Abstract
Copper and zinc are essential metal ions, but toxic in excess. Bacteria have evolved different strategies to control their intracellular concentrations, ensuring proper supply while avoiding toxicity, including the induction of metal-specific as well as non-specific mechanisms. We compared the transcriptional profiles of Salmonella Typhimurium after exposure to either copper or zinc ions in both rich and minimal media. Besides metal-specific regulatory networks many global stress-response pathways react to an excess of either of these metal ions. Copper excess affects both zinc and iron homeostasis by inducing transcription of these metal-specific regulons. In addition to the control of zinc-specific regulons, zinc excess affects the Cpx regulon and the σE envelope-stress responses. Finally, novel metal-specific upregulated genes were detected including a new copper-detoxification pathway that involves the siderophore enterobactin and the outer-membrane protein TolC. This work sheds light onto the transcriptional landscape of Salmonella after copper or zinc overload, and discloses a new mechanism of copper detoxification.
Introduction
Copper (Cu) and zinc (Zn) ions are essential cellular components required for a broad range of enzymes involved in different metabolic pathways (Braymer & Giedroc, 2014). However, even at moderate concentrations, they out-compete other essential metals for their binding sites on enzymes, causing instability, inadequate conformation and malfunction. Cu, which can cycle between two oxidation states, Cu(I) and Cu(II), can displace iron (Fe) from accessible Fe–S clusters in dehydratases and other iron–sulfur proteins (Macomber & Imlay, 2009). Also, as a redox-active metal, it can catalyse the formation of unspecific disulfide bonds and promote the formation of highly reactive oxygen species that lead to oxidative damage of lipids, DNA and proteins (Dupont et al., 2011). To prevent this damage, different signal-response networks exist that rapidly detect a metal overload and induce specific mechanisms for handling, storing and/or trafficking of these ions (Hood & Skaar, 2012; Porcheron et al., 2013).
Salmonella enterica, our working model and one of the major causes of foodborne diseases throughout the world, modulates its gene expression to survive and replicate within host tissues (Bäumler et al., 2011). Essential metal ions like Mg(II), Fe(II) and Mn(II) are responsible for the modulation of signalling mechanisms that affect the expression of virulence factors (Groisman et al., 2013; Hood & Skaar, 2012; Osman & Cavet, 2011). Recent observations demonstrate that nutritional limitation of Zn, one of the most common non-redox transition metals found in enzymes, is a strategy used by eukaryotic cells to limit intracellular proliferation of bacterial pathogens (Liu et al., 2012; Wang et al., 2012). In Salmonella, the high-affinity Zn(II) transporters ZnuABC and ZupT counteract this limitation and help the bacterium to prosper within the host’s tissues. Intracellular bacteria induce expression of ZnuABC, and znuA mutants are impaired in their ability to grow in Caco-2 cells and in phagocytes (Ammendola et al., 2007). This suggests that limitation of Zn availability within the Salmonella-containing vacuole is an active defence process of the host to reduce the multiplication of the pathogen. The znuABC operon is part of the Zur regulon, which has been extensively characterized in Bacillus subtilis and Escherichia coli (Moore & Helmann, 2005; Petrarca et al., 2010). In Salmonella, only two loci have been shown to be under Zur control, znuABC and zinT. The latter encodes an auxiliary periplasmic component of the ZnuABC transporter required during severe Zn shortage (Petrarca et al., 2010). Salmonella also encodes a cytoplasmic Zn(II) exporter, zntA, predicted to be regulated by a MerR-like sensor, ZntR, and to be expressed during Zn overload, as shown in E. coli (Brocklehurst et al., 1999).
Contrary to the limitation of Zn, Mg, Fe and Mn ions, which the host uses to curb infection, there is increasing evidence that the host uses Cu overload rather than limitation to reduce bacterial infection (Achard et al., 2012; Hodgkinson & Petris, 2012; Porcheron et al., 2013). It has been reported that the genes involved in Cu-resistance are necessary for Mycobacterium tuberculosis virulence (Wolschendorf et al., 2011). In Salmonella, a deletion mutant of the periplasmic multicopper oxidase CueO, one of the main Cu-resistance determinants (Espariz et al., 2007), was significantly attenuated for virulence in mice (Achard et al., 2010). Cu-resistance in Salmonella is primarily based on the cue regulon, which includes the cytoplasmic MerR-like sensor CueR. Upon detection of toxic Cu(I) ions, CueR induces the expression of CopA, a cytoplasmic-membrane P-type ATPase; and two periplasmic proteins, CueO (also known as CuiD) and CueP (Pontel & Soncini, 2009). Salmonella also harbours another cue-like regulon, gol, which is involved in resistance to gold ions (Checa et al., 2007). Part of the gol regulon, including the genes encoding the P-type ATPase GolT and the metal-binding protein GolB, is moderately induced by Cu(I) ions, but their influence in Cu-resistance is only observed when cue components are not present (Espariz et al., 2007). Thus, the role of the gol regulon in Cu-resistance appears supplementary.
Two independent genome-wide analyses performed in E. coli (Kershaw et al., 2005; Yamamoto & Ishihama, 2005) revealed that Cu activates not only the expression of specific resistance mechanisms such as the cue regulon and the CusCFBA Cu efflux system (absent in Salmonella), but also genes involved in the general and periplasmic stress response, probably as a secondary defence mechanism against Cu-catalysed cellular injury. This secondary response includes SoxR, a redox sensor that uses oxidation of Fe–S clusters to monitor the redox status of the cell (Kobayashi et al., 2011), and the CpxR–CpxA two-component system, a sensory system which detects misfolded proteins that cause envelope perturbation (Vogt & Raivio, 2012). Here, we analysed the global response of Salmonella to sudden changes in the concentration of Cu. To distinguish metal-specific resistance mechanisms from the response to the cellular damage, we compared the genome-wide transcriptional profile of the bacteria after CuSO4 or ZnSO4 addition. The study was performed in both high and low nutrient environments, because culture conditions can influence metal toxicity, affecting the magnitude of the response. This analysis shows that besides local metal-specific regulatory networks, many global stress-response pathways react to an excess of these metal ions. Furthermore, we show that the siderophore enterobactin and the outer-membrane channel TolC are involved in Cu-resistance, and that the Zur regulon also changes transcription in response to Cu. Finally, genes not previously known to be regulated by Cu ions are described.
Methods
Bacterial strains and growth conditions.
Salmonella enterica serovar Typhimurium (S. Typhimurium) and its derivative strains used in this study are listed in Table 1. Cells were grown at 37 °C in Luria–Bertani (LB), SLB (LB without NaCl) or M9 broth, or on LB-agar plates. Ampicillin was used at 100 µg ml−1, kanamycin at 25 µg ml−1 and chloramphenicol at 10 µg ml−1. When necessary, CuSO4, ZnSO4 or FeSO4 was added to the cultures or plates at different concentrations. General reagents and chemicals were from Sigma and the culture media were from Difco. Except when indicated, molecular biology reagents were from Invitrogen. The oligonucleotides used are listed in Table S1, available in the online Supplementary Material.
Table 1. Bacterial strains used in this study.
| Strain | Relevant genotype | Reference or source |
| 14028s | Wild-type | ATCC |
| PB10146 | ΔtolC | This study |
| PB10184 | ΔtolC cueO : : KnR | This study |
| PB10462 | Δzur | This study |
| PB10464 | entE : : MudJ | This study |
| PB10465 | entE : : MudJ ΔcueO | This study |
| PB10481 | entE : : MudJ ΔtolC | This study |
| PB6127 | ΔcueP | Pontel & Soncini (2009) |
| PB7937 | ΔcueO | Pontel & Soncini (2009) |
Sample preparation for tiling array.
