, Fuji Photo Film, Tokyo Japan) equipped with Hoya +2 and +4 close-up lenses (Hoya Corp., Tokyo, Japan). All thin sections were histologically evaluated by two experienced, board-certified pathologists. The border of each detected metastatic lesion was demarcated in the examined thin sections. This approach proved to be effective for detecting micrometastases > 0.2mm.
Classification of Lymph Nodes
Based on histologic evaluations, lymph nodes were classified into the following five categories:
No-metastasis: lymph nodes that are entirely without any metastatic foci.
≥ 50%-metastases: lymph nodes containing at least one metastatic focus with a metastatic volume in excess of 50% of the node volume.
< 50%-metastases: lymph nodes with metastatic foci having a maximum dimension > 2mm, but not containing any metastatic focus with a volume in excess of 50% of the node volume. (See Fig. 1).
Micrometastases: lymph nodes with metastatic foci having a maximum dimension ≤ 2mm but > 0.2mm. (See Fig. 2).
ITC: lymph nodes with foci consisting only of isolated tumor cells or cell clusters having a maximum cluster dimension ≤ 0.2mm.
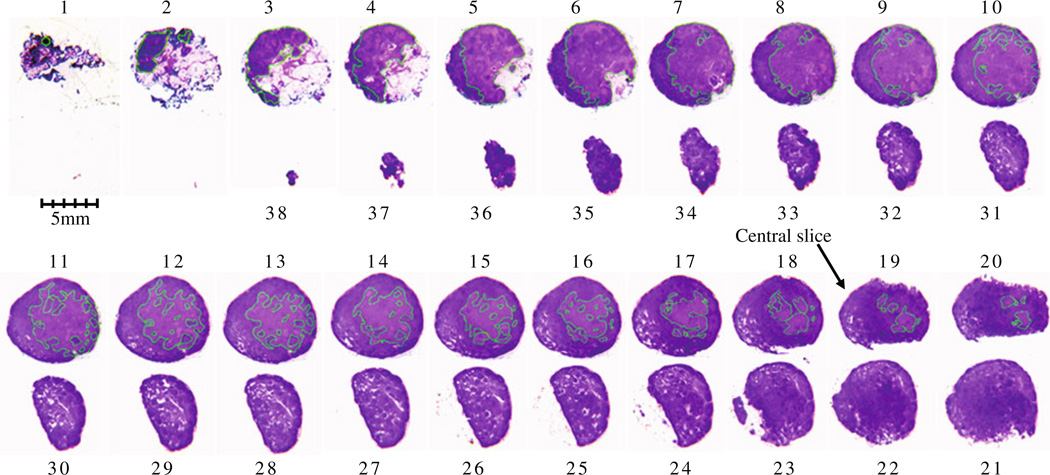
Fig. 1.
Serial histological slices of a node with < 50% metastasis; H&E staining, ×1. Metastatic foci are demarcated in each slice. Metastatic foci are present in slices No.1 to No. 20, but absent in slices No.21 to No.38. The central slice of this lymph node is No.19 where metastatic foci are present
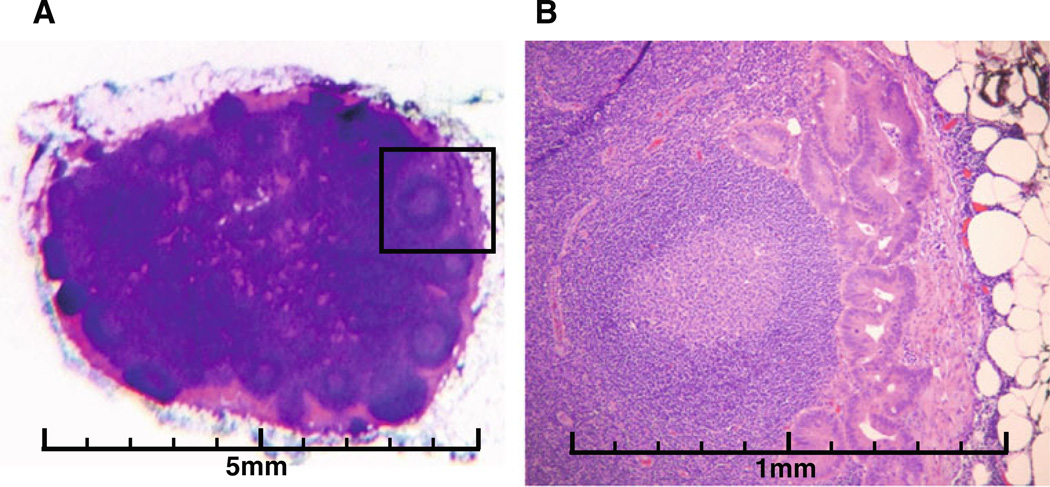
Fig. 2.
