ABSTRACT
Cell polarization is a fundamental process that underlies epithelial morphogenesis, cell motility, cell division and organogenesis. Loss of polarity predisposes tissues to developmental disorders and contributes to cancer progression. The formation and establishment of epithelial cell polarity is mediated by the cooperation of polarity protein complexes, namely the Crumbs, partitioning defective (Par) and Scribble complexes, with Rho family GTPases, including RhoA, Rac1 and Cdc42. The activation of different GTPases triggers distinct downstream signaling pathways to modulate protein–protein interactions and cytoskeletal remodeling. The spatio-temporal activation and inactivation of these small GTPases is tightly controlled by a complex interconnected network of different regulatory proteins, including guanine-nucleotide-exchange factors (GEFs), GTPase-activating proteins (GAPs), and guanine-nucleotide-dissociation inhibitors (GDIs). In this Commentary, we focus on current understanding on how polarity complexes interact with GEFs and GAPs to control the precise location and activation of Rho GTPases (Crumbs for RhoA, Par for Rac1, and Scribble for Cdc42) to promote apical–basal polarization in mammalian epithelial cells. The mutual exclusion of GTPase activities, especially that of RhoA and Rac1, which is well established, provides a mechanism through which polarity complexes that act through distinct Rho GTPases function as cellular rheostats to fine-tune specific downstream pathways to differentiate and preserve the apical and basolateral domains.
This article is part of a Minifocus on Establishing polarity. For further reading, please see related articles: ‘ERM proteins at a glance’ by Andrea McClatchey (J. Cell Sci. 127, 3199–3204). ‘Integrins and epithelial cell polarity’ by Jessica Lee and Charles Streuli (J. Cell Sci. 127, 3217–3215).
KEY WORDS: Crumbs, Par3, Rho GTPase, Scribble, Cell adhesion, Cell polarity
Introduction
Cell polarization, which refers to several subcellular compartmentalization events that result in intracellular polarity, anterior–posterior axis formation or left–right asymmetry, is a crucial process in the development of multicellular organisms. Cell polarity is governed by the coordinated action of three main protein complexes, so called ‘polarity complexes’, and their interactions with cell–cell adhesion receptors and Rho family GTPases. By serving as scaffolding platforms and signaling modules, these complexes regulate protein–protein interactions, protein phosphorylation and downstream signaling events of GTPases to impact on cytoskeletal remodeling, vesicle trafficking and cell morphogenesis. The Rho GTPases are molecular on–off switches that regulate the cytoskeleton and play important roles in the establishment and maintenance of cell adhesion, as well as cell polarity and directed cell migration.
Apical–basal polarization in epithelial cells
In epithelial cells, apical–basal polarization is achieved by the targeting of ‘polarity protein complexes’ to specific localities of the plasma membrane, thus establishing functional and structural specializations for each plasma membrane subdomain. Genetic studies performed primarily in Drosophila melanogaster and Caenorhabditis elegans have identified three key protein complexes that are involved in the establishment of cellular polarity, including the Crumbs (comprising Crb, Pals1 and PATJ), partitioning defective [Par, comprising Par3, Par6 and atypical protein kinase C (aPKC)] and Scribble (comprising Scrib, Dlg and Lgl) complexes (Bilder et al., 2000; Etemad-Moghadam et al., 1995; Jurgens et al., 1984; Kemphues et al., 1988; Tepass et al., 1990). Of note, some of these polarity complex members consist of several isoforms; specific isoforms will be indicated below when necessary or the generic name will be mentioned. Through either cooperative or antagonistic actions, these evolutionarily conserved polarity complexes modulate local signaling and function in concert to institute cellular asymmetry (Betschinger et al., 2003; Bilder et al., 2003; Fletcher et al., 2012; Hurd et al., 2003; Hutterer et al., 2004; McCaffrey and Macara, 2009; Plant et al., 2003; Tanentzapf and Tepass, 2003; Wirtz-Peitz and Knoblich, 2006; Yamanaka et al., 2003). Although similar, it is important to point out that differences exist in junction organization and polarity complex function between flies, worms and mammals, which have been reviewed elsewhere (Knust and Bossinger, 2002; St Johnston and Ahringer, 2010). Fig. 1 depicts the three main polarity complexes, and highlights their cooperative and antagonistic crosstalk that regulates epithelial apical–basal polarity. Importantly, the composition of polarity complexes can be different at different cellular locations, which contributes to the establishment of cell polarity. For example, although Bazooka (the Drosophila ortholog of Par3) and aPKC can directly interact with Par6 (Petronczki and Knoblich, 2001; Wodarz et al., 2000), Bazooka localizes basally to the Par6–aPKC complex, which is controlled by aPKC-mediated Bazooka phosphorylation (Fig. 1) (Doerflinger et al., 2010; Morais-de-Sá et al., 2010).
