Abstract
l,d-Transpeptidases (Ldts) catalyse the formation of 3–3 cross-links in peptidoglycans (PGs); however, the role of these enzymes in cell envelope physiology is not well understood. Mycobacterial PG contains a higher percentage of 3–3 cross-links (~30–80 %) than the PG in most other bacteria, suggesting that they are particularly important to mycobacterial cell wall biology. The genomes of Mycobacterium tuberculosis and Mycobacterium smegmatis encode multiple Ldt genes, but it is not clear if they are redundant. We compared the sequences of the Ldt proteins from 18 mycobacterial genomes and found that they can be grouped into six classes. We then constructed M. smegmatis strains lacking single or multiple Ldt genes to determine the physiological consequence of the loss of these enzymes. We report that of the single mutants, only one, ΔldtC (MSMEG_0929, class 5), displayed an increased susceptibility to imipenem – a carbapenem antibiotic that inhibits the Ldt enzymes. The invariant cysteine in the active site of LdtC was required for function, consistent with its role as an Ldt. A triple mutant missing ldtC and both of the class 2 genes displayed hypersusceptibility to antibiotics, lysozyme and d-methionine, and had an altered cellular morphology. These data demonstrated that the distinct classes of mycobacterial Ldts may reflect different, non-redundant functions and that the class 5 Ldt was peculiar in that its loss, alone and with the class 2 proteins, had the most profound effect on phenotype.
Introduction
Bacteria belonging to the genus Mycobacterium, like many members of the order Actinomycetales, have an exceedingly complex cell envelope consisting of a covalently attached core of mycolic acids, arabinogalactan and peptidoglycan (collectively known as the MAPc), associated with non-covalently attached lipids that, together with the mycolic acids, form a lipid bilayer reminiscent of the outer membrane in Gram-negative bacteria (Hett & Rubin, 2008; Lederer, 1971). The mycolyl-arabinogalactan component of the MAPc is anchored by a N-acetylglucosamine-rhamnose linker (McNeil et al., 1990) attached to muramic acid residues in the peptidoglycan (PG), which is similar in basic structure to that of Escherichia coli, consisting of alternating N-acetylglucosamine and N-acylmuramic acid residues with l-alanyl (or glycyl)-d-iso-glutaminyl-meso-diaminopimelyl-d-alanyl-d-alanine peptides attached to the muramyl sugars (Schleifer & Kandler, 1972). Notably, mycobacterial PG has various modifications, including N-glycolylation of the muramyl residues, amidation of diaminopimelic acid (DAP) as well as amidation of d-glutamate to D-iso-glutamine (Azuma et al., 1970; Schleifer & Kandler, 1972). The mycobacterial PG is also highly cross-linked with peptides involved in a direct cross-link between meso-DAP and d-alanine (also known as a 4–3 cross-link) or between two meso-DAP residues (known as a 3–3 cross-link) (Wietzerbin et al., 1974). It was originally reported that about two-thirds of the cross-links are in the 4–3 configuration, whilst one-third are in the 3–3 configuration (Quintela et al., 1995; Wietzerbin et al., 1974). More recent work shows that the percentage of 3–3 cross-links is in the 60–80 % range in the PG of M. tuberculosis and Mycobacterium abscessus cells (Kumar et al., 2012; Lavollay et al., 2008, 2011). Both types of linkages are widespread in bacteria, but the percentage of 3–3 linkages in mycobacteria is particularly high compared with most other bacteria. A 3–3 linkage is likely to be less flexible compared with a 4–3 linkage and thus one would expect that 3–3 linkages would increase the rigidity of the PG, which could aid in stabilizing the complex mycobacterial cell envelope.
Formation of the 4–3 linkages are catalysed by classical penicillin sensitive d,d-transpeptidases, [the so-called penicillin-binding proteins (PBPs)], whilst generation of the 3–3 linkages is catalysed by a novel set of penicillin-insensitive l,d-transpeptidases (Ldts) that were only discovered in 2002 (Mainardi et al., 2002). These enzymes, which belong to the YkuD superfamily, were first discovered in enterococci, but have since been found in many different bacteria (Magnet et al., 2007a, b; Mainardi et al., 2002). They catalyse the formation of PG cross-links and, in E. coli, also couple Braun's lipoprotein to the PG (Magnet et al., 2007a). Bacteria often have more than one gene encoding Ldt-type enzymes and it is not entirely clear why this is so, underscoring that we know very little about the role of 3–3 cross-links in PG physiology. There are five genes encoding Ldt-type enzymes in M. tuberculosis and six in the saprophyte M. smegmatis. Recombinant versions of the five enzymes from M. tuberculosis are active in vitro, and a M. tuberculosis ΔldtMt2 mutant is hypersusceptible to amoxicillin and attenuated in the mouse model (Cordillot et al., 2013; Gupta et al., 2010; Lavollay et al., 2008). An ΔldtMt1 mutant of M. tuberculosis has no phenotype, but the double ΔldtMt1 ΔldtMt2 mutant has a synergistic phenotype including altered cell shape and protein secretion (Schoonmaker et al., 2014). We report here the genetic analysis of the six Ldt-type enzymes in M. smegmatis and homologues of M. tuberculosis. Our results showed that the mycobacterial Ldts could be grouped into distinct classes based upon homology and differential characteristics within each class of proteins, suggesting that these enzymes may not be entirely redundant.
Methods
Bacterial strains and growth conditions.
Bacterial strains used in this study are listed in Table 1. E. coli strain DH10B was used as the cloning strain of all complementing plasmids and E. coli strain HB101 was used for construction of DNA molecules bearing a resolvable hygromycin resistance marker (res-hyg-res). E. coli strains were grown in Luria Burtani (LB) medium supplemented with 50 µg kanamycin ml−1 or 50 µg apramycin ml−1 (Sigma), or 200 µg hygromycin ml−1 (Roche). The M. smegmatis strain PM965 is a derivative of strain mc2155 containing a deletion of the major β-lactamase (ΔblaS), and was used as the WT strain to construct all mutants (Raymond et al., 2005). Mycobacterial cultures were grown in Middlebrook (Becton Dickinson) medium, 7H9 (liquid) or 7H10 (agar), supplemented with 0.2 % glycerol, 0.05 % Tween 80, and, when required, the antibiotics kanamycin, hygromycin and apramycin were used at concentrations of 25, 100 and 10 µg ml−1, respectively. All strains constructed in this study are available through the corresponding author.
Table 1. Strains and plasmids.