For the microarray experiments, an overnight culture of Salmonella Typhimurium 14028s was used to inoculate 100 ml flasks of SLB or M9. The cultures were grown to an OD630 of 0.55 to 0.65 and treated with either CuSO4 or ZnSO4 for 10 min. A control non-treated culture was processed in parallel. CuSO4 was added at a final concentration of 1000 µM or 10 µM in SLB and M9, respectively, while ZnSO4 was used at 250 µM or 50 µM in SLB or M9, respectively. Metal treatment was stopped by the addition of 20 ml of 5 % acidic phenol in ethanol and incubation of the cultures on ice for 20 min. Total RNA was extracted using the SV Total RNA Isolation kit (Promega) according to the manufacturer’s indications and its quality was assured by both agarose gel electrophoresis and spectrophotometric measurement. For an additional quality control of the RNA samples used in the arrays, the presence of mRNA coding for copA and cueO (Cu-responsive genes) and zntA (Zn-responsive gene) was assessed by real-time PCR. RNA samples were stored at −80 °C.
Tiling microarray design and procedures.
The custom 385k microarray was designed using 46 to 50mer oligonucleotides, based on the S. Typhimurium LT2 (NC_003197.1) and 14028s (CP001363.1: complete genome and CP001362.1: plasmid) genomes, with a moving window of about 12 bases. Control and duplicate oligonucleotides were included.
Microarray hybridizations were done essentially as described (Evans et al., 2011; Fink et al., 2007). Briefly, 10 µg total RNA from cells untreated or treated with Cu or Zn were labelled using Super Script II reverse transcriptase (Invitrogen) in the presence of a random hexamer, dNTPs and fluorescent dye dCTP Cy3 or Cy5 (Amersham). The labelled cDNA was purified using the PCR purification kit (Qiagen) and the concentration was determined using the Nanodrop ND1000 (Thermo). Equivalent amounts of cDNA from each condition were hybridized onto the tiling array at 42 °C overnight according to the instructions of the Nimblegen hybridization kit (Roche). Arrays were scanned using a GenePix 4000B laser scanner (Molecular Devices) at 5 µm resolution. The signal intensities were quantified using NimbleScan software v2.4 (NimbleGen Systems) and the data were normalized and analysed using LIMMA (Linear Models for Microarray Data) statistics at WebarrayDB (www.webarraydb.org) (Xia et al., 2009). WebarrayDB was set to process the data using the function ‘Half’ for background correction. For each probe, log2(Ia/Ib) was calculated, where Ia is the mean intensity of the probe obtained for the sample subjected to metal treatment and Ib is the mean intensity of the probe obtained for the control sample (without metal added). Then data were compressed to single ORF resolution using the median of M-values (log2 ratio) for the group of probes representing the transcribed strand that covers one ORF. Finally, quantile normalization was used to ensure that the intensities had the same empirical distribution across arrays and across channels. The data comparison between the different growth conditions was done using Excel and Venn diagrams (http://bioinfogp.cnb.csic.es/tools/venny/index.html), with the setting described in each figure legend.
We chose to use a curated overannotation of S. Typhimurium 14028s for data summary, which was composed of the official NCBI annotation, an automatic RAST annotation, an annotation produced by the J. Craig Venter Institute, and sRNA and pseudogene candidates compiled from the literature and obtained from J. Vogel, Institute for Molecular Infection Biology, University of Würzburg, Würzburg, Germany.
All microarray results are based on two independent biological replicates. In every one of these experiments a technical replicate was done. The whole dataset of information was deposited at GEO (http://www.ncbi.nlm.nih.gov/geo/) under accession number GSE35328.
Bacterial molecular biology techniques.
Salmonella strains carrying deletions on the chromosome were constructed using Lambda Red-mediated recombination and then moved into the wild-type 14028s background by P22 transduction, basically as previously described (Checa et al., 2007). When necessary, the antibiotic-resistance cassette inserted at the deletion point was removed using the temperature-sensitive plasmid pCP20 carrying the FLP recombinase (Cherepanov & Wackernagel, 1995).
PCR protocols.
Quantitative real-time reverse transcription-PCR (qRT-PCR) or semiquantitative RT-PCR assays were used to validate the microarray data and to confirm the pattern of expression of selected genes. The qRT-PCRs were carried out on the same cDNA samples employed in the arrays using specific primer pairs (Table S1) and the 2× PCR mastermix from Biopioneer according to manufacturer’s specifications. Amplifications were performed in an ABI 7900 Fast HT (Applied Biosystems), and the SDS 2.3 software was used to process the data. Final calculations were done in Excel. To ensure accurate quantification of the mRNA levels, three amplifications for each gene were made with 1, 1/10 and 1/100 dilutions of the total RNA. Measured mRNA levels were normalized to the mRNA levels of the housekeeping glyceraldehyde 3-phosphate dehydrogenase (GAPDH) transcript. Normalized values were used to calculate the ratios of the expression levels in the different conditions tested. Semiquantitative RT-PCR was performed using total RNA extracted from mid exponential phase cultures (OD630 0.4–0.7) of the wild-type or the Δzur mutant strain treated with 1 mM CuSO4 or 0.25 mM ZnSO4 for 40 min, or without treatment. As before, a solution of 5 % acidic phenol in ethanol and incubation for 20 min on ice was used to arrest transcription and stop the metal treatment. The RNA samples were prepared using the RNAzol RT reagent (Molecular Research Center), followed by treatment with RQ1 DNase (Promega) to improve sample quality. The quality and quantity of the RNA samples were verified by agarose gel analysis and spectrophotometric measurement, respectively. Corresponding cDNA was generated using Super Script II reverse transcriptase (Invitrogen) in the presence of dNTPs and oligo-dT nucleotides. Approximately 5 ng cDNA template and specific sets of primers (Table S1) were employed to amplify zinT, znuA or rpmE2 genes using Taq DNA Polymerase (Invitrogen) in a Veriti Thermal Cycler (Applied Biosystems). As before, the housekeeping GAPDH fragment was used as internal standard for normalization. Reaction conditions included an initial 5 min denaturation at 94 °C, followed by 25 cycles of 30 s at 94 °C, 30 s at 59 °C, 30 s at 72 °C and a final 5 min incubation at 72 °C. The intensities of the RT-PCR products on 1 % agarose gels were quantified with the Molecular Imager ChemiDoc XRS+ System (Bio-Rad) and the volume tool of the Quantity One software (Bio-Rad). A background value was subtracted by using a mean background reading from an empty lane at the position of the PCR product band. Normalized values were used to calculate relative expression as the ratio between the RT-PCR product corresponding to the gene of interest and the housekeeping GAPDH fragment in each condition.
β-Galactosidase activity and inhibition assays.
The levels of expression of the lacZ reporter gene were measured essentially as described (Pérez Audero et al., 2010) using total cell extracts from the entE : : MudJ strain grown overnight on LB or M9 medium in the presence of CuSO4, FeSO4 or both, at the indicated final concentrations, or without metal added.
Cu-inhibition assays were performed using overnight cultures of the wild-type or the indicated mutant strains diluted 1 : 100 into fresh LB medium containing increasing concentrations of CuSO4. The experiments were done in a final volume of 1 ml. After 15 h of incubation at 37 °C with shaking, the OD630 was recorded. Each experiment was performed in triplicate.
Cu-sensitivity assays in the absence of oxygen were performed essentially as described previously (Pontel & Soncini, 2009). The plates were incubated at 37 °C for 72 h under anaerobic environments generated using a Gaspak jar system and AnaeroGen sachets (Ooid). Oxygen consumption was verified using anaerobic indicators (Ooid). The MIC values were determined as the minimal concentration of CuSO4 in which no colonies appeared.