Fig. 1.
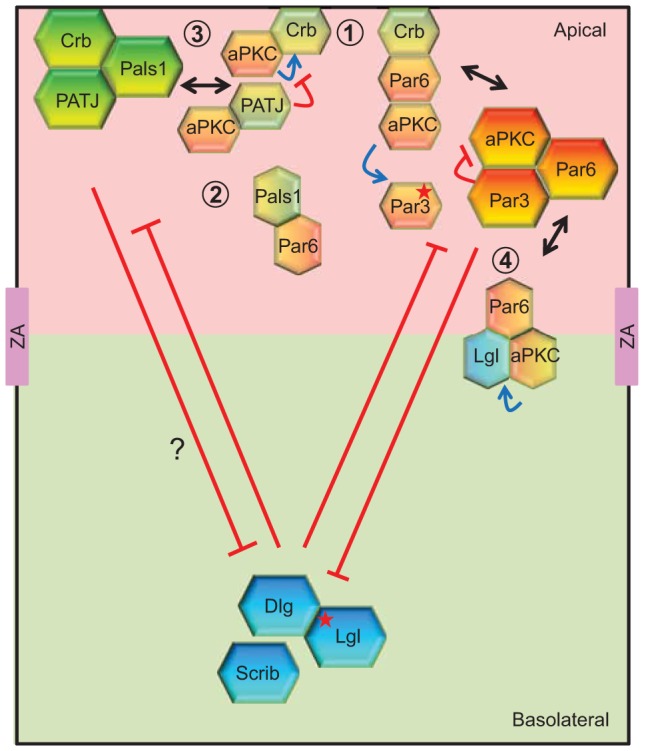
The polarity complex ‘triangle’ – cooperative and antagonistic crosstalk to regulate epithelial apical–basal polarity. The three main protein complexes that regulate epithelial polarization are the apical Crumbs (Crb, Pals1 and PATJ) and Par (Par3, Par6 and aPKC) complexes, and the basolateral Scribble complex (Scrib, Dlg and Lgl). The interaction of Dlg with Scribble is probably indirect (Mathew et al., 2002), and with Lgl is phosphorylation-dependent (red star) (Zhu et al., 2014). Apical and basolateral domains are demarcated by the zonula adherens (ZA), a specialized cell–cell adhesion zone. Members of these three complexes interconnect with each other, allowing mutual exclusion and positive feedback (red, blue and black lines) between different complexes at distinct locations. For example, Crb interacts with the Par6–aPKC complex (Hurd et al., 2003; Kempkens et al., 2006), which is required for the regulation of Par3 localization (Morais-de-Sá et al., 2010; Walther and Pichaud, 2010), modulating junction formation (Lemmers et al., 2004), as well as epithelial morphogenesis and polarity (1) (Nam and Choi, 2003; Walther and Pichaud, 2010). Par6, in turn, interferes with the binding of PATJ to Pals1 (2) (Wang et al., 2004). Although the enzymatic activity of aPKC is inhibited by Par3 in the Par complex (Lin et al., 2000), aPKC can phosphorylate Par3, Crumbs and Lgl (blue arrow), thus bridging and regulating all three polarity complexes (Morais-de-Sá et al., 2010; Plant et al., 2003; Sotillos et al., 2004). Par3 phosphorylation results in its more basal localization relative to the Par6–aPKC complex (Morais-de-Sá et al., 2010). Phosphorylation of Crumbs is crucial for its apical localization and might be inhibited by the presence of PATJ (3) (Sotillos et al., 2004). Lgl competes with Par3 for binding to the Par6–aPKC complex (4); however, once phosphorylated by aPKC, Lgl is released and localizes to the basolateral area (Yamanaka et al., 2003). Despite the importance of the Scribble complex in the basolateral exclusion of Crumbs (Bilder et al., 2000; Bilder and Perrimon, 2000), the physical interactions between members of these two complexes that could underlie this effect are currently unclear.