Micrometastases in a lymph node. (a): the entire node, H&E staining, ×1. (b): magnification of the area with a box shown in (a), H&E staining, ×25. (c): magnification of the area with a box shown in (b), H&E staining, ×100
Number and Location of Metastases in Each Lymph Node
From each series of histology sections, we were able to determine the location and extent of the metastatic foci in 3 dimensions (3D). The number, size and location of metastatic foci in each node were recorded along with the presence or absence of metastatic tissue in the “central slice.” The central slice was defined as the middle slice of all slices; e.g., the 15th slice when 30 slices were examined in a given node. For < 50%-metastasis and for micrometastases, the total number of slices examined in each node and the number of slices where metastatic foci were present were counted. The proportion (fraction) of the number of slices with metastatic foci over the total number of slices was calculated for each node. In addition, the presence or absence of metastatic tissue in the “central” slice was determined.
Results
Metastases were found in 54/311 (17.4%) of all selected lymph nodes; positive nodes were present in 29 of 90 patients (32.2%). Category-1 nodes comprised 257/311 (82.6%) of the selected nodes. Category-2, ≥ 50%-metastasis were found in 39/311 (12.5%) of the nodes; category-3 < 50%-metastasis were found in 3/311 (1.0%) of the nodes; and category-4 micrometastases were found in 12/311 (3.9%) of the nodes. No category-5 ITCs were found. These results are summarized in Table 1.
Table 1.
Patients, lymph nodes, and slices based on lymph-node category
| Node Category | Number of Patients | Number of Lymph Nodes | Number of Slices |
|---|---|---|---|
| No-metastases | 61 (67.8%) | 257 (82.6%) | 5,967 (75.1%) |
| Metastases | 29 (32.2%) | 54 (17.4%) | 1,976 (24.9%) |
| ≥ 50%-metastases | 17 (18.9%) | 39 (12.5%) | 1,616 (20.3%) |
| < 50%-metastases | 3 (3.3%) | 3 (1.0%) | 78 (1.0%) |
| Micrometastases | 9 (10.0%) | 12 (3.9%) | 282 (3.6%) |
| ITC | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Total | 90 | 311 | 7943 |
| Average Per Patient | 3.5 | 25.5 | |
| Standard Deviation | 1.3 | 11.1 |
In the 39 lymph nodes with ≥ 50%-metastasis, the average number of slices examined was 40.4 (range=18 to 75; SD=14.0) depending on the size of the node. Metastatic tissue was present in more than a half of the serial histologic slices including all the central slices.
In the 3 lymph nodes with < 50%-metastases, the average number of slices examined was 26.3 (range=18 to 39; SD=11.6) depending on the size of the lymph node. The size dimensions of these 3 nodes are noted in Table 2. The rate of metastatic slices over all slices was 61.2% (range=51.3 to 77.8%; SD=14.5%). In the central slices, metastases were present in all of 3 lymph nodes (100%). These results are summarized in Table 2.
Table 2.
Characteristics of lymph nodes with < 50% metastatic voolume
| Node Number | Length (mm) |
Width (mm) |
Height (mm) |
Slice Separation (µm) |
Number of Slices |
Number of Metastatic Slices |
Central-slice Metastases |
|---|---|---|---|---|---|---|---|
| 1 | 9.4 | 6 | 5.9 | 100 | 22 | 12 (54.5%) | Yes |
| 2 | 6 | 5.5 | 4.9 | 100 | 18 | 14 (77.8%) | Yes |
| 3 | 9.6 | 9 | 6.3 | 50 | 38 | 20 (52.6%) | Yes |
| Total | 78 | 46 (61.2%) | |||||
| Average Per Node | 8.3 | 6.8 | 5.7 | 26 | 15.3 | ||
| Standard Deviation | 2 | 1.9 | 0.7 | 10.6 | 4.2 |
In the 12 lymph nodes with micrometastases, the average number of slices examined was 23.5 (range=8 to 44; SD=11.1) depending on the size of the lymph node. The sizes of these 12 nodes are noted in Table 3. The rate of metastatic slices over all slices was 39.4% (range= 6.3 to 81.3%; SD=25.8%) In the central slices of each node, metastases were present in 6 of 12 lymph nodes (50%). These 6 nodes represented 1.9% of 311 nodes (total number) and 11.1% of 54 metastatic nodes. These results are summarized in Table 3.