Results and Discussion
A genome-wide landscape of the Salmonella response to Cu and Zn ions
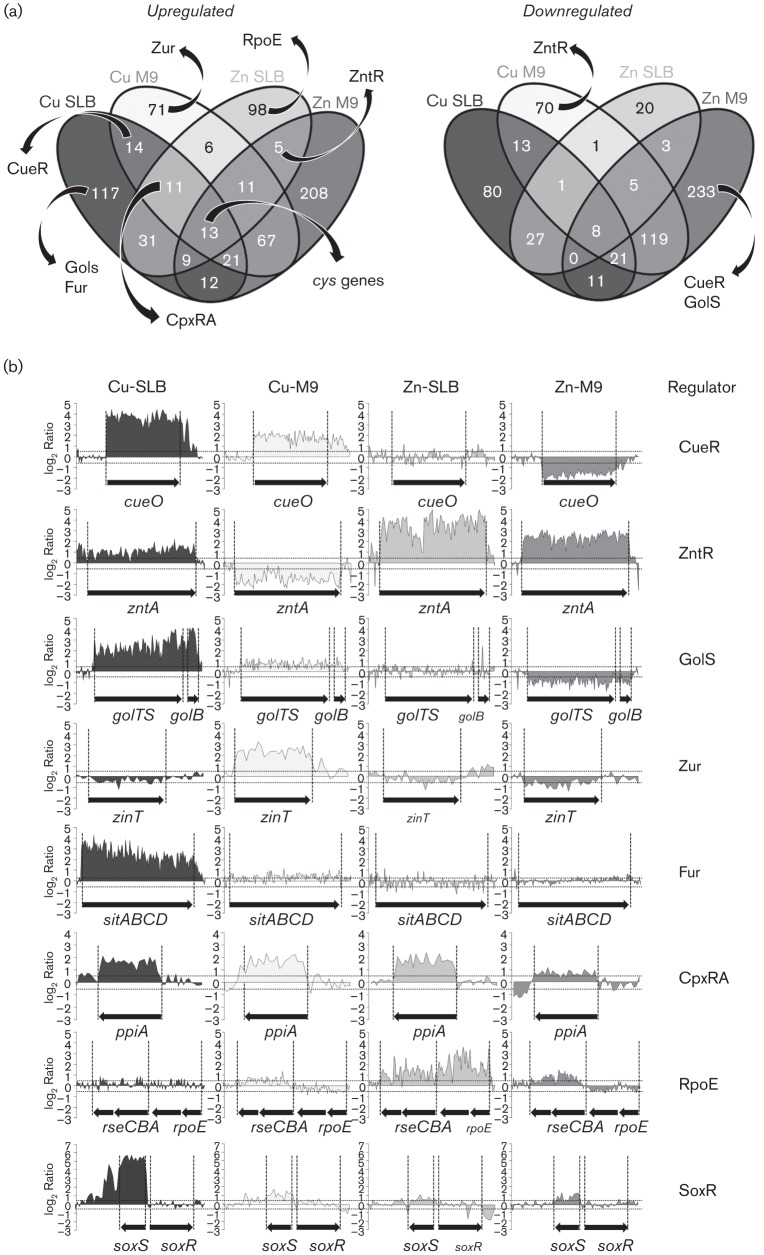
We used a NimbleGen tiling array (Morales et al., 2013) to analyse and compare the genome-wide response of Salmonella after a 10 min exposure to sublethal concentrations of CuSO4 or ZnSO4 when cells were grown either in rich (SLB) or in defined nutrient (M9) culture medium. This approach generates intensity values for 46- to 50-mer probes that cover both strands of the S. Typhimurium 14028s chromosome and plasmid (See Methods). To simplify the data mining, we restricted the analysis to annotated ORFs and putative ORFs that were manually annotated. Using the WebarrayDB tool, we calculated the M-values and the corresponding P-values for each of the 6106 analysed ORFs in each condition tested. These values are shown in Table S2.
We established a threshold of M-values >0.5 or <−0.5 (corresponding to a change in transcription of more than 1.41-fold in either direction) to define activation or repression of transcription by the addition of Cu(II) or Zn(II). From the 6106 ORFs analysed, 117 ORFs were induced and 80 were repressed in SLB in the presence of Cu (SLB vs SLB+Cu), while in M9 medium, a total of 71 ORFs were activated and 70 were repressed (M9 vs M9+Cu). In addition, 98 ORFs were induced when cells were grown in SLB in the presence of Zn (SLB vs SLB+Zn) and 208 when the cells were grown in minimal medium (M9 vs M9+Zn). The number of Zn-repressed genes dropped to 20 in SLB and increased to 233 in M9. The changes in the transcription pattern observed in each condition were mainly attributed to the addition of the metal and/or the intrinsic characteristics of the culture medium, because no significant changes were observed in pH and oxygen level in culture media after metal addition (data not shown).
A consortium of global and specific regulatory pathways is induced in response to copper
Venn diagrams were used to identify common as well as specific features in the Salmonella responses to Cu or Zn. This analysis generated 15 clusters of activated and 15 clusters of repressed transcripts, defined by the groups of ORFs that increased or reduced their transcription in response to the different conditions tested (Fig. 1a). The complete list of genes in each cluster is shown in Tables S3 and S4. The different groups contained genes previously described to belong to global or metal-specific regulons, most of them previously linked to the defence against these metal stresses. The transcriptional profiles for representative loci belonging to these regulons are illustrated in Fig. 1(b). For simplicity, we limit our analysis to these regulons.
Fig. 1.
Genome-wide comparison of the copper and zinc response in Salmonella Typhimurium 14028s. (a) Samples were subjected to different metal stress conditions and labelled cDNA was applied to tiling arrays (see Methods). Venn diagrams were used to visualize up- or downregulated genes. Upregulated genes were defined as those with an M-value (log2 differential expression relative to untreated) >0.5 while downregulated genes were those with an M-value <−0.5. Numbers indicate genes that show statistically significant differential expression in each cluster. The complete list of genes in each cluster is detailed in Tables S3 and S4. The presence of genes belonging to previously known regulons or pathways in each cluster is depicted. (b) WebArrayDB analysis using quantile normalization for all sense strand probes for representative genes of stress-responsive regulons highlighted in (a). The names of genes and the regulator(s) that control their transcription are indicated as well as the conditions tested. The mean change in intensity of two biological replicates, log2(Ia/Ib), for each probe within the ORF is plotted against the genome location. Dotted lines indicate the thresholds for up- and downregulation.
The status of genes previously described as part of Cu- or Zn-responsive regulons was inspected. As expected, genes known to be controlled by the Cu sensor CueR, i.e. copA, cueO and cueP, were found within the group of Cu-upregulated genes in both SLB and M9, while the ZntR-controlled zntA gene, encoding the Zn(II) transporter, was found among the induced transcripts in the presence of Zn ions in both media (Fig. 1, Table S3). Interestingly, the Cu-responsive cue genes and the Zn(II) transporter were selectively repressed in the presence of the other metal but only in M9, a condition that might exacerbate the toxic effects of each of these ions (Fig. 1, Table S4). The pattern of induction/repression of the CueR-controlled cueO and copA genes as well as the ZntR-controlled zntA gene in the conditions tested was verified by real-time reverse transcription-PCR (qRT-PCR) (Fig. 2). As previously reported, we also noticed the activation by Cu ions of two of the three loci controlled by the Au sensor GolS (i.e. golB and golTS, but not gesABC), but unlike the cue regulon, induction by CuSO4 was observed only in SLB-growing cells, and not in M9 (Fig. 1, Table S3).
Fig. 2.
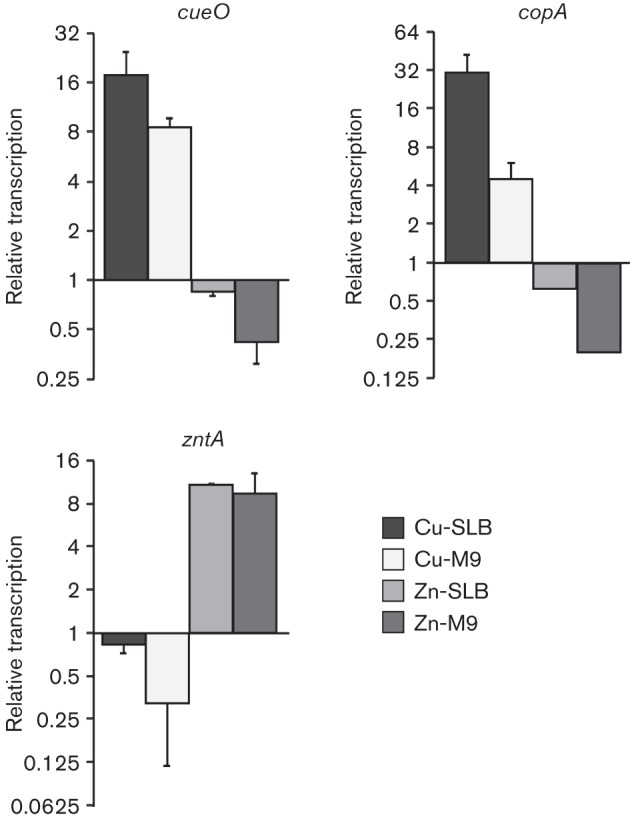
Transcriptional response of known Cu- or Zn-resistance determinants after metal exposure. Real-time RT-PCR was used to determine the levels of transcription of the CueR-controlled genes cueO and copA and the ZntR-controlled zntA obtained after 10 min incubation in the presence of 1000 µM CuSO4 or 250 µM ZnSO4 in SLB (Cu-SLB or Zn-SLB, respectively) or 10 µM CuSO4 or 50 µM ZnSO4 in M9 medium (Cu-M9 or Zn-M9, respectively). Transcription levels were first normalized to the housekeeping gene GAPDH and then relative to the levels obtained in the absence of metal. The RNA samples were the same ones as employed for tiling arrays shown in Fig. 1. Data correspond to mean values of two independent experiments performed in triplicate. Error bars depict sd.