A key mechanism involved in cellular asymmetry is differential protein trafficking and retention to the apical and basolateral membrane subdomains. The formation of cell–cell contacts facilitates epithelial cell polarity, in part by coordinating protein sorting, targeting and distribution of basolateral and apical proteins to their proper membrane destinations (Apodaca et al., 2012). These different protein distributions then contribute to distinct signaling events at discrete locations, modulating cytoskeletal dynamics and remodeling to promote and maintain epithelial polarity.
The establishment of intercellular adhesion is typically a pre-requisite for mammalian epithelial polarization. E-cadherin clustering (Adams et al., 1998; Adams and Nelson, 1998), association of nectin with afadin (Ooshio et al., 2007) and localization of the tight junction protein junction adhesion molecule A (JAM-A, encoded by F11R) at the nascent cell–cell adhesion site are necessary for the recruitment of molecules such as the polarity protein Par3 (Ebnet et al., 2001; Itoh et al., 2001) and the activation of Rho GTPases, namely Rac1 and Cdc42, to initiate signaling cascades (Kawakatsu et al., 2002; Kim et al., 2000; Nakagawa et al., 2001). Nascent adhesion sites contain several structural and signaling proteins that, upon further junction maturation, separate from one another to form a belt-like adherens junction and a mature tight junction. The crosstalk between polarity complexes, Rho GTPases and adhesion complexes, spatiotemporally control junction maturation and epithelial polarization (Iden and Collard, 2008).
Rho GTPases in epithelial polarization
The Rho GTPases cycle between an active GTP-bound state and an inactive GDP-bound state (Etienne-Manneville and Hall, 2002) (Fig. 2A). The activated GTPases interact with downstream effectors, such as kinases, as well as actin and microtubule cytoskeletal regulators, to control a variety of cellular processes. Guanine-nucleotide-exchange factors (GEFs), which catalyze the exchange of GDP for GTP, activate Rho GTPase signaling (García-Mata and Burridge, 2007; Schmidt and Hall, 2002). By contrast, Rho proteins are inactivated by GTPase-activating proteins (GAPs), which catalyze the hydrolysis of GTP to GDP (Lamarche and Hall, 1994; Moon and Zheng, 2003). The inactive Rho GTPases can subsequently be sequestered in the cytoplasm in their GDP-bound state through interactions with the guanine-nucleotide-dissociation inhibitors (GDIs) (Garcia-Mata et al., 2011; Nobes and Hall, 1994; Olofsson, 1999). To regulate complicated cellular events, such as cell polarity, the localization and signaling of these GTPases has to be tightly controlled. Indeed, at least 80 GEFs (Meller et al., 2005; Rossman et al., 2005; Schmidt and Hall, 2002) and 50 GAPs (Peck et al., 2002) have been identified in the human genome, suggesting the presence of a complex GTPase–GEF–GAP network in the cell. The subcellular localization and function of these crucial Rho GTPase regulators on epithelial homeostasis and apical–basal polarity are just starting to become appreciated (Fig. 2B). Importantly, in cancer, a disease that is highly related to loss of cell polarity, altered Rho GTPase signaling is very common, primarily owing to mutations or aberrant expression of Rho GTPase regulators (Cook et al., 2013), although mutations and especially altered expression of Rho GTPases themselves have also been observed (Grise et al., 2009; Kawazu et al., 2013).
Fig. 2.