Table 3.
Characteristics of Micrometastatic Nodes
| Node Number | Length (mm) |
Wide (mm) |
Height (mm) |
Slice Separation (µm) |
Number of Slices |
Number of Metastatic Slices |
Central-slice Metastases |
|---|---|---|---|---|---|---|---|
| 1 | 4.2 | 2.7 | 1.9 | 50 | 8 | 4 (50.0%) | Yes |
| 2 | 5.9 | 4.4 | 3.3 | 50 | 20 | 14 (70.0%) | Yes |
| 3 | 3.4 | 4.2 | 3 | 50 | 16 | 12 (75.0%) | Yes |
| 4 | 6.2 | 4.9 | 4.6 | 50 | 32 | 7 (21.9%) | No |
| 5 | 8.5 | 7.6 | 5.3 | 50 | 38 | 6 (15.8%) | No |
| 6 | 9.6 | 7.8 | 6.2 | 50 | 44 | 24 (54.5%) | Yes |
| 7 | 7.7 | 5.4 | 4.8 | 50 | 24 | 6 (25.0%) | No |
| 8 | 14.1 | 8.8 | 7.3 | 100 | 32 | 2 (6.3%) | No |
| 9 | 5 | 4.4 | 3.7 | 50 | 20 | 6 (30.0%) | No |
| 10 | 3.2 | 2.7 | 2.2 | 50 | 14 | 2 (14.3%) | No |
| 11 | 4.6 | 3.3 | 2.1 | 50 | 14 | 4 (28.6%) | Yes |
| 12 | 2 | 2 | 1.4 | 50 | 16 | 13 (81.3%) | Yes |
| Total | 282 | 100 (39.4%) | |||||
| Average per node | 6.2 | 4.8 | 3.8 | 23.5 | 8.3 | ||
| Standard Deviation | 3.3 | 2.2 | 1.8 | 11.1 | 6.4 |
Using H&E staining, we did not detect any ITC unless it was accompanied by micrometastases or larger metastatic foci.
Discussion
Currently, approximately 20% to 30% of patients with node-negative CRC develop locoregional recurrence or distant metastases and die from CRC within 5 years, likely due to failure to detect lymph node metastases using standard histological node-evaluation methods [2–4]. Similarly, in the case of breast cancer, at least 10% of sentinel and formal node dissections result in missed micrometastases. Tan et al. showed that 83 of 368 (23%) apparently node-negative patients actually had metastases to their nodes; 59 of the 83 (71%) false-negative cases had metastases in only one node [5]. Moreover, 61 of the 83 (73%) false-negative cases had a maximum dimension of no more than 0.2mm, and 17 of the 83 (20%) had a maximum dimension of 0.3 to 2.0mm.
Many studies of different types of cancers have reported clinically significant micrometastases or occult tumor cells in lymph nodes. A few studies of gastric cancer have demonstrated that micrometastases are an important prognostic factor in initially staged N0 gastric cancer [6–8]; a study of sentinel lymph nodes of breast cancer patients showed that sentinel-node micrometastases were associated with additional positive nodes and with distant recurrence [9], and a study of esophageal cancer suggested that micrometastases were useful for determining prognosis [10]. In the cases of CRC, the value of micrometastases for prognosis has been controversial. Many studies of micrometastases of lymph nodes in CRC reported that micrometastases in lymph nodes have no prognostic significance for patients with histologically node-negative CRC [11–17]. In contrast, recent studies are increasingly demonstrating that nodal micrometastases are a significant factor in prognosis [18–23]. Bilchik et al. showed Targeted nodal methods in CRC provide an elegant way of performing focused analysis on a limited number of LNs and thereby improving staging accuracy. However, the method will continue to be investigational until the biologic and prognostic role of micrometastases in CRC is better defined [24].