The transcriptome analysis revealed the activation of at least three loci controlled by the Zn-responsive Zur repressor in the presence of CuSO4, znuA, zinT and rpmE2-rpmJ_1 (Fig. 1, Tables S3 and S4). Interestingly, this activation occurred only in cells grown in M9. The first two genes, znuA and zinT, were reported previously to be repressed by Zn in Salmonella (Petrarca et al., 2010), but Cu-directed activation was not noticed. In contrast, the Zn-dependent modulation of expression of the ribosomal proteins coding genes rpmE2-rpmJ_1 in Salmonella has been predicted by in silico analysis as part of the Zur regulon (Panina et al., 2003), but effective regulation had not yet been demonstrated. Homologues to rpmE2 and rpmJ_1 were reported to be under control of Zur in Bacillus subtilis and Streptomyces coelicolor (Gabriel & Helmann, 2009; Shin et al., 2007). Using semiquantitative RT-PCR we verified not only the repression by Zn and the Zur-dependent expression of znuA, zinT and rpmE2-rpmJ_1, but also their induction by Cu (Fig. 3). It is possible that competition between these two metal ions is the cause of the expression of the Zur-regulated genes by means of the excess of Cu in M9 medium leading to an increase in the availability of Zn ions.
Fig. 3.
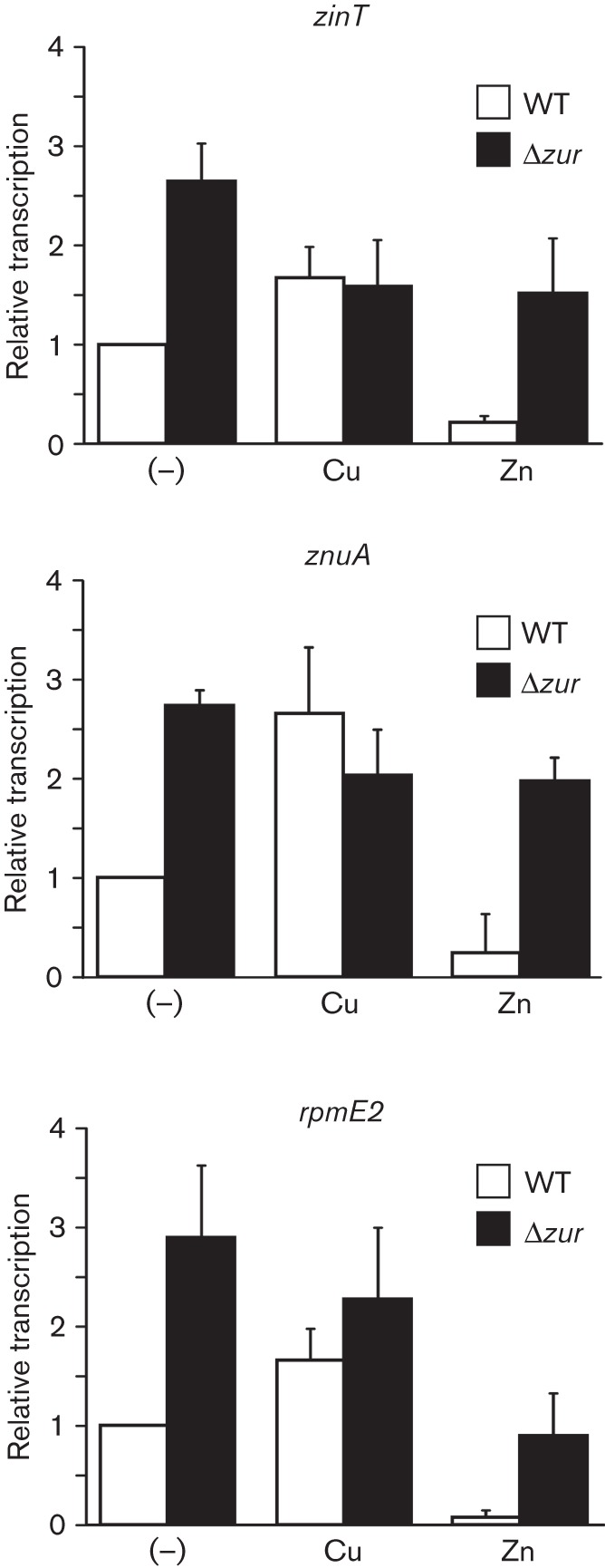
The Zur-regulated genes zinT, znuA and rpmE2 are activated by Cu ions. The levels of transcription of each gene were determined by RT-PCR using samples obtained from the wild-type or the Δzur mutant strain grown in M9 medium after 40 min exposure to 10 µM CuSO4 (Cu) or 50 µM ZnSO4 (Zn), or without metal (–). After electrophoretic analysis of the amplification products the bands were quantified by densitometry (representative agarose gels are shown in Fig. S1). In each condition, the values were normalized against those of the housekeeping gene GAPDH. The graphs show the levels of transcription of zinT, znuA or rpmE2 obtained in each strain and condition, relative to the levels obtained in the wild-type strain with no metal added. Data correspond to mean values of three independent experiments performed in triplicate. Error bars depict sd.
Cu-overloading not only disturbs Zn-homeostasis but also affects the intracellular balance of other transition metals. One of the toxic effects of Cu is the destabilization of iron–sulfur clusters of proteins (Macomber & Imlay, 2009), altering Fe-homeostasis. Thus, in the microarrays, we particularly focused on genes transcriptionally controlled by the Fe-binding Fur repressor such as the sitABCD and the entCEBA operons (Tsolis et al., 1995). As predicted, these were activated by Cu in SLB (Fig. 1, Table S3). The activation of Fur-regulated genes in response to Cu stress and the resulting increased Fe-acquisition would prevent improper metal supplementation of proteins due to competition between Cu and Fe ions for binding sites. As we show below, at least some of these genes can also play a direct role in Cu tolerance.
In E. coli, a set of genes under the control of the periplasmic stress responsive CpxA–CpxR two-component system was reported to be induced in the presence of Cu ions (Yamamoto & Ishihama, 2005). Activation of this regulatory network also occurs in the presence of Zn ions (Lee et al., 2005), although the mechanism for CpxA activation appears to be different in the presence of Cu as compared with Zn (Vogt & Raivio, 2012). Our microarray data revealed the activation of at least five CpxR-controlled genes (including ppiA) when Salmonella was grown in the presence of CuSO4 in both SLB and M9, as well as in the presence of ZnSO4 in SLB (Fig. 1, Table S3). These genes were among the 11 ORFs reported to be upregulated in E. coli as a consequence of Cu-overloading. Noticeably, genes involved in the defence against envelope stress but controlled by the alternative σE factor, such as rseA, rseB and rseC, were induced in response to Zn ions but not to Cu ions in SLB (Fig. 1, Table S3). These results support the notion that, although both Cu and Zn disturb periplasmic homeostasis, the molecular targets may differ between these stressors.