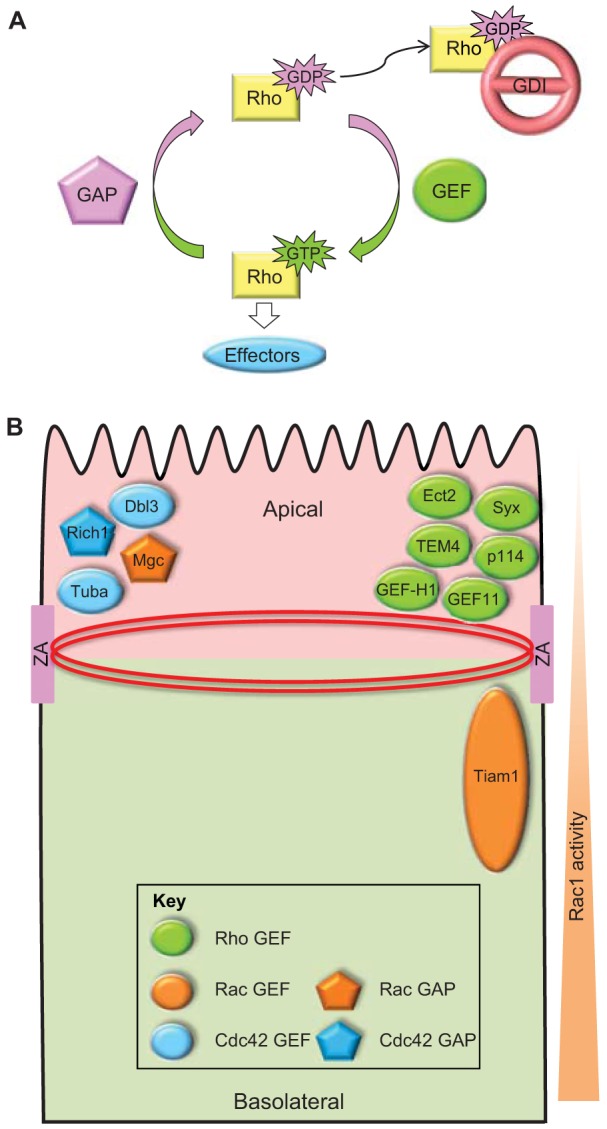
The activation of Rho family GTPases is spatio-temporally controlled by regulatory molecules. (A) Rho family GTPases, including RhoA, Rac1 and Cdc42 cycle between the active GTP-bound and the inactive GDP-bound states. Once activated, Rho GTPases interact with downstream effectors, which lead to diverse signaling pathways and biological processes, such as cytoskeletal organization, cell junction formation, cell polarization and cell motility. The on–off cycle of Rho family GTPases is tightly controlled by three classes of regulatory proteins: GEFs catalyze the exchange of GDP for GTP; GAPs enhance GTPase activity to hydrolyze GTP back to GDP; and GDIs associate with GDP-bound Rho GTPases and prevent nucleotide dissociation. (B) In polarized mammalian epithelial cells, several GEFs and GAPs have been shown to localize either apically at mature junctions (zonula adherens, ZA) that encompass the circumferential actomyosin ring or to sub-apical areas of cell–cell contact. Localization of the Cdc42 GEF Tuba at apical junctions activates Cdc42 to promote junctional actin organization (Otani et al., 2006) and lumenogenesis (Bryant et al., 2010; Qin et al., 2010). Interestingly, the Cdc42 GAP Rich1, which also localizes to apical junctions, is required for junction integrity (Wells et al., 2006). A number of RhoA GEFs have been identified at apical junctions. Specifically, Ect2, Syx, p114RhoGEF (p114), TEM4 and ARHGEF11 (GEF11) promote the localization and activity of RhoA at the ZA to support junction integrity (Ngok et al., 2012; Ratheesh et al., 2012; Terry et al., 2011; Itoh et al., 2012; Ngok et al., 2013). In contrast, GEF-H1-regulated RhoA activity is negatively controlled to prevent junction disassembly (Aijaz et al., 2005; Guillemot et al., 2008; Samarin et al., 2007). Although the precise localization of activated RhoA and Cdc42 in polarized epithelial cells is still unclear, an apical–basal gradient of Rac1 activity has been observed with higher Rac1 activity at more basal regions due to the localized activation of the RacGEF TIAM1, which is required for proper junction formation (Mack et al., 2012). Consistent with this, MgcRacGAP localizes and acts apically (Guillemot et al., 2014). Collectively, the studies support a key role for the precise spatio-temporal activation of Rho family GTPases in junction formation and epithelial organization.