| Strain or plasmid | Description | Reference or source |
| M. smegmatis strains | ||
| mc2155 | ept-1 | Snapper et al. (1990) |
| PM965 | ept-1 rpsL4 ΔblaS | Raymond et al. (2005) |
| PM2102 | PM965/pJV53 | This study |
| PM2110 | PM965 ldtC : : res-hyg-res | This study |
| PM2115 | PM965 ldtC : : res | This study |
| PM2232 | PM965 ldtB : : res-hyg-res | This study |
| PM2239 | PM965 ldtF : : res-hyg-res | This study |
| PM2543 | PM965 ldtA : : aacC41 | This study |
| PM2687 | PM965 ldtG : : res-hyg-res | This study |
| PM2688 | PM965 ldtE : : res-hyg-res | This study |
| PM2269 | PM965 ldtB : : res-hyg-res ldtF : : res | This study |
| PM2544 | PM965 ldtB : : res-hyg-res ldtF : : res ldtA : : aacC41 | This study |
| PM2546 | PM965 ldtB : : res-hyg-res ldtF : : res ldtC : : aacC41 | This study |
| PM2855 | PM965 ldtB : : res ldtF : : res ldtC : : aacC41 | This study |
| PM2683 | PM965 ldtB : : res ldtF : : res ldtA : : aacC41 ldtC : : res-hyg-res | This study |
| PM2650 | PM965/pMV261 | |
| PM2705 | PM965 ldtB : : res ldtF : : res,ldtA : : aacC41,ldtG : : res-hyg-res | This study |
| PM2706 | PM965 ldtB : : res ldtF : : res,ldtA : : aacC41, ldtE : : res-hyg-res | This study |
| PM2116 | PM2110/pMV261 | This study |
| PM2117 | PM2110/pMP850 | This study |
| PM2118 | PM2110/pMP855 | This study |
| PM2934 | PM2110/pMP1081 | This study |
| PM2935 | PM2110/pMP1086 | This study |
| PM2938 | PM2110/pMP1097 | This study |
| PM2939 | PM2110/pMP1145 | This study |
| PM2562 | PM2546/pMV261 | This study |
| PM2563 | PM2546/pMP850 | This study |
| PM2565 | PM2546/pMP855 | This study |
| PM2566 | PM2546/pMP891 | This study |
| PM2567 | PM2546/pMP894 | This study |
| PM2568 | PM2546/pMP1041 | This study |
| PM3070 | PM965/pMN437 | This study |
| PM3071 | PM2115/pMN437 | This study |
| PM3072 | PM2855/pMN437 | This study |
| E. coli strains | ||
| DH10B | F− mcrA Δ(mrr-hsd RMS-mcrBC) ϕ80ΔlacZΔM15 ΔlacX74 deoR recA1 φaraD139 Δ(ara,leu)7697 galU galK λ− rpsL endA1 nupG | Lab collection |
| HB101 | F− hsdS20 (rB− mB−) supE44 recA13 ara-14 galK2 proA2 lacY1 rpsL20 xly-5 mtl-1 | Lab collection |
| Plasmids | ||
| pJV53 | Kmr recombineering plasmid | van Kessel & Hatfull (2008) |
| pMV261 | Kmr E. coli–mycobacterium shuttle vector, contains groEL promoter, pAL500 oriM, ColE1 | Stover et al. (1991) |
| pMP854 | Kmr pMV261 tnpR (γδ resolvase) | This study |
| pMP850 | pMV261 Rv0483 lprQ+ (ldtC) | This study |
| pMP855 | pMV261 MSMEG_0929+ (ldtC) | This study |
| pMP891 | pMV261 MSMEG_1322+ (ldtF) | This study |
| pMP894 | pMV261 MSMEG_4745+ (ldtB) | This study |
| pMP1041 | pMV261 Rv2518 lppS+ (ldtB) | This study |
| pMP1081 | pMV261 MSMEG_0929 (ldtC) C-terminal c-Myc His6 | This study |
| pMP1086 | pMV261 MSMEG_0929 (ldtC) C360A C-terminal c-Myc His6 | This study |
| pMP1097 | pMV261 MSMEG_0929 (ldtC) N358H C-terminal c-Myc His6 | This study |
| pMP1145 | pMV261 MSMEG_0929 (ldtC) ΔPRR C-terminal c-Myc His6 | This study |
| pMN437 | pMS2 (Kmr E. coli–mycobacterium shuttle vector) with codon-optimized gfp | Song et al. (2008) |
Plasmids and DNA methods.
DNA manipulations were performed as described previously (Asubel et al., 1987). All genes for complementation were amplified from M. smegmatis strain mc2155 using iProof (Bio-Rad) with primers containing the desired restriction sites and cloned into the E. coli–mycobacteria shuttle vector pMV261. The MSMEG_0929 alleles containing site-directed mutations were constructed using splice overlap extension as described previously (Ho et al., 1989). Plasmids were purified using Qiagen columns and sequenced by ACGT. Oligonucleotides were manufactured by Invitrogen Life Technologies and restriction and DNA modification enzymes were obtained from Fermentas or New England Biolabs. Detailed descriptions of plasmid and allele construction can be obtained from the corresponding author. All plasmids constructed in this study are available through the corresponding author.
Reverse transcription (RT)-PCR analysis.
RNA was prepared from 50 ml WT PM965 culture at mid-exponential phase. Pelleted cells were lysed using the FastRNA Blue kit from MP Biomedicals according to the manufacturer's directions. RNA was treated with TurboDNase according to the manufacturer's protocol. Reverse transcriptase reactions were done using 1 µg purified RNA with Superscript II (Invitrogen) for each primer pair in duplicate, with a control reaction lacking the reverse transcriptase. First-strand synthesis was done at 42 °C and subsequent PCR was done using iProof polymerase (Bio-Rad). Reaction products were analysed using a 1.8 % NuSieve agarose gel (Cambrix).
Construction of M. smegmatis Ldt mutants.
Single and multiple M. smegmatis ldt strains were constructed using the recombineering method as described previously (van Kessel & Hatfull, 2008). Briefly, host strains carrying the plasmid pJV53, which encodes the Che9c mycobacteriophage recombineering proteins (gp60, gp61), were transformed with insertionally mutated deletion alleles of all six genes (MSMEG_0929, MSMEG_1322, MSMEG_4745, MSMEG_3528, MSMEG_0674 and MSMEG_0233) disrupted by an apramycin (aacC41) gene or a res-hyg-res cassette. All clones were selected on media containing the appropriate antibiotic and were subsequently cured of the pJV53 plasmid by subculture in the absence of antibiotic selection. When necessary, strains were resolved of their res-hyg-res cassette by expression of the plasmid-encoded γδ resolvase (tnpR) from pMP854 and the strains cured of the plasmid as described above for pJV53 (van Kessel & Hatfull, 2008). All clones were verified by either PCR or Southern blot.
Immunoblotting.
Protein from whole-cell lysates were separated on a 10–12 % Bistris SDS-PAGE denaturing gel (Invitrogen), and immunoblotted using PVDF membranes and probed with mouse anti-cMyc antibodies (Invitrogen). Detection was done using rabbit anti-mouse antibodies conjugated to alkaline phosphatase and the WesternBreeze chemiluminescence system (GE Healthcare) according to the manufacturer’s protocol.
Antibiotic sensitivity assay.
Antibiotic sensitivity assays were performed using the disc diffusion method. M. smegmatis strains grown to the mid-exponential phase of growth were used to inoculate 0.7 % top agar and seeded onto 7H10 medium for confluent growth. Sensi-Discs impregnated with imipenem (10 µg), meropenem (10 µg), ertapenem (10 µg), rifampicin (25 µg), isoniazid (5 µg), ethambutol (25 µg), ceftriaxone (30 µg) or vancomycin (30 µg) (Becton, Dickinson), or ampicillin (50 µg) on sterile paper discs were placed in the middle of the plate. Plates were incubated at 37°C for 48 h after which the diameter of the zone of inhibition was measured in millimetres.
Lysozyme sensitivity assay.
M. smegmatis strains were grown to the mid-exponential phase of growth in triplicate cultures, which were plated in duplicate for viable cell counts on 7H10 medium or 7H10 supplemented with 0.2 mg lysozyme ml−1 (MP Biochemicals). The c.f.u. were recorded after 72–96 h incubation at 37 °C.
d-Methionine sensitivity assay.
M. smegmatis strains were grown to the mid-exponential phase of growth in triplicate cultures, which were plated in duplicate for viable cell counts on 7H10 medium or 7H10 supplemented with 15 mM d-methionine (Sigma). The c.f.u. were recorded after 72–96 h incubation at 37 °C.
Microscopy.
M. smegmatis strains expressing gfp from plasmid pMN437 (Song et al., 2008) were grown to the stationary phase of growth. Cells were harvested, washed once with PBS/glycerol (25 %) solution and subsequently wet mounted on a microscope slide. Cells were visualized by an Olympus BX41 fluorescent microscope with a ×100 oil immersion objective.