One reason for the controversy regarding the significance of micrometastases in lymph nodes is the dependence of their detectability on the histological methods used to evaluate the nodes. In the majority of studies on CRC lymph nodes, only a single thin section of each node was examined for the purpose of finding micrometastases [11–13, 18, 19]. If a lymph node is examined with only a single slice, then very severe under sampling occurs, and a micrometastasis is very likely to be missed. Conversely, if a lymph node is examined with multiple slices, sampling improves, and the likelihood of detecting micrometastases increases as the number of cut sections examined increases. Noura evaluated the detection rate of micrometastases as a function of the number of sections used, and showed that the frequency of micrometastases detected in lymph nodes increased from 3.8% (33/878) using one slice to 6.3% (55/878) using two slices and to 11.8% (104/878) using five slices [14]. Sasaki et al. examined occult metastases in lymph nodes in 19 Dukes’ stages A and B patients by cutting 10 slices, and demonstrated occult metastases in 90 of 268 nodes (33.6%) [20]. Yasuda et al. examined micrometastases of lymph node in 42 Dukes’ stage B patients by cutting 5 slices, showing micrometastases in 136 of 1,013 nodes (13.4%) [22]. Messerini et al. examined micrometastases in the lymph nodes of 42 stage IIA patients by cutting 6 slices on average, and showed micrometastases in lymph node in 44 of 8,266 nodes (0.5%) [17]. Furthermore, Palma et al. examined lymph-node micrometastases in 38 Dukes’ stage B patients by cutting 3 slices, and found micrometastases in 7 of 383 nodes (1.82%) [15]. In our present study, micrometastases in lymph nodes from Stage I-IV patients were detected in 12 of 311 (3.9%) nodes, but half of them would have been overlooked using a single central slice for evaluation. (See Table 1.)
A variety of methods are available to detect micrometastases or ITC in lymph nodes such as immuno-histochemical (IHC) staining, reverse transcriptase-polymerase chain reaction (RT-PCR), and finely spaced serial sectioning. Most of previous studies on micrometastases or ITC in lymph nodes have used IHC or RT-PCR [11–23]. IHC and RT-PCR have been shown to be sensitive techniques for detecting small clusters of tumor cells in comparison with traditional H&E staining. Iddings et al. reviewed multiple studies and found that micrometastases were identified in 179/566 (32%) of node-negative patients when IHC was used, and in 64/173 (37%) of node-negative patients when RT-PCR was used [25]. In our present study, micrometastases were examined only by H&E staining using closely spaced serial sections of lymph nodes without IHC or RT-PCR. Therefore, ITCs and possibly some micrometastases might have been missed. However, ITCs are currently considered to be clinically insignificant in determining prognosis, because ITCs typically do not show evidence of metastatic activity by proliferation, of induction of a stromal reaction, or of vascular or lymphatic sinus-wall invasion [26]. Therefore, we did not use IHC or RT-PCR in this study. Rather, we examined a much larger number of slices per node than other studies on lymph nodes of CRC patients. Ishii et al. investigated the occurrence of micrometastases in 1,028 lymph nodes of 35 gastric-cancer patients using a total of 24,094 slices (average=23.4 slices per node) with IHC; this study revealed micrometastases in only 6 /1,028 (0.6%) of the lymph nodes [27]. In our study, the total number of CRC nodes examined was 311; the number of slices examined was 7,943; and the average number of slices examined per node was 25.5. We detected metastases in 17.4% (54/311) of our examined nodes, and we observed lymph-node micrometastases in 10% (9/90) of the patients and in 3.9% (12/311) of the nodes. (See Table 1.) While other studies suggest that approximately 30% of sampled nodes contain metastases, our data may reflect earlier stages of CRC [31].