Early studies implicated RhoA and Rac1 in the structural regulation of tight junctions and cell polarization (Jou and Nelson, 1998; Jou et al., 1998). Cdc42 was also postulated to support polarization through the promotion of adherens junction formation and stability (Fukuhara et al., 2003; Kodama et al., 1999). Recent investigations further confirm the importance of Rho GTPases in epithelial polarization. GTP-Rac1 is necessary for the activation of Par-complex-associated signaling to initiate tight junction morphogenesis and epithelial polarization (Johansson et al., 2000; Mack et al., 2012; Mertens et al., 2005; Noda et al., 2001). Active Cdc42, which also binds members of the Par complex, is involved in junction formation, apical–basal polarization and acini lumen formation in three-dimensional cultures (Bray et al., 2011; Fukuhara et al., 2003; Johansson et al., 2000; Lin et al., 2000; Qiu et al., 2000; Yamanaka et al., 2001). The specific mechanisms that are regulated by RhoA are somewhat unclear, but its function in mediating actomyosin contractility and in generating the cortical actomyosin ring during polarization indicates that it is indispensable for epithelial polarization (Ivanov et al., 2005; Vaezi et al., 2002).
Owing to the complexity of polarity-complex-mediated signaling and the broad spectrum of proteins involved, this Commentary will focus on summarizing how polarity complexes regulate the apical–basal polarization of mammalian epithelial cells by influencing Rho GTPase signaling. Specific physical and functional interactions of the three main polarity complexes with regulators of Rho GTPases will be reviewed, and their link to RhoA, Rac1 or Cdc42 signaling will be highlighted. The picture emerging from these studies suggests that polarity complexes differentially affect Rho GTPase activation in a spatiotemporal manner, leading to the establishment and maintenance of cellular asymmetry.
Polarity complexes and their associated GEFs and GAPs
The Par complex – Tiam1, Ect2 and p190RhoGAP
The Par complex, which consists of core members Par3, Par6 and aPKC (the two mammalian isozymes PKCζ and PKCι), is perhaps the best-studied polarity complex. These members form a tripartite complex or duo complexes, which are regulated by protein phosphorylation and the activation of small GTPases (McCaffrey and Macara, 2009) (Fig. 1). In mammalian cells, the Par complex is found primarily at the tight junctions and promotes a broad range of tight-junction- and polarity-related cellular events. Importantly, its activation of Rho family GTPase signaling is considered a major cue for initiating epithelial polarization.
Initially discovered as crucial proteins for proper partitioning of germline-specific P-granules in embryos of C. elegans (Kemphues et al., 1988), the Par proteins are highly conserved molecules that are required for establishing mammalian cell polarity (Kemphues, 2000; Macara, 2004). Par3 self-associates (Mizuno et al., 2003), binds the tight junction adhesion molecules JAM-A, JAM-B and JAM-C (Ebnet et al., 2003; Ebnet et al., 2001), and can interact directly with aPKC (Horikoshi et al., 2009; Hung and Kemphues, 1999; Izumi et al., 1998; Joberty et al., 2000; Lin et al., 2000). Several studies have provided evidence showing that the phosphorylation of Par3 by aPKC is crucial for the establishment of tight junctions and cell polarity. Overexpression of a phospho-deficient Par3 mutant results in cell polarity defects (Nagai-Tamai et al., 2002), and the induced-expression of an aPKC-binding-deficient Par3 mutant fails to facilitate tight junction formation (Hirose et al., 2002). Consistent with these findings, depletion of Par3 disrupts epithelial cell morphogenesis in three-dimensional cell culture, whereas the introduction of a wild-type Par3, but not an aPKC-uncoupled Par3 mutant, rescues the observed defect (Horikoshi et al., 2009). A recent study has revealed the function of an additional Par3-binding partner, the FERM-domain protein willin (also known as FRMD6) (Ishiuchi and Takeichi, 2011), in defining the apical domain of epithelial cells.