As a redox-active metal, long-term exposure of Salmonella to Cu enhances transcription of genes belonging to the SoxR/SoxS regulon involved in the response to oxidative stress, as previously noted for E. coli (Kershaw et al., 2005; Kimura & Nishioka, 1997). In Enterobacteria, the only known target of the redox sensor SoxR is soxS, encoding a transcription factor that activates genes required to counteract oxidative stress (Kobayashi et al., 2014). In our experiments, we observed a marked upregulation of soxS in the presence of CuSO4 in SLB, but no induction of any known SoxS-controlled genes (Fig. 1b, Table S3). It is possible that our chosen time point (10 min post metal addition) was too soon to observe the induction of these genes. Alternatively, a second, Cu-independent, oxidative-stress signal may be required to enhance the activation of SoxS-regulated genes under excess of Cu ions.
A total of 13 ORFs were induced in all four assayed conditions; presence of either Cu or Zn ions in cells grown either in SLB or in SM9. Among these activated transcripts, four encode proteins involved in cysteine biosynthesis (cysD, cysJ, cysK and cysT) (Fig. 1a, Table S3). In E. coli, these xenologous genes were also reported to be induced by Zn, but not by Cu (Yamamoto et al., 2011). Note, however, that Yamamoto et al. used a lower concentration of Cu(II) (5 µM) compared with our experimental model, and CuCl2 (as opposed to our CuSO4). The other genes also induced at least 1.4-fold in all tested conditions were the RNA-binding protein coding gene yhbY, two genes (flmB and pmrF) involved in cell-wall synthesis, spr, which codes for a lipoprotein involved in thermo-resistance, and three genes with unknown functions (Table S3). The products of these genes are probably required for the defence against non-specific metal injury or play a central role in global stress response. Eight ORFs were repressed in all assayed conditions, including: cydA and cydB, encoding the cytochrome d terminal oxidase; napF, encoding a ferredoxin-type protein involved in electron transfer to the periplasmic nitrate reductase NapA; manX and manY, mannose-specific PTS transporters; and two other genes encoding proteins with unknown functions (Table S4).
The Fe-siderophore enterobactin and TolC contribute to copper resistance
The link between Fe- and Cu-homeostasis in bacteria has remained elusive, and only a few lines of evidence connect the mechanisms that handle these two transition metals. Previous reports indicate that in E. coli the multi-copper oxidase CueO, which is expressed in the presence of Cu, is not only able to oxidize Cu(I) to Cu(II) in the periplasm, but also catecholate-containing ligands such as enterobactin (Grass et al., 2004). Because this siderophore is able to reduce Cu(II) (Kamau & Jordan, 2002), it was proposed that enterobactin oxidation by CueO plays an additional role in Cu resistance by avoiding the generation of the more toxic Cu(I). In addition, the oxidized form of 2,3-dihydroxybenzoic acid (DHB), an intermediate in enterobactin biosynthesis, was shown to stably bind Cu ions, acting as a Cu sink (Grass et al., 2004). Recently, the interplay between two siderophores and Cu was described in the uropathogenic E. coli (UPEC). In this species, yersiniabactin but not enterobactin protects bacteria from intracellular killing by sequestering host-derived Cu(II) outside the bacterial cell and preventing its catechol-mediated reduction to Cu(I) (Chaturvedi et al., 2012). Moreover, the Cu(II)–yersiniabactin complex was found to have superoxide dismutase activity protecting bacteria from oxidative stress inside phagocytic vesicles (Chaturvedi et al., 2014).
The expression analysis performed here revealed the induction of the Fur-controlled entCEBA locus, encoding enzymes involved in the biosynthesis of enterobactin, after a 10 min exposure to CuSO4 in SLB (Fig. 4a). To confirm this induction as well as the repression by Fe(II) – an expected behaviour for a Fur-regulated locus (Tsolis et al., 1995) – we employed a strain carrying an entE : : MudJ transcriptional fusion (Table S1). As shown in Fig. 4(b), entE : : MudJ expression increased more than fourfold in the presence of 0.25 mM CuSO4, while repression was observed in the presence of FeSO4. In M9, where the basal expression of the reporter gene was high due to the deprivation of Fe, no induction by Cu was detected (Fig. 4b). However, the simultaneous addition of FeSO4 and CuSO4 could not completely abrogate transcriptions of the entC : : MudJ fusion and some Cu-mediated activation was still detected (Fig. 4b).
Fig. 4.
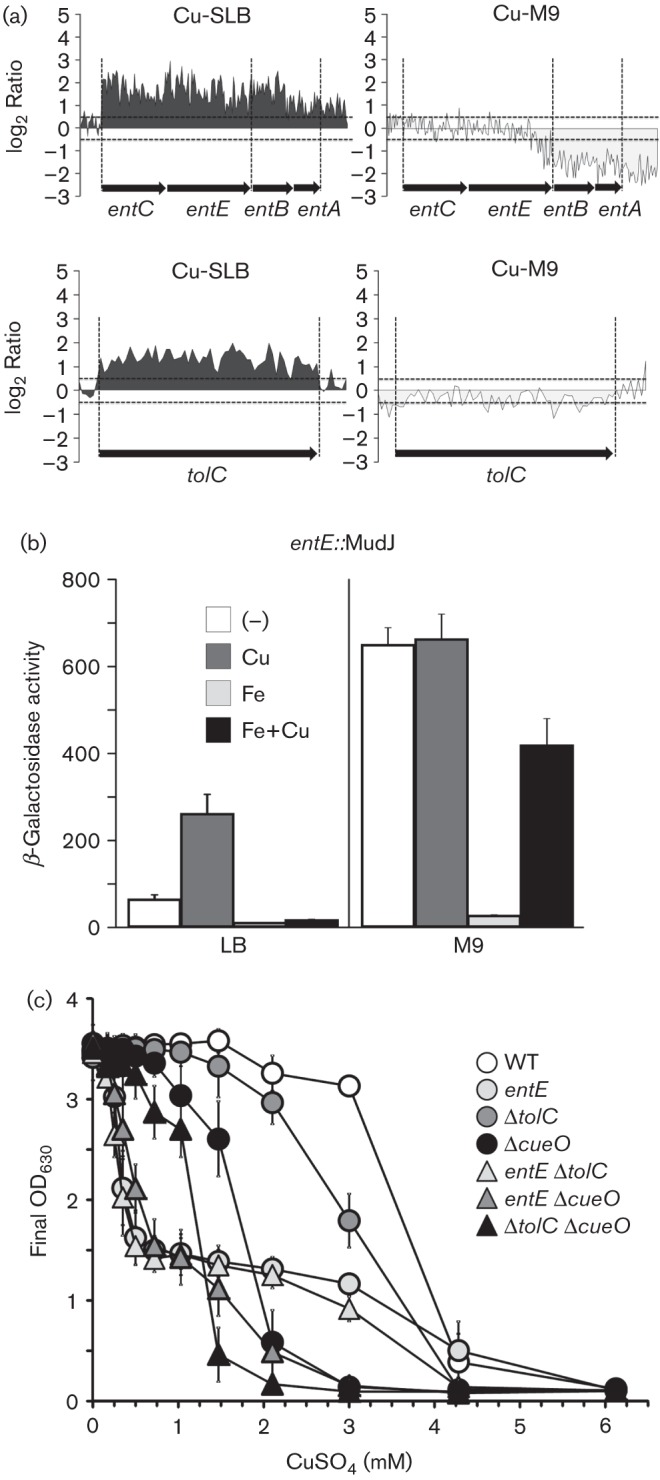
The genes involved in enterobactin biosynthesis and tolC are required for Cu-resistance. (a) WebArrayDB analysis for ent and tolC loci done in SLB or M9 medium in the presence of Cu ions as described in Fig. 1. (b) β-galactosidase activity from a strain carrying an entE : : MudJ transcriptional fusion cultured overnight in LB or M9 without (–) or with the addition of CuSO4 (Cu), FeSO4 (Fe) or both metal salts (Fe+Cu). The metal salts were used at a final concentration of 250 µM in LB or 10 µM in M9. The data correspond to mean values of four independent experiments performed in duplicate. Error bars depict standard deviations. (c) The final OD630 of the cultures of the wild-type strain (WT) or the mutants in the indicated genes was recorded after 15 h of incubation in the presence of the specified concentrations of CuSO4. Data correspond to mean values of three independent experiments performed in triplicate. Error bars depict standard deviations.