In our study, lymph nodes were microscopically examined over their entire volume at 50-µm intervals for smaller nodes and 100-µm intervals for larger nodes. The number of slices histologically examined in micrometastatic cases ranged from 8 to 44 depending on the size of the lymph node. The rate of micrometastatic slices over all slices in each node varied from 6.3% to 81.3%. Micrometastases were present in the central slices of each lymph node in 6 of 12 nodes (50%). This indicated that overlooked micrometastases would have occurred in 50% of micrometastatic nodes if traditional methods using a single central section had been performed; these micrometastatic foci would have been overlooked because of the lateral off-center location of the foci with respect to the orientation of the sectioning plane.
Conclusions
In conclusion, we detected metastases in 17.4% of all lymph nodes and micrometastases in 3.9% of all lymph nodes by entire-volume serial histological examination. The detectability of micrometastases depends on the location of micrometastatic foci in a lymph node and the number and/ or direction of histological slice sectioning. This study suggested that micrometastases would have been missed by traditional single-slice sectioning in 50% of micrometastatic lymph nodes, or 1.9% of all nodes. Considering the clinical significance of micrometastases in the lymph nodes of CRC patients, the entire-volume serial histological method would be advantageous over the traditional single-slice method. However, a multiple-slice method is extremely time consuming, and in a realistic clinical setting, it would not be practical for application to all lymph nodes dissected from all patients with CRC. In order to identify micrometastases as a routine procedure, a method is needed that can examine the entire volume of a lymph node rapidly, e.g., within a few minutes per node. We currently are investigating quantitative high-frequency ultrasound as a basis for such a method, and preliminary results have demonstrated very encouraging results in studies of 83 lymph nodes from CRC patients [28–30].
Acknowledgments
This research was supported in part by NIH grant CA100183 and by the Riverside Research Institute Biomedical Engineering Research Fund.
Contributor Information
Masaki Hata, Email: masakihata4999@yahoo.co.jp, c/o Junji Machi, 405 N. Kuakini Street, Suite 601, Honolulu, HI 96817, USA; Department of Surgery, University of Hawaii and Kuakini Medical Center, Honolulu, HI, USA; Department of Coloproctological Surgery, Juntendo University School of Medicine, Tokyo, Japan.
Junji Machi, Email: junji@hawaii.edu, Department of Surgery, University of Hawaii and Kuakini Medical Center, Honolulu, HI, USA.
Jonathan Mamou, Email: JMamou@RiversideResearch.org, Riverside Research Institute, New York, NY, USA.
Eugene T. Yanagihara, Email: kplety@earthlink.net, Department of Pathology, University of Hawaii and Kuakini Medical Center, Honolulu, HI, USA.
Emi Saegusa-Beecroft, Email: emilysaegusa@hotmail.com, Department of Surgery, University of Hawaii and Kuakini Medical Center, Honolulu, HI, USA.
Gregory K. Kobayashi, Email: gkk808@gmail.com, Department of Pathology, University of Hawaii and Kuakini Medical Center, Honolulu, HI, USA.
Clifford C. M. Wong, Email: clifford.wong@mail.com, Department of Pathology, University of Hawaii and Kuakini Medical Center, Honolulu, HI, USA.
Conway Fung, Email: sleepysea2000@yahoo.com, Department of Pathology, University of Hawaii and Kuakini Medical Center, Honolulu, HI, USA.
Ernest J. Feleppa, Email: efeleppa@RiversideResearch.org, Riverside Research Institute, New York, NY, USA.
Kazuhiro Sakamoto, Email: kazusaka@med.juntendo.ac.jp, Department of Coloproctological Surgery, Juntendo University School of Medicine, Tokyo, Japan.
