Abstract
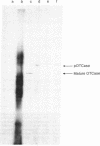
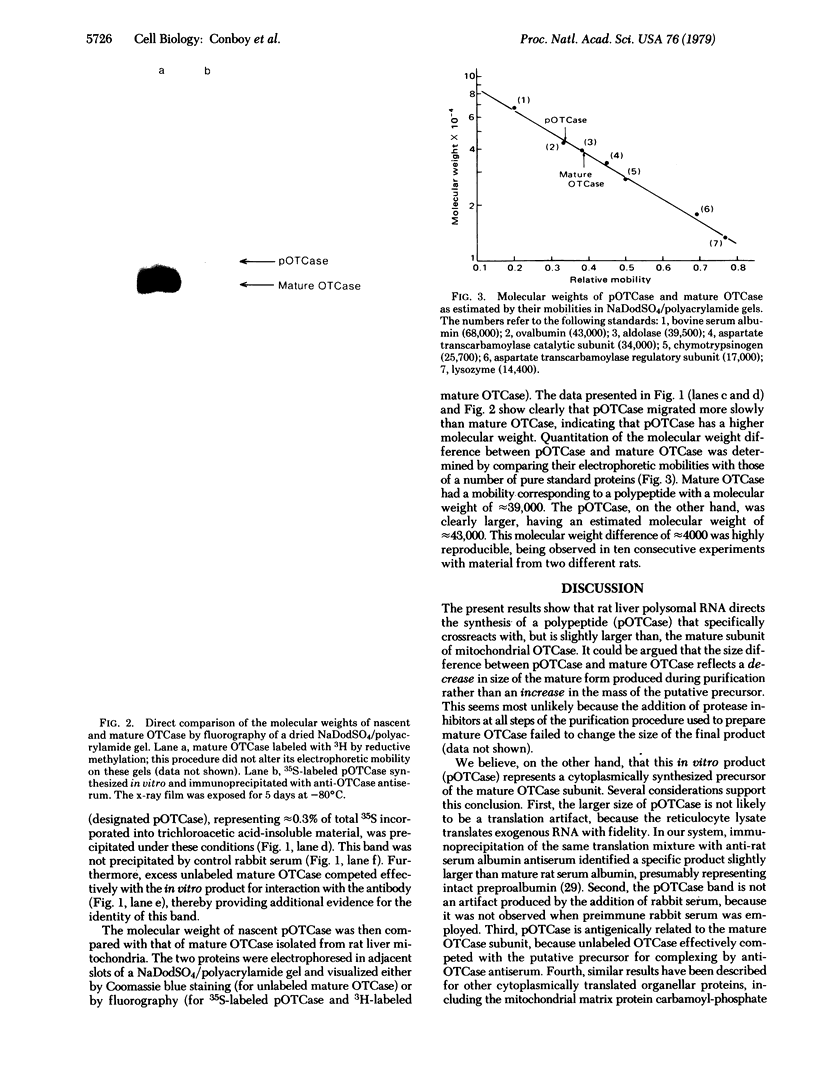
Ornithine transcarbamoylase (OTCase; ornithine carbamoyltransferase; carbamoyl phosphate:L-ornithine carbamoyltransferase, EC 2.1.3.3), a major mitochondrial matrix enzyme in ureotelic animals, is synthesized on cytoplasmic ribosomes and translocated across both mitochondrial membranes to the matrix. In an attempt to identify the primary translation product (or an early intermediate) that is the substrate for this transport process, we translated rat liver polysomal RNA in vitro by using the rabbit reticulocyte lysate system. Immunoprecipitation of the [35S]methionine-labeled translation mixture was performed by using monospecific OTCase antiserum and the immunoadsorbent Staphylococcus aureus. Approximately 0.3% of total trichloroacetic acid-insoluble 35S-labeled material was specifically precipitated. Analysis of the precipitate by fluorography of a dried sodium dodecyl sulfate/polyacrylamide gel showed a single major translation product whose mobility corresponded to a polypeptide of 43,000 daltons, a value approximately 4000 daltons greater than that noted for the "mature" OTCase subunit isolated from rat liver. This translation product was not precipitated by preimmune rabbit serum, and excess unlabeled mature OTCase competed with it for interaction with OTCase antiserum. These results suggested that rat liver OTCase, like a number of other cytoplasmically synthesized organellar proteins, is initially made as a larger precursor that contains an amino acid sequence necessary to confer on OTCase its transport properties. The potential application of these findings to the study of inherited complete OTCase deficiency in humans is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassford P., Beckwith J. Escherichia coli mutants accumulating the precursor of a secreted protein in the cytoplasm. Nature. 1979 Feb 15;277(5697):538–541. doi: 10.1038/277538a0. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Campbell A. G., Rosenberg L. E., Snodgrass P. J., Nuzum C. T. Ornithine transcarbamylase deficiency: a cause of lethal neonatal hyperammonemia in males. N Engl J Med. 1973 Jan 4;288(1):1–6. doi: 10.1056/NEJM197301042880101. [DOI] [PubMed] [Google Scholar]
- Chua N. H., Schmidt G. W. Post-translational transport into intact chloroplasts of a precursor to the small subunit of ribulose-1,5-bisphosphate carboxylase. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6110–6114. doi: 10.1073/pnas.75.12.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S. The polypeptides of rat liver mitochondria: identification of a 36,000 dalton polypeptide as the subunit of ornithine transcarbamylase. Biochem Biophys Res Commun. 1976 Aug 23;71(4):1118–1124. doi: 10.1016/0006-291x(76)90769-5. [DOI] [PubMed] [Google Scholar]
- Cremer K. J., Bodemer M., Summers W. P., Summers W. C., Gesteland R. F. In vitro suppression of UAG and UGA mutants in the thymidine kinase gene of herpes simplex virus. Proc Natl Acad Sci U S A. 1979 Jan;76(1):430–434. doi: 10.1073/pnas.76.1.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer K., Bodemer M., Summers W. C. Characterization of the mRNA for herpes simplex virus thymidine kinase by cell-free synthesis of active enzyme. Nucleic Acids Res. 1978 Jul;5(7):2333–2344. doi: 10.1093/nar/5.7.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté C., Solioz M., Schatz G. Biogenesis of the cytochrome bc1 complex of yeast mitochondria. A precursor form of the cytoplasmically made subunit V. J Biol Chem. 1979 Mar 10;254(5):1437–1439. [PubMed] [Google Scholar]