Results
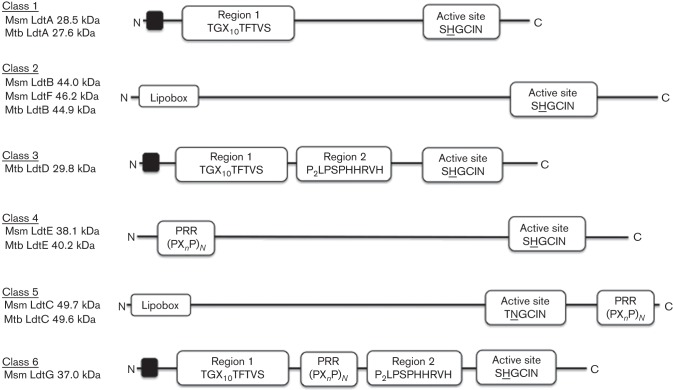
The genome of M. smegmatis contains six genes encoding Ldts similar to those originally identified in enterococci and then found in a variety of Gram-negative and Gram-positive bacteria. Homologues of these genes are conserved among the mycobacterial genomes sequenced to date; however, the number of Ldts varies among members of the species. In M. tuberculosis, there are five Ldt paralogues that, depending on the strain, have been given various gene name designations. For clarity in this study we have followed a new naming scheme for the Ldts, based upon the M. tuberculosis H37Rv annotated genome (http://tuberculist.epfl.ch) and will refer to them as ldtA to ldtG (Table 2), which is consistent with established bacterial genetic nomenclature and the names of Ldts in other bacteria. Sequence-based alignments (Larkin et al., 2007) with the translated Ldts in 18 mycobacterial genomes in GenBank demonstrated that the proteins can be organized into six distinct classes (Fig. 1). For simplicity, the classes are numbered according to the original LdtMt numerical designations (see Table 2). All classes contain a similar active-site region, which is the hallmark of all Ldts, distinguished by a catalytic cysteine residue, located near the C termini of the proteins (Biarrotte-Sorin et al., 2006; Erdemli et al., 2012; Kim et al., 2013; Li et al., 2013). The class 5 protein stands out in that the histidine residue in the active-site consensus sequence is replaced with an asparagine (Fig. 1). Classes 5 and 2 are both annotated as lipoproteins, but the class 5 proteins also contain a proline-rich C-terminal region (PRR-C) that is missing in the class 2 proteins. Classes 1, 3 and 6 all contain a conserved Region 1 sequence (TGX10TFTVS), while classes 3 and 6 also share a conserved Region 2 sequence (PPPLPSPHHRVH). The class 6 proteins also contain an internal proline-rich sequence (PRR-I). Of note, there is no class 6 representative in M. tuberculosis and no class 3 protein in M. smegmatis. Finally, class 4 proteins are distinguished by a conserved proline-rich sequence in the N terminus of the protein (PRR-N). Note that M. smegmatis has two distinct class 2 proteins and that M. tuberculosis has only one. (Table 2, and see protein homologies in Tables S1 and S2, available in the online Supplementary Material). All five ldt genes of M. tuberculosis are also found in Mycobacterium leprae, although the class 4 representative is annotated as a pseudogene in the latter species.
Table 2. Mycobacterial Ldt gene nomenclature.
| Class | M. smegmatis | M. tuberculosis | New name | ||
| H37Rv | CDC1551 | Other | |||
| 1 | MSMEG_3528 | Rv0116c | MT0125 | ldtMt1 | ldtA |
| 2 | MSMEG_4745 | Rv2518c | MT2594 | lppS,ldtMt2 | ldtB |
| 2 | MSMEG_1322 | – | – | – | ldtF |
| 3 | – | Rv1433 | MT1477 | ldtMt3 | ldtD |
| 4 | MSMEG_0233 | Rv0192 | MT0202 | ldtMt4 | ldtE |
| 5 | MSMEG_0929 | Rv0483 | MT0501 | lprQ,ldtMt5 | ldtC |
| 6 | MSMEG_0674 | – | – | – | ldtG |
Fig. 1.
Structural classification of mycobacterial Ldts (Msm, M. smegmatis; Mtb, M. tuberculosis). Schematic protein organization of each class of mycobacterial Ldts is shown, derived from analyses of the translated sequences of the Ldt genes in 18 mycobacterial genomes. The proteins can be grouped into six classes. Class 5 and 2 are lipoproteins (Lipobox) and also contain a PRR C terminus. Class 1 proteins contain a conserved Region 1 sequence, whilst class 3 and class 6 share a conserved Region 2 sequence along with the Region 1 sequence. Class 6 proteins also have an internal PRR sequence. Class 4 proteins have a conserved PRR sequence in the N terminus. Black boxes at the N termini of some of the proteins indicate predicted transmembrane helices according to genome annotation. All classes contain the characteristic active-site region, HXX14–17[S/T]HGChN (where h is a hydrophobic residue), containing the catalytic cysteine residues. Note that two residues preceding the cysteine, only class 5 has an asparagine instead of the conserved histidine.
It is remarkable that three of the classes (4, 5 and 6) have extensive proline-rich domains that are similar to those we found previously in the mycobacterial PBPs, PonA1, PonA2 and PonA3 (Patru & Pavelka, 2010). A comparison of these proline-rich regions (PRRs) indicating the PxxP and PxxxP motifs within each protein is shown in Fig. S2.
Phenotypes of deletion mutants
M. smegmatis class 5 mutant, ΔldtC, is hypersusceptible to imipenem.
We created M. smegmatis strains lacking each of the ldt genes, either singly or in various combinations, up to triple and quadruple mutants (Table 1, Fig. S1). As a reporter of cell wall homeostasis, we tested the susceptibility of each of the single-mutant strains against the antibiotics isoniazid, ethambutol, ampicillin, ceftriaxone and vancomycin, all of which target the biosynthesis of different components of the cell envelope. However, none of the single-mutant strains displayed an increase in susceptibility when challenged with these antibiotics (data not shown). It has been shown previously that the Ldt enzymes are sensitive to carbapenem-type antibiotics, which acylate the catalytic cysteine in the active site (Mainardi et al., 2007). Of the M. smegmatis single-mutant strains, only one, PM2110 (ΔldtC), displayed a hypersensitivity to imipenem as shown by an 8 mm increase in the disc diffusion zone diameter compared with the parental strain (PM965) (Table 3). In addition, PM2110 also displayed an increase in susceptibility to ertapenem and meropenem, two other carbapenems (Table 4). Complementation of the ΔldtC strain with either the M. smegmatis (pMP855) or M. tuberculosis (pMP850) version of ldtC+ carried on a multi-copy plasmid fully restored the WT imipenem phenotype (see strains PM2117 and PM2116 in Table 3).
Table 3. Imipenem susceptibilities of M. smegmatis ldt strains.
| Strain | Description | Class | Zone diameter (mm) |
| PM965 | WT | 35±3 | |
| PM2110 | ΔldtC | Δ5 | 43±2 |
| PM2239 | ΔldtB | Δ2 | 34±3 |
| PM2232 | ΔldtF | Δ2 | 34±2 |
| PM2269 | ΔldtB, ΔldtF | Δ2 | 35±1 |
| PM2546 | ΔldtB, ΔldtF, ΔldtC | Δ2, Δ5 | 55±2 |
| PM2544 | ΔldtB, ΔldtF, ΔldtA | Δ2, Δ1 | 34±2 |
| PM2683 | ΔldtB, ΔldtF, ΔldtA, ΔldtC | Δ2, Δ1, Δ5 | 54±2 |
| PM2131 | WT/pMV261 | 32±2 | |
| PM2116 | ΔldtC/pMV261 | Δ5 | 39±1 |
| PM2117 | ΔldtC/ldtC+ (Rv0483) | Δ5/5+ | 29±1 |
| PM2118 | ΔldtC/ldtC+ (msmeg0929) | Δ5/5+ | 28±2 |
| PM2562 | ΔldtB, ΔldtF, ΔldtC/pMV261 | Δ2, Δ5 | 55±2 |
| PM2563 | ΔldtB, ΔldtF, ΔldtC/ldtC+ (Rv0483) | Δ2, Δ5/5+ | 36±1 |
| PM2565 | ΔldtB, ΔldtF, ΔldtC/ldtC+ (MSMEG_0929) | Δ2, Δ5/5+ | 25±1 |
| PM2566 | ΔldtB, ΔldtF, ΔldtC/ldtF+ | Δ2, Δ5/2+ | 38±2 |
| PM2567 | ΔldtB, ΔldtF, ΔldtC/ldtB+ | Δ2, Δ5/2+ | 39±1 |
| PM2568 | ΔldtF, ΔldtB, ΔldtC/ldtB+ (Rv2518c) | Δ2, Δ5/2+ | 35±1 |
| PM2934 | ΔldtC/ldtC C-terminal c-Myc His6 | Δ5/5+ | 27±3 |
| PM2935 | ΔldtC/ldtC C360A C-terminal c-Myc His6 | Δ5/5 | 40±2 |
| PM2937 | ΔldtC/ldtC N358H C-terminal c-Myc His6 | Δ5/5 | 28±2 |
| PM2939 | ΔldtC/ldtC ΔPRR C-terminal c-Myc His6 | Δ5/5 | 30±1 |
Table 4. Antibiotic susceptibilities of select M. smegmatis ldt mutants.