We hypothesized that the Cu-induced increased synthesis of enterobactin would be required either to compensate for the metal deprivation of the Fe–S-containing proteins as a consequence of Cu-induced damage (Macomber & Imlay, 2009) or to chelate the toxic metal, lowering its internalization. It was previously reported that enterobactin is exported through the outer-membrane channel tunnel protein TolC, to acquire Fe from the medium (Bleuel et al., 2005; Newton et al., 2010; Vega & Young, 2014). Interestingly, tolC was also induced by CuSO4 in SLB (Fig. 4a). This prompted us to analyse the role of these genes in Cu resistance. The final OD of Salmonella mutants in ent, tolC and/or cueO was determined after overnight growth in SLB in the presence of increasing concentrations of CuSO4 (Fig. 4c). The entE : : MudJ mutant strain showed a biphasic behaviour. It reached a third of the final OD of the wild-type strain at CuSO4 concentrations up to 0.5 mM (Cu levels that did not affect growth of any of the other tested strains), and this value remained unmodified up to 3 mM CuSO4. The ΔcueO strain showed a sigmoidal curve, reaching half of the OD values of the parental strain at 1.5 mM CuSO4 with no growth at 3 mM CuSO4. The ΔtolC mutant reached half of the OD values of the parental strain at 2.1 mM CuSO4, showing also a sigmoidal Cu-sensitivity curve. The OD curve of the ent ΔcueO double mutant correlated with an additive contribution of both ent and cueO to Cu-resistance. Similarly, the effect of CuSO4 on the final OD of the double mutant ΔtolC ΔcueO resembled an additive contribution of both factors, suggesting independent pathways for Cu-resistance. The double ent ΔtolC mutant, however, exhibited the same sensitivity curve as the single ent mutant, suggesting that the role of TolC in Cu-resistance depends on enterobactin. Overall, the ent loci and to a lesser extent tolC probably contribute to Cu-resistance by employing the same detoxification pathway, which does not involve CueO (Fig. 4).
It has been proposed that inactivation of TolC may affect detoxification of some compounds not as a direct consequence of efflux impairment, but as a result of oxidative damage to membranes caused by the toxic species (Zgurskaya et al., 2011). Considering that one of the main toxic effects of Cu is the inactivation of iron–sulfur dehydratases, which may enhance oxidative stress in the periplasm (Macomber & Imlay, 2009), we evaluated the contribution of both the tolC and ent genes to Cu-resistance in the absence of oxygen. We determined the MIC of CuSO4 in LB agar plates for the ent, ΔtolC and ent ΔtolC mutants compared with the wild-type strain and the ΔcueP mutant, which was reported to be sensitive to Cu in this condition (Pontel & Soncini, 2009). In the absence of oxygen, the MIC values were 0.28±0.03 mM CuSO4 for the entE mutant and 0.19±0.03 mM CuSO4 for both the ΔtolC and the ent ΔtolC mutant. The values for the wild-type strain and the ΔcueP mutant were 0.40±0.07 and 0.16±0.04, respectively. Although it was not possible to distinguish individual contributions of ent and tolC at sublethal Cu levels using this approach, the results confirm the role of the Ent proteins and the outer-membrane channel TolC as part of the ancillary Cu-detoxification pathway.
We observed that although the transcriptional induction is extended to the entire ent locus in Cu-SLB, in M9, the third and fourth genes in the locus, entB and entA, were repressed by Cu (Fig. 4a). Both the entA and entB products, 2,3-dihydro-2,3-dihydroxybenzoate dehydrogenase and isochorismate lyase, respectively, participate in the conversion of chorismate to 2,3-dihydroxybenzoate (DHB), the first part of the enterobactin synthesis pathway (Crosa & Walsh, 2002). Also, the C-terminal aryl carrier domain of the bifunctional EntB protein participates in the final steps of enterobactin synthesis, the condensation of DHB with serine and cyclization. The differential expression of these genes in Cu-SM9 suggests the existence of additional regulatory mechanisms whose physiological relevance remains to be investigated.
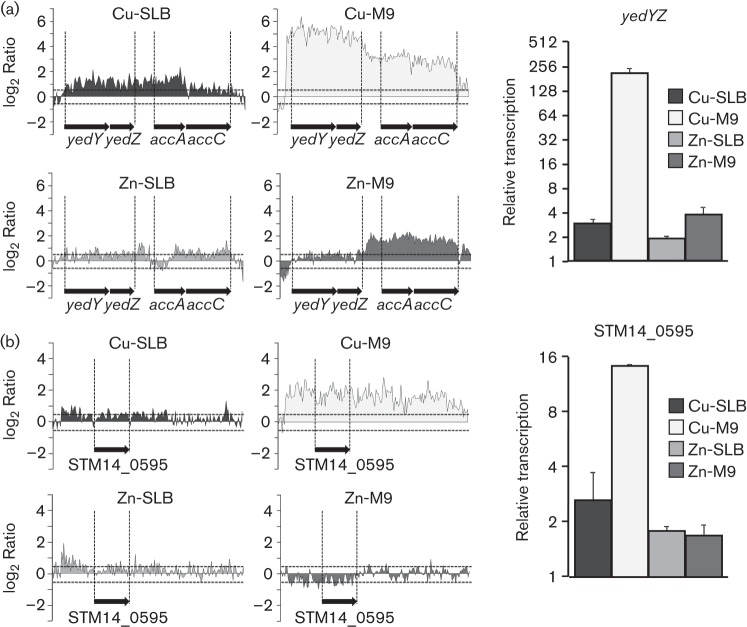
Novel Cu-upregulated genes
Among the upregulated genes in the presence of CuSO4 in M9 (Table S2) are yedYZ and STM14_0595 (Fig. 5). We confirmed the Cu-mediated induction of these genes by real-time PCR. Both genes are conserved in E. coli, although their physiological role and their induction by Cu ions were previously unknown. YedYZ from E. coli was the first molybdo-protein identified in bacteria (Loschi et al., 2004). In the heterodimeric enzyme, the soluble, periplasmic subunit YedY is strongly associated with YedZ, the membrane-bound haem-containing subunit. Although it was characterized in vitro as a sulfite oxidase, it exhibited limited activity towards sulfite as a substrate; thus it was proposed to act as an S- and N-oxide reductase in the periplasm (Brokx et al., 2005). A Campylobacter jejuni mutant deficient in the homologous YedY protein is impaired in chicken colonization and is more sensitive to NO-releasing agents than the wild-type (Hitchcock et al., 2010). Thus, the Cu-mediated activation of the yedYZ genes in Salmonella may help to counteract redox stress and avoid intracellular killing during the infection cycle. STM14_0595, also named ybbA, codes for an uncharacterized ABC transporter. This large family of ATP-binding membrane-associated proteins has been associated with a number of biological processes, including translocation of various substrates across membranes, and also non-transport-related processes such as the biogenesis of outer-membrane proteins, or even translation of RNA and DNA repair (Davidson et al., 2008).
Fig. 5.
Cu upregulates the transcription of yedYZ and a putative ABC transporter. Transcription profile of yedYZ (a) and STM14_0595 (b) obtained after a 10 min exposure to 1000 µM CuSO4 or 250 µM ZnSO4 in SLB (Cu-SLB or Zn-SLB, respectively), or 10 µM CuSO4 or 50 µM ZnSO4 in M9 medium (Cu-M9 or Zn-M9, respectively). Both the tiling arrays and WebArrayDB analysis (left) and real-time RT-PCR quantifications (right) are shown. Transcription levels were first normalized to the expression of GAPDH and then relative to the levels obtained in the absence of metal. The RNA samples were the same as the ones employed for tiling arrays shown in Fig. 1.