References
- 1.Greene FL, Page DL, Fleming ID, et al. AJCC cancer staging manual. 6th ed. New York: Springer; 2002. [Google Scholar]
- 2.Meijerink WJ, van der Pas MH, van der Peet DL, et al. New horizons in colorectal cancer surgery. Surg Endosc. 2009;23:1–3. doi: 10.1007/s00464-008-0254-9. [DOI] [PubMed] [Google Scholar]
- 3.Cohen AM, Kelsen D, Saltz L, et al. Adjuvant therapy for colorectal cancer. Curr Prob Cancer. 1998;22:5–65. [Google Scholar]
- 4.Gunderson LL, Sargent DJ, Tepper JE, et al. Impact of T and N stage and treatment on survival and relapse in adjuvant rectal cancer: a pooled analysis. J Clin Oncol. 2004;22:1785–1796. doi: 10.1200/JCO.2004.08.173. [DOI] [PubMed] [Google Scholar]
- 5.Tan LK, Giri D, Hummer AJ, et al. Occult Axillary Node Metastases in Breast Cancer Are Prognostically Significant: Results in 368 Node-Negative Patients With 20-Year Follow-Up. J Clin Oncol. 2008;26:1803–1809. doi: 10.1200/JCO.2007.12.6425. [DOI] [PubMed] [Google Scholar]
- 6.Maehara Y, Oshiro T, Endo K, et al. Clinical significance of occult micrometastases in lymph nodes from patients with early gastric cancer who died of recurrence. Surgery. 1996;119:397–402. doi: 10.1016/s0039-6060(96)80138-3. [DOI] [PubMed] [Google Scholar]
- 7.Cai J, Ikeguchi M, Maeta M, et al. Micrometastasis in lymph nodes and microinvasion of the muscularis propria in primary lesions of submucosal gastric cancer. Surgery. 2000;127:32–39. doi: 10.1067/msy.2000.100881. [DOI] [PubMed] [Google Scholar]
- 8.Nakajo A, Natsugoe S, Ishigami S, et al. Detection and prediction of micrometastasis in the lymph nodes of patients with pN0 gastric cancer. Ann Surg Oncol. 2001;8:158–162. doi: 10.1007/s10434-001-0158-6. [DOI] [PubMed] [Google Scholar]
- 9.Reed J, Rosman M, Verbanac KM, et al. Prognostic Implications of Isolated Tumor Cells and Micrometastases in Sentinel Nodes of Patients with Invasive Breast Cancer: 10-Year Analysis of Patients Enrolled in the Prospective East Carolina University/ Anne Arundel Medical Center Sentinel Node Multicenter Study. J Am Coll Surg. 2009;208:333–340. doi: 10.1016/j.jamcollsurg.2008.10.036. [DOI] [PubMed] [Google Scholar]
- 10.Izbicki JR, Hosch SB, Pichlmeier U, et al. Prognostic value of immunohistochemically identifiable tumor cells in lymph nodes of patients with completely resected esophageal cancer. N Engl J Med. 1997;337:1188–1194. doi: 10.1056/NEJM199710233371702. [DOI] [PubMed] [Google Scholar]
- 11.Öberg A, Stenling R, Tavelin B, et al. Are lymph node micrometastases of any clinical significance in Dukes stages A and B colorectal cancer? Dis Colon Rectum. 1998;41:1244–1249. doi: 10.1007/BF02258221. [DOI] [PubMed] [Google Scholar]
- 12.Choi H-J, Choi Y-Y, Hong S-H. Incidence and prognostic implications of isolated tumor cells in lymph nodes from patients with Dukes B colorectal carcinoma. Dis Colon Rectum. 2002;45:750–756. doi: 10.1007/s10350-004-6291-0. [DOI] [PubMed] [Google Scholar]
- 13.Fisher ER, Colangelo L, Wieand S, et al. Lack of influence of cytokeratin-positive mini micrometastases in “negative node” patients with colorectal cancer: findings from the National Surgical Adjuvant Breast and Bowel Projects protocols R-01 and C-01. Dis Colon Rectum. 2003;46:1021–1026. doi: 10.1007/s10350-004-7275-9. [DOI] [PubMed] [Google Scholar]
- 14.Noura S, Yamamoto H, Miyake Y, et al. Immunohistochemical assessment of localization and frequency of micrometastasis in lymph nodes of colorectal cancer. Clin Cancer Res. 2002;8:759–767. [PubMed] [Google Scholar]
- 15.Palma RT, Waisberg J, Bromberg SH, et al. Micrometastasis in regional lymph nodes of extirpated colorectal carcinoma: immunohistochemical study using anti-cytokeratin antibodies AE1/AE3. Colorectal Dis. 2003;5:164–168. doi: 10.1046/j.1463-1318.2003.00414.x. [DOI] [PubMed] [Google Scholar]