| Strain | Zone diameter (mm) | ||||||
| Ampicillin | Meropenem | Ertapenem | Vancomycin | Rifampicin | Isoniazid | Ethambutol | |
| PM965 (WT) | 36±0 | 14.5±0.5 | 0±0 | 25±0 | 15±0 | 18±0 | 42.5±0.5 |
| PM2110 (ΔldtC) | 36.5±0.5 | 17.5±0.5 | 9.5±0.5 | 24±0 | 14.5±0.5 | 18±0 | 42±0 |
| PM2546 (ΔldtB, ΔldtF, ΔldtC) | 42.5±0.5 | 20.5±0.5 | 20±0 | 25±0 | 24.5±0.5 | 17.5±0 | 53.5±0.5 |
A M. smegmatis class 5 and 2 triple-mutant strain is hypersusceptible to imipenem.
As the mycobacterial Ldt enzymes have the potential to function in a redundant manner, we tested the sensitivity of the multiple ldt mutant strains to agents that target PG biosynthesis. A triple-mutant strain (PM2546), lacking class 5 (ΔldtC) and both class 2 enzymes (ΔldtB, ΔldtF), displayed an enhanced sensitivity to imipenem with a 20 mm increase in disc diffusion zone diameter compared with the parental strain PM965 and a 12 mm increase compared with the ΔldtC single-mutant strain PM2110 (Table 3). Neither the class 2 single-gene mutants (PM2232, PM2239) nor the class 2 double-gene mutant (PM2269) demonstrated a change in susceptibility compared with the parental strain, suggesting a synergistic defect when the class 5 and class 2 mutations were combined (Table 3). The imipenem sensitivity of the triple-mutant strain could be fully restored to that of WT by complementation with either of the class 5 genes from M. smegmatis (pMP855) or M. tuberculosis (pMP850) (Table 3). Complementation with either of the class 2 genes from M. smegmatis (pMP891, pMP894) or the class 2 gene from M. tuberculosis (pMP1041) was able to restore the imipenem phenotype back to that of the ΔldtC strain PM2110 (Table 3).
We also challenged the strains lacking multiple ldt genes with a diverse panel of antibiotics. The triple-mutant strain PM2546 (ΔldtB ΔldtF ΔldtC) was unique in that, in addition to being hypersusceptible to the carbapenems (imipenem, ertapenem and meropenem), it was also hypersusceptible to rifampicin, ampicillin and ethambutol (Table 4). In the case of the latter two antibiotics, the hypersusceptibility was only seen in the presence of 0.05 % Tween 80 in the agar.
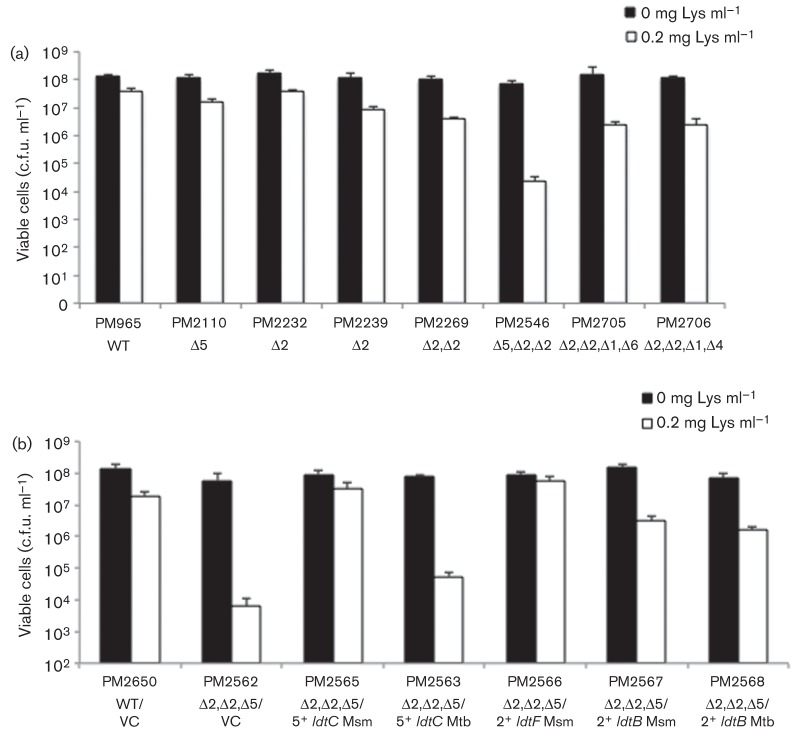
Class 5 and class 2 triple-mutant strain PM2546 is hypersusceptible to lysozyme.
The Ldt mutants were also tested against lysozyme, which cleaves the glycosidic backbone of the PG. Whilst neither the class 5 or 2 single mutants (PM2110, PM2232, PM2239) nor the class 2 double mutant (PM2269) were greatly affected by 0.2 mg lysozyme ml−1 in solid growth media, the triple-mutant strain PM2546, lacking both class 2 genes and the class 5 gene, demonstrated a 3log10 decrease in survival (Fig. 2a). Interestingly, the class 5 ldtC+ and class 2 ldtF+ gene from M. smegmatis were both able to fully complement the lysozyme phenotype when introduced individually; however, the ldtC+ and ldtB+ genes from M. tuberculosis along with the other class 2 gene, ldtB+, from M. smegmatis only showed partial restoration when expressed by themselves in the triple-mutant strain (Fig. 2b).
Fig. 2.
Lysozyme sensitivity of the class 5 and class 2 triple-mutant strain. WT and mutant strains were grown to the mid-exponential phase of growth and plated for viable cell counts on either 7H10 medium or 7H10 supplemented with 0.2 mg lysozyme (Lys) ml−1. (a) Class 5 (PM2110) and class 2 (PM2232, PM2239) single-mutant strains, class 2 double-mutant strain (PM2269), and class 5 and 2 triple-mutant strain (PM2546). (b) Class 5 and class 2 triple-mutant strain was complemented with either pMV261 [vector control (VC)], or pMV261 containing either a class 5 or class 2 gene from M. smegmatis (Msm) or M. tuberculosis (Mtb) Data were analysed using Student’s t-test. All values were significant comparing lysozyme-treated and untreated samples for the same strain (P<0.01), and comparing lysozyme treated strains with the lysozyme-treated WT control (P<0.001).
Class 5 and class 2 triple-mutant strain PM2546 is sensitive to d-methionine.
In addition to the formation of 3–3 cross-links, Ldts have also been shown to incorporate d-amino acids into the fourth position of the peptide chain of the PG in various organisms (Cava et al., 2011). However, an increased amount of d-amino acids in the PG has proven to be toxic to certain organisms, possibly due to inhibition of cross-link formation. To examine this phenomenon, we tested the sensitivity of WT and ldt mutant M. smegmatis strains to d-methionine. As shown in Fig. 3, WT and the ΔldtC mutant PM2110 were able to grow on solid media containing up to 15 mM d-methionine, whilst the triple-mutant strain PM2546, lacking ldtC and the two class 2 genes, displayed a severe growth defect on media containing 15 mM d-methionine compared with media without.