In summary, the results obtained here reveal the complexity of the mechanisms used by Salmonella to detoxify Cu ions, mainly at the cell envelope, probably the preferred target for metal injury during pathogen–host interactions. The arsenal of detoxification strategies in the periplasmic space includes members of the CueR-controlled system, CueO and CueP, and equally important factors previously known to be associated with Fe-acquisition, such as the siderophore enterobactin and the outer-membrane channel TolC. The high Cu-sensitivity exhibited by the ent, ent ΔtolC, ent ΔcueO and ΔtolC ΔcueO mutants suggested an active role of the ent–tolC-mediated pathway in Cu removal rather than its merely being directed to restore metal supply of Fe–S-containing proteins damaged by Cu ions. It remains to be established where enterobactin chelates the toxic metal and, in consequence, whether TolC exports free enterobactin or a Cu-loaded siderophore. The enterobactin-mediated Cu-detoxification pathway might be of particular relevance during intracellular survival where Salmonella is expected to face an environment rich in Cu but deprived in Fe (Achard et al., 2012; Bäumler et al., 2011). Finally, the identification of novel Cu-upregulated genes coding for putative detoxification factors adds more pieces to the complicated and as yet unsolved puzzle of Cu-handling in Salmonella.
Acknowledgements
This work was supported by grants from Agencia Nacional de Promoción Científica y Tecnológica and from the National Scientific and Technical Research Council (CONICET) to S. K. C. and F. C. S. L. B. P. was supported by a postdoctoral fellowship from CONICET and also by short-terms awards from ASM and the A.V.E program from U.N.R. N. L. S. is a fellow of CONICET. S. K. C. and F. C. S. are career investigators of CONICET. S. P. and M. M. acknowledge support from NIH grants AI039557, AI052237, AI073971, AI075093, AI077645, AI083646, NIH contract HHSN272200900040C, USDA grants 2009-03579 and 2011-67017-30127, the Binational Agricultural Research and Development Fund, and a grant from the Center for Produce Safety. F. C. S. is also a career investigator of the Rosario National University Research Council (CIUNR).
Footnotes
Four supplementary tables and one supplementary figure are available with the online version of this paper.
References
- Achard M. E. S., Tree J. J., Holden J. A., Simpfendorfer K. R., Wijburg O. L. C., Strugnell R. A., Schembri M. A., Sweet M. J., Jennings M. P., McEwan A. G. (2010). The multi-copper-ion oxidase CueO of Salmonella enterica serovar Typhimurium is required for systemic virulence. Infect Immun 78, 2312–2319. 10.1128/IAI.01208-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achard M. E., Stafford S. L., Bokil N. J., Chartres J., Bernhardt P. V., Schembri M. A., Sweet M. J., McEwan A. G. (2012). Copper redistribution in murine macrophages in response to Salmonella infection. Biochem J 444, 51–57. 10.1042/BJ20112180 [DOI] [PubMed] [Google Scholar]
- Ammendola S., Pasquali P., Pistoia C., Petrucci P., Petrarca P., Rotilio G., Battistoni A. (2007). High-affinity Zn2+ uptake system ZnuABC is required for bacterial zinc homeostasis in intracellular environments and contributes to the virulence of Salmonella enterica. Infect Immun 75, 5867–5876. 10.1128/IAI.00559-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäumler A. J., Winter S. E., Thiennimitr P., Casadesús J. (2011). Intestinal and chronic infections: Salmonella lifestyles in hostile environments. Environ Microbiol Rep 3, 508–517. 10.1111/j.1758-2229.2011.00242.x [DOI] [PubMed] [Google Scholar]
- Bleuel C., Grosse C., Taudte N., Scherer J., Wesenberg D., Krauss G. J., Nies D. H., Grass G. (2005). TolC is involved in enterobactin efflux across the outer membrane of Escherichia coli. J Bacteriol 187, 6701–6707. 10.1128/JB.187.19.6701-6707.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braymer J. J., Giedroc D. P. (2014). Recent developments in copper and zinc homeostasis in bacterial pathogens. Curr Opin Chem Biol 19, 59–66. 10.1016/j.cbpa.2013.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K. R., Hobman J. L., Lawley B., Blank L., Marshall S. J., Brown N. L., Morby A. P. (1999). ZntR is a Zn(II)-responsive MerR-like transcriptional regulator of zntA in Escherichia coli. Mol Microbiol 31, 893–902. 10.1046/j.1365-2958.1999.01229.x [DOI] [PubMed] [Google Scholar]
- Brokx S. J., Rothery R. A., Zhang G., Ng D. P., Weiner J. H. (2005). Characterization of an Escherichia coli sulfite oxidase homologue reveals the role of a conserved active site cysteine in assembly and function. Biochemistry 44, 10339–10348. 10.1021/bi050621a [DOI] [PubMed] [Google Scholar]
- Chaturvedi K. S., Hung C. S., Crowley J. R., Stapleton A. E., Henderson J. P. (2012). The siderophore yersiniabactin binds copper to protect pathogens during infection. Nat Chem Biol 8, 731–736. 10.1038/nchembio.1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi K. S., Hung C. S., Giblin D. E., Urushidani S., Austin A. M., Dinauer M. C., Henderson J. P. (2014). Cupric yersiniabactin is a virulence-associated superoxide dismutase mimic. ACS Chem Biol 9, 551–561. 10.1021/cb400658k [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checa S. K., Espariz M., Pérez Audero M. E., Botta P. E., Spinelli S. V., Soncini F. C. (2007). Bacterial sensing of and resistance to gold salts. Mol Microbiol 63, 1307–1318. 10.1111/j.1365-2958.2007.05590.x [DOI] [PubMed] [Google Scholar]
- Cherepanov P. P., Wackernagel W. (1995). Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 158, 9–14. 10.1016/0378-1119(95)00193-A [DOI] [PubMed] [Google Scholar]
- Crosa J. H., Walsh C. T. (2002). Genetics and assembly line enzymology of siderophore biosynthesis in bacteria. Microbiol Mol Biol Rev 66, 223–249. 10.1128/MMBR.66.2.223-249.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson A. L., Dassa E., Orelle C., Chen J. (2008). Structure, function, and evolution of bacterial ATP-binding cassette systems. Microbiol Mol Biol Rev 72, 317–364. 10.1128/MMBR.00031-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont C. L., Grass G., Rensing C. (2011). Copper toxicity and the origin of bacterial resistance – new insights and applications. Metallomics 3, 1109–1118. 10.1039/c1mt00107h [DOI] [PubMed] [Google Scholar]
- Espariz M., Checa S. K., Pérez Audero M. E., Pontel L. B., Soncini F. C. (2007). Dissecting the Salmonella response to copper. Microbiology 153, 2989–2997. 10.1099/mic.0.2007/006536-0 [DOI] [PubMed] [Google Scholar]
- Evans M. R., Fink R. C., Vazquez-Torres A., Porwollik S., Jones-Carson J., McClelland M., Hassan H. M. (2011). Analysis of the ArcA regulon in anaerobically grown Salmonella enterica sv. Typhimurium. BMC Microbiol 11, 58. 10.1186/1471-2180-11-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink R. C., Evans M. R., Porwollik S., Vazquez-Torres A., Jones-Carson J., Troxell B., Libby S. J., McClelland M., Hassan H. M. (2007). FNR is a global regulator of virulence and anaerobic metabolism in Salmonella enterica serovar Typhimurium (ATCC 14028s). J Bacteriol 189, 2262–2273. 10.1128/JB.00726-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel S. E., Helmann J. D. (2009). Contributions of Zur-controlled ribosomal proteins to growth under zinc starvation conditions. J Bacteriol 191, 6116–6122. 10.1128/JB.00802-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grass G., Thakali K., Klebba P. E., Thieme D., Müller A., Wildner G. F., Rensing C. (2004). Linkage between catecholate siderophores and the multicopper oxidase CueO in Escherichia coli. J Bacteriol 186, 5826–5833. 10.1128/JB.186.17.5826-5833.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groisman E. A., Hollands K., Kriner M. A., Lee E.-J., Park S.-Y., Pontes M. H. (2013). Bacterial Mg2+ homeostasis, transport, and virulence. Annu Rev Genet 47, 625–646. 10.1146/annurev-genet-051313-051025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock A., Hall S. J., Myers J. D., Mulholland F., Jones M. A., Kelly D. J. (2010). Roles of the twin-arginine translocase and associated chaperones in the biogenesis of the electron transport chains of the human pathogen Campylobacter jejuni. Microbiology 156, 2994–3010. 10.1099/mic.0.042788-0 [DOI] [PubMed] [Google Scholar]