Similar to Par3, Par6 and aPKC are involved in tight junction assembly and regulation of cell polarity (Gao et al., 2002; Joberty et al., 2000; Lemmers et al., 2004; Nagai-Tamai et al., 2002; Suzuki et al., 2002; Yamanaka et al., 2001). Localization of Par6 and activation of aPKC are controlled by Cdc42 (Henrique and Schweisguth, 2003), whose apical enrichment and activation is controlled by the Cdc42 GEF Tuba (also known as DNMBP and ARHGEF36) (Otani et al., 2006). Upon the enrichment of phosphatidylinositol 4,5-bisphosphate [Ptdln(4,5)P2] at the apical domain, activated Cdc42 recruits Par6 and aPKC to initiate polarity establishment and lumenogenesis as shown in three-dimensional culture (Martin-Belmonte et al., 2007). Par6 forms a complex with aPKC (Joberty et al., 2000) and can directly bind Crb3 (a main component of the Crumbs complex), an epithelial-specific event that is proposed to be important for regulating the morphogenesis of the apical membrane (Lemmers et al., 2004). Par6 also binds Pals1 (also known as Mpp5), another member of the Crumbs complex, allowing functional crosstalk between these two polarity complexes (Hurd et al., 2003; Wang et al., 2004). Highlighting its role in actin reorganization, the association of Cdc42 to Par6 regulates adherens junction remodeling in Drosophila through the Arp2/3 complex (Georgiou et al., 2008). A major function of aPKC in the formation of cell polarity is mediated by its kinase activity (Suzuki et al., 2002), which is likely activated by the binding of active Cdc42 to Par6 (Joberty et al., 2000; Lin et al., 2000; Yamanaka et al., 2001). In addition to phosphorylating Par3 (Nagai-Tamai et al., 2002), aPKC phosphorylates Par1/MARK (Hurov et al., 2004; Suzuki et al., 2004) and the Notch antagonist Numb (Smith et al., 2007) to exclude these two proteins from areas along the cell membrane where aPKC localizes and to establish cellular asymmetry. In addition to cell polarity proteins, aPKC phosphorylates JAM-A to regulate the function of tight junctions (Iden et al., 2012), suggesting that the kinase activity of aPKC is crucial in multiple steps involved in tight junction assembly and apical–basal polarization.
Although not a focus of this review, other members in the Par family that are conserved throughout Metazoa, including the serine/threonine kinases Par1/MARK (Elbert et al., 2006; Guo and Kemphues, 1995; Ossipova et al., 2007) and Par4/LKB1 (Baas et al., 2004; Gloerich et al., 2012; Partanen et al., 2007; Tanwar et al., 2012; ten Klooster et al., 2009) and the regulatory molecule Par5/14-3-3 (Ling et al., 2010; Watkins et al., 2008; Winter et al., 2012), are also important regulators of the localization, formation, and function of the Par complex as well as the establishment of cell polarity.