Fig. 3.
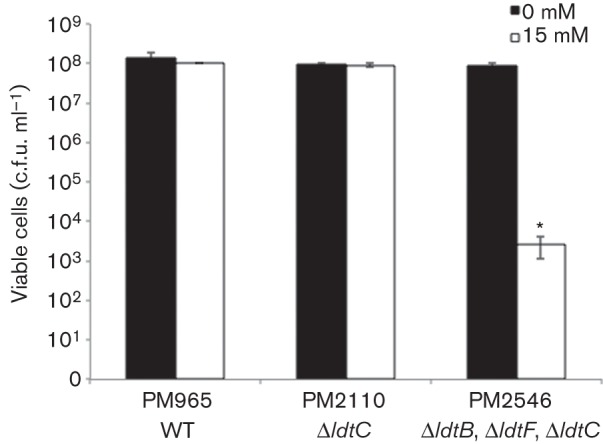
d-Methionine sensitivity of the class 5 and class 2 triple-mutant strain. WT and mutant strains were grown to the mid-exponential phase of growth and plated for viable cell counts on either 7H10 medium or 7H10 supplemented with 15 mM d-methionine. Data were analysed using Student’s t-test, comparing d-methionine-treated and untreated samples for the same strain (P<0.001) *.
Class 5 and class 2 triple-mutant strain PM2546 displays unusual cellular morphology.
The PG layer plays an important role in the viability of bacterial cells, including the maintenance of cell shape and division. To test if the Ldts of M. smegmatis play a role cell shape, we examined the mutant strains microscopically. WT, the class 5 ΔldtC mutant PM2110, and the class 5 and 2 triple mutant PM2546 (ΔldtC ΔldtB ΔldtF) were transformed with a plasmid expressing a gene for the GFP that had been codon-optimized for expression in mycobacteria. The gfp-expressing strains were grown to stationary phase and visualized by fluorescence microscopy (Fig. 4). Only the class 5 and 2 triple mutant displayed an abnormal bulbous cellular morphology, compared with WT and ΔldtC cells that displayed a normal rod-shaped morphology. A class 2 double mutant appeared the same as the WT (data not shown).
Fig. 4.
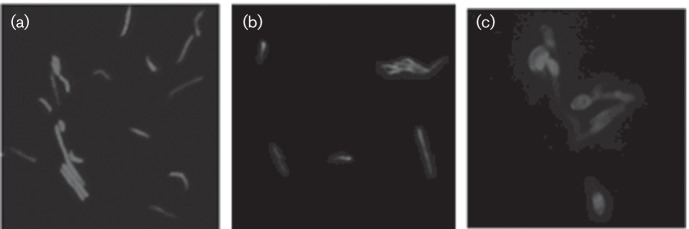
Cellular morphology of the class 5 and class 2 triple-mutant strain. Fluorescence microscopy of stationary-phase M. smegmatis strains expressing gfp from plasmid pMN437. (a) PM3070 (WT), (b) PM3071 (ΔldtC) and (c) PM3072 (ΔldtB, ΔldtF, ΔldtC).
Class 1, class 4 and class 6 ldt genes are expressed.
We only observed phenotypes for strains with class 5 and class 2 mutations, suggesting that the other classes of enzymes may have different roles. However, an alternative explanation is that the genes are not expressed during laboratory culture. Therefore, we performed RT-PCR analysis on RNA prepared from M. smegmatis grown to exponential phase in Middlebrook 7H9 medium, using primer pairs specific for unique regions within the class 1 (ldtA), class 4 (ldtE) and class 6 (ldtG) genes. As shown in Fig. 5, all three genes were expressed under these growth conditions.
Fig. 5.
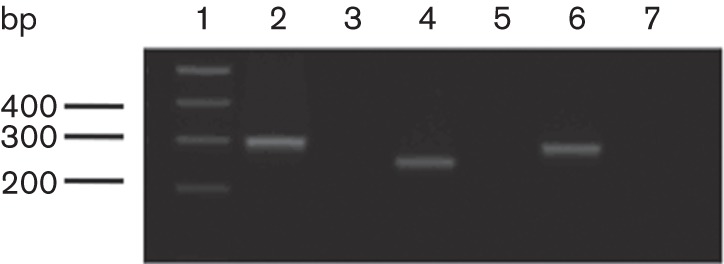
RT-PCR of select ldt genes, performed on RNA purified from exponential-phase cell cultures. Lane 1, DNA size markers (bp); lanes 2 and 3, MSMEG_0223, class 4 (ldtE), 295 bp; lanes 4 and 5, MSMEG_0674, class 6 (ldtG), 248 bp; lanes 6 and 7,: MSMEG_3528, class 1, (ldtA) 278 bp. Reactions in lanes 2, 4 and 6 included reverse transcriptase, whilst the control reactions in lanes 3, 5 and 7 lacked the enzyme.
Loss of the class 1 gene ldtA has no effect on susceptibility.
We sought to determine if combining class 2 and class 1 gene deletions would have a phenotypic effect on the cells similar to combining class 2 and class 5 deletions as described above. We tested a triple mutant, PM2544, lacking both class 2 genes (ldtB, ldtF), and the class 1 gene ldtA, and found that it had no change in susceptibility to imipenem (Table 3). In contrast, the quadruple-mutant strain PM2683, which lacks the same genes of PM2544 but also the class 5 gene ldtC, exhibited an increase in susceptibility to imipenem that was equivalent to the susceptibility seen with the triple class 5 and class 2 mutant PM2546 (Table 3). This indicates that loss of the class 1 gene, ldtA, had no effect on imipenem susceptibility and underscores the importance of the class 5 gene to the imipenem phenotype.
We also examined the effect of the loss of the class 1 gene ldtA on the lysozyme phenotype of the double class 2 deletion strain (PM2269) in the context of deleting either the class 6 or class 4 gene. As shown in Fig. 2, there was a slight increase in lysozyme susceptibility in strain PM2269, which lacked both class 2 genes (ΔldtB, ΔldtF), compared with WT. Loss of the class 1 gene (ΔldtA) in this background, in combination with a class 6 deletion (ΔldtG, strain PM2705) or with a class 4 deletion (ΔldtE, strain PM2706), did not change the lysozyme susceptibility of the strains, compared with the double class 2 mutant PM2269.
Site-directed mutagenesis of LdtC
ΔldtC imipenem phenotype is dependent on the active-site cysteine residue of the protein.
We chose to perform a mutational analysis on the LdtC protein as the ΔldtC mutant was the only single-gene deletion mutant that displayed an antibiotic susceptibility phenotype. The signature active-site sequence HXX14–17[S/T]HGChN (where h is a hydrophobic residue), with the cysteine being the site of catalytic activity, is characteristic of all Ldt enzymes. We constructed a mutant version of the M. smegmatis LdtC in which the active-site Cys360 was substituted with an alanine residue (C360A). The ability of the C360A allele to complement the M. smegmatis ΔldtC strain PM2110 was assessed in the imipenem disc diffusion assay. As expected, the allele failed to restore the WT imipenem phenotype (Table 3). Western blot analysis of cellular extracts of the M. smegmatis ΔldtC strain expressing His-tagged versions of LdtC and LdtC C360A demonstrated that the mutant protein was expressed comparably to the WT LdtC (Fig. S3).
Other ldtC alleles can fully complement the ΔldtC mutant strain.
Class 5 Ldts have an active site that is slightly divergent from that seen in the other five Ldt classes, with an asparagine residue positioned two residues prior to the catalytic cysteine, whilst all other Ldt enzymes contain a histidine residue (Fig. 1). To determine the significance of the Asn358 in LdtC we constructed a mutant allele in which that residue was replaced with a histidine (N358H). Complementation of the M. smegmatis ldtC strain with the N358H allele was able to restore the WT imipenem phenotype (Table 3).