- 16.Kronberg U, Lo’pez-Kostner F, Soto G, et al. Detection of lymphatic micrometastasis in patients with stages I and II colorectal cancer: impact on five-year survival. Dis Colon Rectum. 2004;47:1151–1157. doi: 10.1007/s10350-004-0560-9. [DOI] [PubMed] [Google Scholar]
- 17.Messerini L, Cianchi F, Cortesini C, et al. Incidence and prognostic significance of occult tumor cells in lymph nodes from patients with stage IIA colorectal carcinoma. Hum Pathol. 2006;37:1259–1267. doi: 10.1016/j.humpath.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 18.Clarke G, Ryan E, O’Keane JC, et al. The detection of cytokeratins in lymph node of Duke’s B colorectal cancer subjects predicts a poor outcome. Eur J Gastroenterol Hepatol. 2000;12:549–552. doi: 10.1097/00042737-200012050-00012. [DOI] [PubMed] [Google Scholar]
- 19.Shimoyama M, Yamazaki T, Suda T, et al. Prognostic significance of lateral lymph node micrometastases in lower rectal cancer. Dis Colon Rectum. 2003;46:333–339. doi: 10.1007/s10350-004-6552-y. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki M, Watanabe H, Jass JR, et al. Occult lymph node metastases detected by cytokeratin immunohistochemistry predicts recurrence in “node-negative” colorectal cancer. J Gastroenterol. 1997;32:758–764. doi: 10.1007/BF02936951. [DOI] [PubMed] [Google Scholar]
- 21.Isaka N, Nozue M, Doy M, et al. Prognostic significance of perirectal lymph node micrometastases in Dukes’ B rectal carcinoma: an immunohistochemical study by CAM5.2. Clin Cancer Res. 1999;5:2065–2068. [PubMed] [Google Scholar]
- 22.Yasuda K, Adachi Y, Shiraishi N, et al. Pattern of lymph node micrometastasis and prognosis of patients with colorectal cancer. Ann Surg Oncol. 2001;8:300–304. doi: 10.1007/s10434-001-0300-5. [DOI] [PubMed] [Google Scholar]
- 23.Bukholm IRK, Bondi J, Wiik P, et al. Presence of isolated tumour cells in mesenteric lymph nodes predicts poor prognosis in patients with stage II colon cancer. Eur J Surg Oncol. 2003;29:862–866. doi: 10.1016/j.ejso.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Bilchik AJ, Stojadinovich A, Wainberg Z, et al. Targeted lymph node evaluation in colorectal cancer: A decade of progress! J Surg Oncol. 2009;99:273–274. doi: 10.1002/jso.21231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iddings D, Ahmad A, Elashoff D, et al. The Prognostic Effect of Micrometastases in Previously Staged Lymph Node Negative (N0) Colorectal Carcinoma: A Meta-analysis. Ann Surg Oncol. 2006;13:1386–1392. doi: 10.1245/s10434-006-9120-y. [DOI] [PubMed] [Google Scholar]
- 26.Hermanek P, Hutter RV, Sobin LH, et al. International Union Against Cancer. Classification of isolated tumor cells and micrometastasis. Cancer. 1999;86:2668–2673. [PubMed] [Google Scholar]
- 27.Ishii K, Kinami S, Funaki K, et al. Detection of sentinel and non-sentinel lymph node micrometastases by complete serial sectioning and immunohistochemical analysis for gastric cancer. J Exp Clin Cancer Res. 2008;27:1–7. doi: 10.1186/1756-9966-27-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feleppa E, Mamou J, Hata M, et al. In: Ultrasonic detection of metastases in dissected lymph nodes of cancer patients Acoustical Imaging, Vol.30. Hua Lee., editor. NY: Klewer Academic; 2009. (in press) [Google Scholar]
- 29.Mamou J, Coron A, Hata M, et al. High-frequency quantitative ultrasound imaging of cancerous lymph-nodes. JJAP. 2009;48 07GK08-1: 07GK08-8. [Google Scholar]
- 30.Mamou J, Coron A, Hata M, et al. Three-dimensional High-frequency Characterization of Cancerous Lymph Nodes. Ultrasound Med Biol. 2010;36:361–375. doi: 10.1016/j.ultrasmedbio.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pheby D, Levine D, Pitcher R, et al. Lymph-node harvests directly influence the staging of colorectal cancer: evidence of a regional audit. J Clin Pathol. 2004;57:43–47. doi: 10.1136/jcp.57.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]