- Dobberstein B., Blobel G., Chua N. H. In vitro synthesis and processing of a putative precursor for the small subunit of ribulose-1,5-bisphosphate carboxylase of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1082–1085. doi: 10.1073/pnas.74.3.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble J. G., Lehninger A. L. Transport of ornithine and citrulline across the mitochondrial membrane. J Biol Chem. 1973 Jan 25;248(2):610–618. [PubMed] [Google Scholar]
- Kalousek F., François B., Rosenberg L. E. Isolation and characterization of ornithine transcarbamylase from normal human liver. J Biol Chem. 1978 Jun 10;253(11):3939–3944. [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Livingston D. M. Immunoaffinity chromatography of proteins. Methods Enzymol. 1974;34:723–731. doi: 10.1016/s0076-6879(74)34094-3. [DOI] [PubMed] [Google Scholar]
- Maccecchini M. L., Rudin Y., Blobel G., Schatz G. Import of proteins into mitochondria: precursor forms of the extramitochondrially made F1-ATPase subunits in yeast. Proc Natl Acad Sci U S A. 1979 Jan;76(1):343–347. doi: 10.1073/pnas.76.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall M., Cohen P. P. Ornithine transcarbamylase from Streptococcus faecalis and bovine liver. I. Isolation and subunit structure. J Biol Chem. 1972 Mar 25;247(6):1641–1653. [PubMed] [Google Scholar]
- Means G. E. Reductive alkylation of amino groups. Methods Enzymol. 1977;47:469–478. doi: 10.1016/0076-6879(77)47047-2. [DOI] [PubMed] [Google Scholar]
- Michel R., Wachter E., Sebald W. Synthesis of a larger precursor for the proteolipid subunit of the mitochondrial ATPase complex of Neurospora crassa in a cell-free wheat germ system. FEBS Lett. 1979 May 15;101(2):373–376. doi: 10.1016/0014-5793(79)81047-9. [DOI] [PubMed] [Google Scholar]
- Palacios R., Palmiter R. D., Schimke R. T. Identification and isolation of ovalbumin-synthesizing polysomes. I. Specific binding of 125 I-anti-ovalbumin to polysomes. J Biol Chem. 1972 Apr 25;247(8):2316–2321. [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Ricciuti F. C., Gelehrter T. D., Rosenberg L. E. X-chromosome inactivation in human liver: confirmation of X-linkage of ornithine transcarbamylase. Am J Hum Genet. 1976 Jul;28(4):332–338. [PMC free article] [PubMed] [Google Scholar]
- Shore G. C., Carignan P., Raymond Y. In vitro synthesis of a putative precursor to the mitochondrial enzyme, carbamyl phosphate synthetase. J Biol Chem. 1979 May 10;254(9):3141–3144. [PubMed] [Google Scholar]
- Short E. M., Conn H. O., Snodgrass P. J., Campbell A. G., Rosenberg L. E. Evidence for x-linked dominant inheritance of ornithine transcarbamylase deficiency. N Engl J Med. 1973 Jan 4;288(1):7–12. doi: 10.1056/NEJM197301042880102. [DOI] [PubMed] [Google Scholar]
- Strauss A. W., Donohue A. M., Bennett C. D., Rodkey J. A., Alberts A. W. Rat liver preproalbumin: in vitro synthesis and partial amino acid sequence. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1358–1362. doi: 10.1073/pnas.74.4.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M., Schimke R. T. Synthesis of rat liver albumin in a rabbit reticulocyte cell-free protein-synthesizing system. J Biol Chem. 1973 Nov 25;248(22):7661–7668. [PubMed] [Google Scholar]
- Walk R. A., Hock B. Cell-free synthesis of glyoxysomal malate dehydrogenase. Biochem Biophys Res Commun. 1978 Mar 30;81(2):636–643. doi: 10.1016/0006-291x(78)91583-8. [DOI] [PubMed] [Google Scholar]