The C terminus of the LdtC protein contains a region that is rich in proline residues (Fig. 1). PRRs have been demonstrated to modulate protein–protein interactions as well as play a role in signal transduction in both prokaryotic and eukaryotic organisms. To investigate the role of the C-terminal PRR in LdtC function, we constructed a truncated version of the protein deleted for 40 aa containing the PXXP motif (ΔPRR). Complementation of the ΔldtC mutant strain with LdtC-ΔPRR showed no difference from the strain complemented with WT LdtC when challenged with imipenem (Table 3).
LdtC active-site cysteine is required for lysozyme resistance.
To investigate if the residues and domains of LdtC described above were also important to the lysozyme phenotype, we transformed the triple-mutant strain PM2546 (ΔltdB, ΔltdF, ΔltdC) with plasmids bearing the WT, C360A, N358H and ΔPRR alleles, and then assayed for survival in the 0.2 mg lysozyme ml−1 challenge. Similar to the imipenem results, C360A was the only LdtC mutant unable to complement the lysozyme phenotype of PM2546 (data not shown).
Discussion
The crystal structures of the M. tuberculosis LdtA and LdtB proteins have been reported recently, and it was shown that the Ldts are composed of two major domains: an IgD-like domain and the catalytic domain (Both et al., 2013; Correale et al., 2013; Erdemli et al., 2012; Gupta et al., 2010; Li et al., 2013). It has been proposed that the mycobacterial Ldts can be separated into two groups based upon size, resulting from whether or not they have one or two IgD-like domains. In this study, we have demonstrated that the mycobacterial Ldt enzymes can be divided into six classes based on conserved regions in the protein sequences. Most of the regions we have identified were not noticed previously as they are outside of sequences used for the production of recombinant proteins for crystallization and structural analysis. However, one group did note the sequence we call Region 2 in the class 3 and 6 proteins, in an alignment that showed it as a sequence inserted just within the catalytic domain of the protein (Erdemli et al., 2012).
The class 5 protein, LdtC, appears to be unique because the mutant lacking ldtC was the only single ldt mutant with increased sensitivity to imipenem, which is known to inhibit the Ldts. Structure–function analysis of LdtC showed that the ability of the ldtC gene to complement the mutant is dependent on the Cys360 residue in the active site, consistent with other enzymes of the Ldt family. Recently, it was shown that recombinant M. tuberculosis LdtC can perform the 3–3 cross-linking reaction in vitro, but it is not inhibited by carbapenems (Cordillot et al., 2013). As ldtC genes from both M. tuberculosis and M. smegmatis fully complement the imipenem phenotype of the ldtC mutant, it would appear that, with regard to carbapenem resistance, the enzymes are equivalent in the two species. We noted a divergence in the active site in LdtC in which an invariant His was changed to an Asp residue; however, we showed that a mutant allele with the Asp358 replaced with a histidine residue was able to fully complement the ΔldtC strain when challenged with imipenem, indicating that the asparagine substitution did not play a part in the resistance of LdtC to imipenem. Therefore, there may be other divergent residues in LdtC involved with the carbapenem resistance of this enzyme.
Our data suggest that in the absence of the M. smegmatis LdtC, the cells become more susceptible to imipenem by inactivation of the remaining five Ldts. In all our mutants, a strong antibiotic, lysozyme, or morphological phenotype is only seen in strains lacking LdtC, leading to the conclusion that it is the primary Ldt enzyme in this organism. It has been proposed by others that even though LdtC is resistant to carbapenems, it is incapable of compensating for the other Ldt enzymes as mycobacteria can be killed by carbapenems (Cordillot et al., 2013). However, it is not clear if killing of mycobacteria by these drugs is due to inhibition of Ldts or the classical PBPs, which catalyse standard 4–3 linkages in the PG. The 3–3 cross-linking pathway has not been shown conclusively to be essential to any bacteria in the absence of antibiotics.
A strain devoid of class 5 and class 2 Ldts demonstrated a synergistic defect in response to challenge with carbapenems as well as a hypersusceptibility to ampicillin, rifampicin, ethambutol and lysozyme. These data suggest an important role for the class 2 Ldts in the cell envelope organization as well. In M. tuberculosis, class 2 is represented by a single protein, LdtB, and a M. tuberculosis strain containing a transposon insertion within ldtB has been shown to possess multiple phenotypes, such as altered colony morphology, a hypersusceptibility to amoxicillin and attenuation in the mouse model of infection (Gupta et al., 2010). Although the single class 2 mutant strains and the double class 2 mutant of M. smegmatis had no phenotype, when combined with a class 5 mutation, the phenotype of this resultant triple-mutant strain (PM2546) was enhanced significantly.
The antibiotic and lysozyme phenotypes of the triple-mutant strain PM2546 may have manifested through a number of different mechanisms. Loss of class 5 and class 2 Ldts may decrease the amount of 3–3 cross-linking to the point of compromising the rigidity of the PG. Imipenem and ampicillin are both β-lactam antibiotics, and in the absence or decrease of 3–3 cross-linking enzymes these antibiotics can also target PBPs and inhibit 4–3 cross-links as well. In a similar fashion, a decrease in 3–3 cross-links may make the strain more susceptible to killing by lysozyme. Alternatively, because PG is the structural scaffold for the MAPc, decreasing the rigidity of the PG by a loss of Ldt activity may disrupt the permeability barrier of the cell envelope, allowing better penetration of antibiotics and lysozyme. However, this explanation does not agree with a previous study in which a M. smegmatis mutant lacking ldtB was found in a screen for resistance to ubiquitinated peptides, which resulted from decreased envelope permeability (Purdy et al., 2009). A thorough analysis of the composition of the envelope in these mutants may help explain the antibiotic and lysozyme phenotypes.
In addition to 3–3 cross-link formation, Ldts have also been found to incorporate non-canonical d-amino acids (NCDAAs) into PG during the stationary phase in many organisms (Horcajo et al., 2012). In Vibrio cholerae, the incorporation of NCDAAs has been shown to be a mechanism of resistance to osmotic stress as well as a mechanism for regulating the amount of PG per cell (Cava et al., 2011). It is unknown whether mycobacteria manufacture NCDAAs and incorporate them into their PG; however, we have seen that increasing concentrations of NCDAAs, such as d-methionine, in the culture medium can be toxic to WT cells at a concentration of 30 mM (data not shown). In addition, we have shown that the triple-mutant strain PM2546 also displayed increased sensitivity to d-methionine, suggesting that the PG is less able to tolerate alterations to its primary structure in the absence of these enzymes. The greater defect shown in the triple-mutant strain in the presence of d-methionine may be the result of a decrease in 3–3 cross-links affecting the rigidity of the cell wall, which is then less able to withstand the toxic effects of d-methionine.
Our analysis of the mycobacterial Ldt enzymes suggests that they may not be entirely redundant in function. Clearly, there is some redundancy in the two class 2 proteins ldtB and ldtF, as deletion of both was required to reveal a phenotype in conjunction with loss of the class 5 protein. However, the complementation analysis showed that there might be some subtle differences in function between the two class 2 proteins. The M. tuberculosis ldtB gene was able to complement the imipenem phenotype of the triple mutant PM2546 to the same extent as ldtB or ldtF from M. smegmatis suggesting that these enzymes may have equivalent functions. However, the lysozyme phenotype of the triple mutant was only partially restored by the ldtB genes of M. tuberculosis and M. smegmatis, while the ldtF gene of M. smegmatis was fully functional.
The class 1, 3 and 6 proteins are very closely related to each other, and thus may play some additional role that is distinct from the class 5 and 2 enzymes. We found no alteration in the phenotype of M. smegmatis mutants lacking class 1 or 6 genes. However, others have shown that whilst an M. tuberculosis mutant lacking the class 1 gene, ldtA, has no phenotype, adding a ldtA deletion to the ldtB mutant acerbates the ΔldtB phenotype and adds a morphological defect as well as alterations in protein secretion (Schoonmaker et al., 2014). We did not observe this synergy in our experiments, which may suggest some differences in the way some of these proteins function between the different species. A similar situation was seen with the ldtC genes, in that the genes from both M. smegmatis and M. tuberculosis could fully complement the imipenem phenotype of the ΔldtC mutant, but only the M. smegmatis gene fully complemented the lysozyme defect of the class 5 and class 2 triple mutant.