T-cell lymphoma invasion and metastasis 1 (Tiam1), a Rac1-specific GEF, is an established regulator of cell adhesion and epithelial polarity that associates with the Par complex (Lambert et al., 2002; Mertens et al., 2006). The recruitment of Tiam1 to the Par complex by Par3 is required for tight junction biogenesis (Chen and Macara, 2005; Mertens et al., 2005). Interestingly, these studies have shown that either impaired Tiam1–Rac1 signaling (Mertens et al., 2005) or constitutive Rac1 activation due to Par3 knockdown results in deficient tight junction assembly (Chen and Macara, 2005). The conflicting results might be reconciled by the observation that a Tiam1-mediated active Rac1 gradient along areas of cell–cell contact is essential for maintaining proper organization of epithelial monolayers, and is controlled by Par3 and a complex containing β2-syntrophin (Mack et al., 2012) (Fig. 2B). The data suggest that binding of activated Cdc42 to the Par complex and subsequent fine-tuning of Rac1 activation by Par3-bound Tiam1 are crucial events for the initiation and maintenance of epithelial polarity (Chen and Macara, 2005; Joberty et al., 2000; Mertens et al., 2005; Nishimura et al., 2005). In addition to Tiam1, the Rho GEF protein epithelial cell transforming sequence 2 (Ect2), which can activate RhoA, Rac1 and Cdc42 activity in vitro, also interacts with the Par complex through Par6 and has been proposed to influence epithelial polarity by increasing Rac1 and Cdc42 activity (Liu et al., 2004; Liu et al., 2006). Combined, these studies indicate that the Par complex affects localized activation of Rho family GTPases, especially that of Rac1 (Fig. 3), to promote the formation of tight junctions and to establish the boundaries of the apical and basolateral membrane domains in polarized epithelial cells.
Fig. 3.
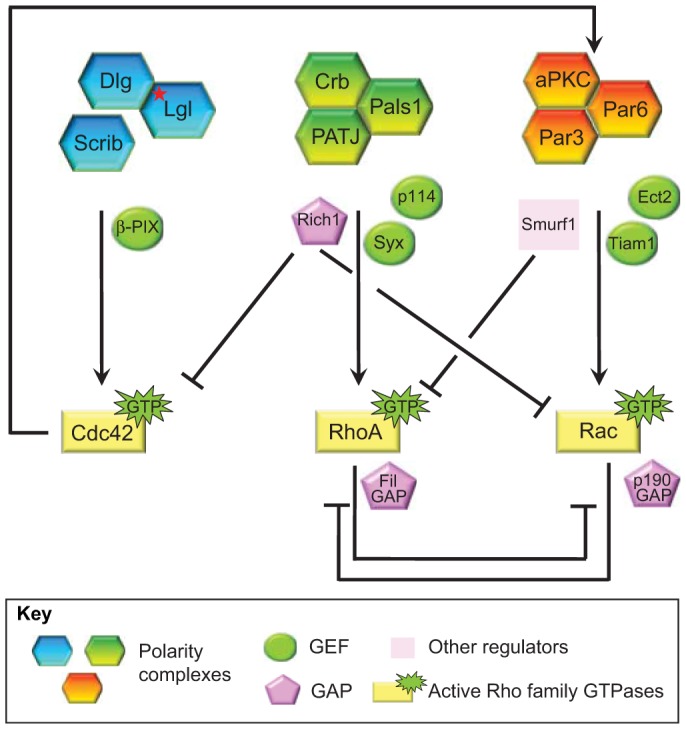
Crosstalk between polarity complexes and Rho family GTPases. The Scribble polarity complex is coupled to Cdc42 activation through the Rac and Cdc42 GEF β-PIX (Osmani et al., 2006). Upon Cdc42 activation, the Par complex mediates the activation of Rac1 through the Rac GEF Tiam1 (Mack et al., 2012; Mertens et al., 2005). Rac1 activation can also be induced by the Par complex by the Rho GEF Ect2 (Liu et al., 2004). In response to TGF-β stimulation, the Par complex interacts with the E3 ubiquitin ligase Smurf1, resulting in RhoA degradation (Ozdamar et al., 2005). By contrast, the Crumbs complex recruits Rho GEFs to the apical domain to signal RhoA activation. For instance, p114RhoGEF (p114) is recruited by PATJ to cell–cell junctions (Nakajima and Tanoue, 2011; Terry et al., 2011), whereas the Rho GEF Syx is recruited to cell junctions by Mupp1 (Ngok et al., 2012), a paralog of PATJ, resulting in the local activation of RhoA and downstream signaling. The Crumbs complex also interacts with the Rac and Cdc42 GAP Rich1 through the Pals1–Amot complex and downregulates the activities of Rac1 and Cdc42 (Wells et al., 2006; Yi et al., 2011). Although they might not directly associate with polarity complexes, p190RhoGAP and FilGAP can mediate mutual antagonism of RhoA and Rac1 signaling downstream of polarity complexes.