- Hodgkinson V., Petris M. J. (2012). Copper homeostasis at the host–pathogen interface. J Biol Chem 287, 13549–13555. 10.1074/jbc.R111.316406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood M. I., Skaar E. P. (2012). Nutritional immunity: transition metals at the pathogen–host interface. Nat Rev Microbiol 10, 525–537. 10.1038/nrmicro2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamau P., Jordan R. B. (2002). Kinetic study of the oxidation of catechol by aqueous copper(II). Inorg Chem 41, 3076–3083. 10.1021/ic010978c [DOI] [PubMed] [Google Scholar]
- Kershaw C. J., Brown N. L., Constantinidou C., Patel M. D., Hobman J. L. (2005). The expression profile of Escherichia coli K-12 in response to minimal, optimal and excess copper concentrations. Microbiology 151, 1187–1198. 10.1099/mic.0.27650-0 [DOI] [PubMed] [Google Scholar]
- Kimura T., Nishioka H. (1997). Intracellular generation of superoxide by copper sulphate in Escherichia coli. Mutat Res 389, 237–242. 10.1016/S1383-5718(96)00153-2 [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Mizuno M., Fujikawa M., Mizutani Y. (2011). Protein conformational changes of the oxidative stress sensor, SoxR, upon redox changes of the [2Fe-2S] cluster probed with ultraviolet resonance Raman spectroscopy. Biochemistry 50, 9468–9474. 10.1021/bi201526y [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Fujikawa M., Kozawa T. (2014). Oxidative stress sensing by the iron-sulfur cluster in the transcription factor, SoxR. J Inorg Biochem 133, 87–91. 10.1016/j.jinorgbio.2013.11.008 [DOI] [PubMed] [Google Scholar]
- Lee L. J., Barrett J. A., Poole R. K. (2005). Genome-wide transcriptional response of chemostat-cultured Escherichia coli to zinc. J Bacteriol 187, 1124–1134. 10.1128/JB.187.3.1124-1134.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. Z., Jellbauer S., Poe A. J., Ton V., Pesciaroli M., Kehl-Fie T. E., Restrepo N. A., Hosking M. P., Edwards R. A. & other authors (2012). Zinc sequestration by the neutrophil protein calprotectin enhances Salmonella growth in the inflamed gut. Cell Host Microbe 11, 227–239. 10.1016/j.chom.2012.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loschi L., Brokx S. J., Hills T. L., Zhang G., Bertero M. G., Lovering A. L., Weiner J. H., Strynadka N. C. J. (2004). Structural and biochemical identification of a novel bacterial oxidoreductase. J Biol Chem 279, 50391–50400. 10.1074/jbc.M408876200 [DOI] [PubMed] [Google Scholar]
- Macomber L., Imlay J. A. (2009). The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc Natl Acad Sci U S A 106, 8344–8349. 10.1073/pnas.0812808106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C. M., Helmann J. D. (2005). Metal ion homeostasis in Bacillus subtilis. Curr Opin Microbiol 8, 188–195. 10.1016/j.mib.2005.02.007 [DOI] [PubMed] [Google Scholar]
- Morales E. H., Collao B., Desai P. T., Calderón I. L., Gil F., Luraschi R., Porwollik S., McClelland M., Saavedra C. P. (2013). Probing the ArcA regulon under aerobic/ROS conditions in Salmonella enterica serovar Typhimurium. BMC Genomics 14, 626. 10.1186/1471-2164-14-626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton S. M., Trinh V., Pi H., Klebba P. E. (2010). Direct measurements of the outer membrane stage of ferric enterobactin transport: postuptake binding. J Biol Chem 285, 17488–17497. 10.1074/jbc.M109.100206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman D., Cavet J. S. (2011). Metal sensing in Salmonella: implications for pathogenesis. Adv Microb Physiol 58, 175–232. [DOI] [PubMed] [Google Scholar]
- Panina E. M., Mironov A. A., Gelfand M. S. (2003). Comparative genomics of bacterial zinc regulons: enhanced ion transport, pathogenesis, and rearrangement of ribosomal proteins. Proc Natl Acad Sci U S A 100, 9912–9917. 10.1073/pnas.1733691100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez Audero M. E., Podoroska B. M., Ibáñez M. M., Cauerhff A., Checa S. K., Soncini F. C. (2010). Target transcription binding sites differentiate two groups of MerR-monovalent metal ion sensors. Mol Microbiol 78, 853–865. 10.1111/j.1365-2958.2010.07370.x [DOI] [PubMed] [Google Scholar]
- Petrarca P., Ammendola S., Pasquali P., Battistoni A. (2010). The Zur-regulated ZinT protein is an auxiliary component of the high-affinity ZnuABC zinc transporter that facilitates metal recruitment during severe zinc shortage. J Bacteriol 192, 1553–1564. 10.1128/JB.01310-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontel L. B., Soncini F. C. (2009). Alternative periplasmic copper-resistance mechanisms in Gram negative bacteria. Mol Microbiol 73, 212–225. 10.1111/j.1365-2958.2009.06763.x [DOI] [PubMed] [Google Scholar]
- Porcheron G., Garénaux A., Proulx J., Sabri M., Dozois C. M. (2013). Iron, copper, zinc, and manganese transport and regulation in pathogenic Enterobacteria: correlations between strains, site of infection and the relative importance of the different metal transport systems for virulence. Front Cell Infect Microbiol 3, 90. 10.3389/fcimb.2013.00090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J.-H., Oh S.-Y., Kim S.-J., Roe J.-H. (2007). The zinc-responsive regulator Zur controls a zinc uptake system and some ribosomal proteins in Streptomyces coelicolor A3(2). J Bacteriol 189, 4070–4077. 10.1128/JB.01851-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsolis R. M., Bäumler A. J., Stojiljkovic I., Heffron F. (1995). Fur regulon of Salmonella typhimurium: identification of new iron-regulated genes. J Bacteriol 177, 4628–4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega D. E., Young K. D. (2014). Accumulation of periplasmic enterobactin impairs the growth and morphology of Escherichia coli tolC mutants. Mol Microbiol 91, 508–521. 10.1111/mmi.12473 [DOI] [PubMed] [Google Scholar]
- Vogt S. L., Raivio T. L. (2012). Just scratching the surface: an expanding view of the Cpx envelope stress response. FEMS Microbiol Lett 326, 2–11. 10.1111/j.1574-6968.2011.02406.x [DOI] [PubMed] [Google Scholar]
- Wang D., Hosteen O., Fierke C. A. (2012). ZntR-mediated transcription of zntA responds to nanomolar intracellular free zinc. J Inorg Biochem 111, 173–181. 10.1016/j.jinorgbio.2012.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolschendorf F., Ackart D., Shrestha T. B., Hascall-Dove L., Nolan S., Lamichhane G., Wang Y., Bossmann S. H., Basaraba R. J., Niederweis M. (2011). Copper resistance is essential for virulence of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 108, 1621–1626. 10.1073/pnas.1009261108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X.-Q., McClelland M., Porwollik S., Song W., Cong X., Wang Y. (2009). WebArrayDB: cross-platform microarray data analysis and public data repository. Bioinformatics 25, 2425–2429. 10.1093/bioinformatics/btp430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Ishihama A. (2005). Transcriptional response of Escherichia coli to external copper. Mol Microbiol 56, 215–227. 10.1111/j.1365-2958.2005.04532.x [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Oshima T., Nonaka G., Ito H., Ishihama A. (2011). Induction of the Escherichia coli cysK gene by genetic and environmental factors. FEMS Microbiol Lett 323, 88–95. 10.1111/j.1574-6968.2011.02364.x [DOI] [PubMed] [Google Scholar]
- Zgurskaya H. I., Krishnamoorthy G., Ntreh A., Lu S. (2011). Mechanism and function of the outer membrane channel TolC in multidrug resistance and physiology of enterobacteria. Front Microbiol 2, 189. [DOI] [PMC free article] [PubMed] [Google Scholar]