In E. coli, there are five Ldt enzymes – two that catalyse 3–3 linkage formation and three that are responsible for cross-linking the major outer membrane lipoprotein, Lpp (Braun’s lipoprotein), to the PG for cell envelope stability (Magnet et al., 2007a; Sanders & Pavelka, 2013). Mycobacteria do not have an lpp homologue and to date have not been found to cross-link proteins to the PG. However, proteins of unknown function have been isolated from purified cell wall preparations in organisms such as M. tuberculosis, Mycobacterium chelonae and Mycobacterium leprae, suggesting that mycobacteria may carry out this function (Brennan, 1989; Hirschfield et al., 1990; Magnet et al., 2007a). Covalent attachment of proteins to the PG is found in both Gram-negative and Gram-positive bacteria alike. In Gram-positive organisms this is performed by sortases, which have been shown to be related to the Ldts (Dramsi et al., 2008). In this regard, it is tempting to speculate that perhaps some of the Ldts in mycobacteria may couple proteins to the cell wall. One candidate for this is the class 4 LdtD protein of M. tuberculosis, which was reported recently to be acylated by carbapenem antibiotics, but unable to carry out 3–3 linkage formation in vitro (Cordillot et al., 2013).
The class 4 protein, with its unusual N-terminal PRR, is an enigma as we did not see a phenotype with any class 4 mutant. PRRs are also present in the class 5 and class 6 Ldt enzymes. Loss of the C-terminal PRR of the class 5 LdtC enzyme did not affect the function of the protein in the imipenem or lysozyme challenge, but this does not mean that the region is not important for some other function of the protein. We have noted previously that similar PRRs are present in the mycobacterial PBPs PonA1, PonA2, and PonA3, which catalyse 4–3 linkage formation in the PG (Patru & Pavelka, 2010). The PonA1 protein, which is involved with cell division, has both N-terminal and C-terminal PRRs, whilst PonA2, which has a role in cell survival under non-replicating conditions, has a C-terminal PRR similar to that seen in PonA3. Strikingly, all these proteins have similar PxxP/PxxxP motifs with variation in runs of prolines and repeat sequences. The location of such domains in several different kinds of proteins involved with PG synthesis in mycobacteria argues for an underlying theme in the regulation of cell wall metabolism. Eukaryotic proteins with PxxP regions are known to interact with SH3 domains in signalling cascades and similar SH3b domains are often found in cell wall hydrolases in bacteria (Kay et al., 2000; Lu et al., 2006; Whisstock & Lesk, 1999; Williamson, 1994). Thus, it is possible that the PRRs in the Ldt enzymes described in this study interact with other proteins involved with PG turnover.
Acknowledgements
This work was supported by the National Institutes of Health [grants AI073772 (M. S. P.) and T32 AI007362 (A. S. K)]. We would like to thank M. Niederweis for providing pMN437 and E. Katich for the construction of pMP854.
Abbreviations:
- DAP
diaminopimelic acid
- Ldt
l,d-transpeptidase
- MAPc
core of mycolic acids, arabinogalactan and peptidoglycan
- NCDAA
non-canonical d-amino acid
- PBP
penicillin-binding protein
- PG
peptidoglycan
- PRR
proline-rich region
- RT
reverse transcription
Footnotes
Three supplementary figures and two supplementary tables are available with the online version of this paper.
References
- Asubel F., Brent R., Kingston R. E., Moore D. D., Seidman J. G., Smith J. A., Struhl K. (1987). Current Protocols in Molecular Biology. New York: Greene/Wiley Interscience. [Google Scholar]
- Azuma I., Thomas D. W., Adam A., Ghuysen J. M., Bonaly R., Petit J. F., Lederer E. (1970). Occurrence of N-glycolylmuramic acid in bacterial cell walls. A preliminary survey. Biochim Biophys Acta 208, 444–451. 10.1016/0304-4165(70)90217-5 [DOI] [PubMed] [Google Scholar]
- Biarrotte-Sorin S., Hugonnet J. E., Delfosse V., Mainardi J. L., Gutmann L., Arthur M., Mayer C. (2006). Crystal structure of a novel beta-lactam-insensitive peptidoglycan transpeptidase. J Mol Biol 359, 533–538. 10.1016/j.jmb.2006.03.014 [DOI] [PubMed] [Google Scholar]
- Böth D., Steiner E. M., Stadler D., Lindqvist Y., Schnell R., Schneider G. (2013). Structure of LdtMt2, an l,d-transpeptidase from Mycobacterium tuberculosis. Acta Crystallogr D Biol Crystallogr 69, 432–441. 10.1107/S0907444912049268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan P. J. (1989). Structure of mycobacteria: recent developments in defining cell wall carbohydrates and proteins. Rev Infect Dis 11 (Suppl 2), S420–S430. 10.1093/clinids/11.Supplement_2.S420 [DOI] [PubMed] [Google Scholar]
- Cava F., de Pedro M. A., Lam H., Davis B. M., Waldor M. K. (2011). Distinct pathways for modification of the bacterial cell wall by non-canonical d-amino acids. EMBO J 30, 3442–3453. 10.1038/emboj.2011.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordillot M., Dubée V., Triboulet S., Dubost L., Marie A., Hugonnet J. E., Arthur M., Mainardi J. L. (2013). In vitro cross-linking of Mycobacterium tuberculosis peptidoglycan by l,d-transpeptidases and inactivation of these enzymes by carbapenems. Antimicrob Agents Chemother 57, 5940–5945. 10.1128/AAC.01663-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correale S., Ruggiero A., Capparelli R., Pedone E., Berisio R. (2013). Structures of free and inhibited forms of the l,d-transpeptidase LdtMt1 from Mycobacterium tuberculosis. Acta Crystallogr D Biol Crystallogr 69, 1697–1706. 10.1107/S0907444913013085 [DOI] [PubMed] [Google Scholar]
- Dramsi S., Magnet S., Davison S., Arthur M. (2008). Covalent attachment of proteins to peptidoglycan. FEMS Microbiol Rev 32, 307–320. 10.1111/j.1574-6976.2008.00102.x [DOI] [PubMed] [Google Scholar]
- Erdemli S. B., Gupta R., Bishai W. R., Lamichhane G., Amzel L. M., Bianchet M. A. (2012). Targeting the cell wall of Mycobacterium tuberculosis: structure and mechanism of l,d-transpeptidase 2. Structure 20, 2103–2115. 10.1016/j.str.2012.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R., Lavollay M., Mainardi J. L., Arthur M., Bishai W. R., Lamichhane G. (2010). The Mycobacterium tuberculosis protein LdtMt2 is a nonclassical transpeptidase required for virulence and resistance to amoxicillin. Nat Med 16, 466–469. 10.1038/nm.2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hett E. C., Rubin E. J. (2008). Bacterial growth and cell division: a mycobacterial perspective. Microbiol Mol Biol Rev 72, 126–156. 10.1128/MMBR.00028-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschfield G. R., McNeil M., Brennan P. J. (1990). Peptidoglycan-associated polypeptides of Mycobacterium tuberculosis. J Bacteriol 172, 1005–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. (1989). Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77, 51–59. 10.1016/0378-1119(89)90358-2 [DOI] [PubMed] [Google Scholar]
- Horcajo P., de Pedro M. A., Cava F. (2012). Peptidoglycan plasticity in bacteria: stress-induced peptidoglycan editing by noncanonical d-amino acids. Microb Drug Resist 18, 306–313. 10.1089/mdr.2012.0009 [DOI] [PubMed] [Google Scholar]
- Kay B. K., Williamson M. P., Sudol M. (2000). The importance of being proline: the interaction of proline-rich motifs in signaling proteins with their cognate domains. FASEB J 14, 231–241. [PubMed] [Google Scholar]
- Kim H. S., Kim J., Im H. N., Yoon J. Y., An D. R., Yoon H. J., Kim J. Y., Min H. K., Kim S. J. & other authors (2013). Structural basis for the inhibition of Mycobacterium tuberculosis l,d-transpeptidase by meropenem, a drug effective against extensively drug-resistant strains. Acta Crystallogr D Biol Crystallogr 69, 420–431. 10.1107/S0907444912048998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P., Arora K., Lloyd J. R., Lee I. Y., Nair V., Fischer E., Boshoff H. I., Barry C. E., III (2012). Meropenem inhibits d,d-carboxypeptidase activity in Mycobacterium tuberculosis. Mol Microbiol 86, 367–381. 10.1111/j.1365-2958.2012.08199.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin M. A., Blackshields G., Brown N. P., Chenna R., McGettigan P. A., McWilliam H., Valentin F., Wallace I. M., Wilm A. & other authors (2007). Clustal W and Clustal X version 2.0. Bioinformatics 23, 2947–2948. 10.1093/bioinformatics/btm404 [DOI] [PubMed] [Google Scholar]
- Lavollay M., Arthur M., Fourgeaud M., Dubost L., Marie A., Veziris N., Blanot D., Gutmann L., Mainardi J. L. (2008). The peptidoglycan of stationary-phase Mycobacterium tuberculosis predominantly contains cross-links generated by l,d-transpeptidation. J Bacteriol 190, 4360–4366. 10.1128/JB.00239-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavollay M., Fourgeaud M., Herrmann J. L., Dubost L., Marie A., Gutmann L., Arthur M., Mainardi J. L. (2011). The peptidoglycan of Mycobacterium abscessus is predominantly cross-linked by l,d-transpeptidases. J Bacteriol 193, 778–782. 10.1128/JB.00606-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer E. (1971). The mycobacterial cell wall. Pure Appl Chem 25, 135–165. 10.1351/pac197125010135 [DOI] [PubMed] [Google Scholar]
- Li W. J., Li D. F., Hu Y. L., Zhang X. E., Bi L. J., Wang D. C. (2013). Crystal structure of l,d-transpeptidase LdtMt2 in complex with meropenem reveals the mechanism of carbapenem against Mycobacterium tuberculosis. Cell Res 23, 728–731. 10.1038/cr.2013.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J. Z., Fujiwara T., Komatsuzawa H., Sugai M., Sakon J. (2006). Cell wall-targeting domain of glycylglycine endopeptidase distinguishes among peptidoglycan cross-bridges. J Biol Chem 281, 549–558. 10.1074/jbc.M509691200 [DOI] [PubMed] [Google Scholar]
- Magnet S., Bellais S., Dubost L., Fourgeaud M., Mainardi J. L., Petit-Frère S., Marie A., Mengin-Lecreulx D., Arthur M., Gutmann L. (2007a). Identification of the l,d-transpeptidases responsible for attachment of the Braun lipoprotein to Escherichia coli peptidoglycan. J Bacteriol 189, 3927–3931. 10.1128/JB.00084-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnet S., Arbeloa A., Mainardi J. L., Hugonnet J. E., Fourgeaud M., Dubost L., Marie A., Delfosse V., Mayer C. & other authors (2007b). Specificity of l,d-transpeptidases from Gram-positive bacteria producing different peptidoglycan chemotypes. J Biol Chem 282, 13151–13159. 10.1074/jbc.M610911200 [DOI] [PubMed] [Google Scholar]
- Mainardi J. L., Morel V., Fourgeaud M., Cremniter J., Blanot D., Legrand R., Frehel C., Arthur M., Van Heijenoort J., Gutmann L. (2002). Balance between two transpeptidation mechanisms determines the expression of beta-lactam resistance in Enterococcus faecium. J Biol Chem 277, 35801–35807. 10.1074/jbc.M204319200 [DOI] [PubMed] [Google Scholar]
- Mainardi J. L., Hugonnet J. E., Rusconi F., Fourgeaud M., Dubost L., Moumi A. N., Delfosse V., Mayer C., Gutmann L. & other authors (2007). Unexpected inhibition of peptidoglycan ld-transpeptidase from Enterococcus faecium by the beta-lactam imipenem. J Biol Chem 282, 30414–30422. 10.1074/jbc.M704286200 [DOI] [PubMed] [Google Scholar]
- McNeil M., Daffe M., Brennan P. J. (1990). Evidence for the nature of the link between the arabinogalactan and peptidoglycan of mycobacterial cell walls. J Biol Chem 265, 18200–18206. [PubMed] [Google Scholar]
- Patru M. M., Pavelka M. S., Jr (2010). A role for the class A penicillin-binding protein PonA2 in the survival of Mycobacterium smegmatis under conditions of nonreplication. J Bacteriol 192, 3043–3054. 10.1128/JB.00025-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdy G. E., Niederweis M., Russell D. G. (2009). Decreased outer membrane permeability protects mycobacteria from killing by ubiquitin-derived peptides. Mol Microbiol 73, 844–857. 10.1111/j.1365-2958.2009.06801.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintela J. C., Caparrós M., de Pedro M. A. (1995). Variability of peptidoglycan structural parameters in Gram-negative bacteria. FEMS Microbiol Lett 125, 95–100. 10.1111/j.1574-6968.1995.tb07341.x [DOI] [PubMed] [Google Scholar]
- Raymond J. B., Mahapatra S., Crick D. C., Pavelka M. S., Jr (2005). Identification of the namH gene, encoding the hydroxylase responsible for the N-glycolylation of the mycobacterial peptidoglycan. J Biol Chem 280, 326–333. [DOI] [PubMed] [Google Scholar]
- Sanders A. N., Pavelka M. S., Jr (2013). Phenotypic analysis of Escherichia coli mutants lacking l,d-transpeptidases. Microbiology 159, 1842–1852. 10.1099/mic.0.069211-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. (1972). Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev 36, 407–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoonmaker M. K., Bishai W. R., Lamichhane G. (2014). Nonclassical transpeptidases of Mycobacterium tuberculosis alter cell size, morphology, the cytosolic matrix, protein localization, virulence, and resistance to β-lactams. J Bacteriol 196, 1394–1402. 10.1128/JB.01396-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper S. B., Melton R. E., Mustafa S., Kieser T., Jacobs W. R., Jr (1990). Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol Microbiol 4, 1911–1919. 10.1111/j.1365-2958.1990.tb02040.x [DOI] [PubMed] [Google Scholar]
- Song H., Sandie R., Wang Y., Andrade-Navarro M. A., Niederweis M. (2008). Identification of outer membrane proteins of Mycobacterium tuberculosis. Tuberculosis (Edinb) 88, 526–544. 10.1016/j.tube.2008.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover C. K., de la Cruz V. F., Fuerst T. R., Burlein J. E., Benson L. A., Bennett L. T., Bansal G. P., Young J. F., Lee M. H. & other authors (1991). New use of BCG for recombinant vaccines. Nature 351, 456–460. 10.1038/351456a0 [DOI] [PubMed] [Google Scholar]
- van Kessel J. C., Hatfull G. F. (2008). Mycobacterial recombineering. Methods Mol Biol 435, 203–215. 10.1007/978-1-59745-232-8_15 [DOI] [PubMed] [Google Scholar]
- Whisstock J. C., Lesk A. M. (1999). SH3 domains in prokaryotes. Trends Biochem Sci 24, 132–133. 10.1016/S0968-0004(99)01366-3 [DOI] [PubMed] [Google Scholar]
- Wietzerbin J., Das B. C., Petit J. F., Lederer E., Leyh-Bouille M., Ghuysen J. M. (1974). Occurrence of d-alanyl-(d)-meso-diaminopimelic acid and meso-diaminopimelyl-meso-diaminopimelic acid interpeptide linkages in the peptidoglycan of Mycobacteria. Biochemistry 13, 3471–3476. 10.1021/bi00714a008 [DOI] [PubMed] [Google Scholar]
- Williamson M. P. (1994). The structure and function of proline-rich regions in proteins. Biochem J 297, 249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]