Currently, no mammalian Rho GAP has been shown to physically associate with the Par complex. Nevertheless, the C. elegans Rho GAP PAC-1, which resides at cell contacts, has been shown to restrict Par6 localization to contact-free areas to regulate radial polarization during gastrulation (Anderson et al., 2008). Moreover, RhoA activity is antagonized by p190RhoGAP (also known as ARHGAP35), which is recruited by activated Rac1 to cell–cell junctions (Nimnual et al., 2003; Wildenberg et al., 2006). p190RhoGAP has been proposed to inactivate RhoA downstream of Par6–aPKC in dendritic spines (Zhang and Macara, 2008). Given the presence of a GTP-Rac1 gradient, where incremental Rac1 activity is found towards sub-apical junctions along the axis of cell–cell contact (Mack et al., 2012), it is probable that Rac1 and p190RhoGAP restrict RhoA activity towards the apical junctions to help define the apical domain in polarized epithelial cells (Fig. 3). Furthermore, Par6 can target the degradation of RhoA by the E3 ubiquitin ligase Smurf1 downstream of transforming growth factor β (TGF-β) signaling at the tight junction (Ozdamar et al., 2005), providing an additional mechanism to limit the local availability of RhoA (Fig. 3).
The Crumbs complex – Syx, p114RhoGEF, Rich1 and FilGAP
Similar to the Par complex, the Crumbs polarity complex (Crb–Pals1–PATJ) participates in the development of functional tight junctions and apical–basal polarity in epithelial monolayers. Early studies in Drosophila described Crumbs as an epidermal growth factor (EGF)-like protein and indicated its importance in the development and organization of epithelia (Tepass et al., 1990; Wodarz et al., 1993; Wodarz et al., 1995). Crumbs is important for the proper distribution of Drosophila E-cadherin and Armadillo (the ortholog of β-catenin) in Drosophila epithelia (Grawe et al., 1996; Klebes and Knust, 2000). In mammals, three members of the Crumbs family have been identified (den Hollander et al., 2002; den Hollander et al., 1999; Makarova et al., 2003; van den Hurk et al., 2005); among them, Crb3, is the most widely expressed member in mammalian epithelial cells, and is localized at the apical region of polarized epithelial cells (Lemmers et al., 2004; Makarova et al., 2003). Snail-mediated disruption of Crb3 expression alters cell polarity, and overexpression of Crb3 induces formation of tight junctions in cells that do not normally do so (Fogg et al., 2005; Whiteman et al., 2008). Importantly, Crb3-knockout mice die soon after birth and exhibit severe defects in epithelial morphogenesis, lung development and perturbed organization of multiple organs (Whiteman et al., 2014).
Protein associated with Lin Seven 1 (Pals1), a linker protein that interacts directly with Crb3, is also necessary for proper epithelial polarization and acini formation of cells grown in three-dimensional culture (Makarova et al., 2003; Roh et al., 2003; Roh et al., 2002b; Straight et al., 2004). Consistent with the Drosophila studies showing that Stardust (the ortholog of Pals1), together with Bazooka (the ortholog of Par3) and Armadillo (the ortholog of β-catenin), is necessary for adherens junction assembly and cell polarity (Krahn et al., 2010; Müller and Wieschaus, 1996), mammalian Pals1 is involved in adherens junction maintenance by mediating E-cadherin delivery to the cell surface (Wang et al., 2007). Pals1-associated tight junction protein (PATJ), the third member of the Crumbs polarity complex, is a scaffolding protein that is targeted to the tight junctions by zona occludens-3 (ZO-3, also known as TJP3) and recruits Pals1 to the junctions (Lemmers et al., 2002; Roh et al., 2002a). Harboring multiple postsynaptic density/discs large/zonula occludens (PDZ) domains, PATJ associates with nectins, JAM-A and claudin-1 as well as ZO-3 (Adachi et al., 2009; Lemmers et al., 2002; Roh et al., 2002a; Roh et al., 2002b). In Drosophila, Patj is required for embryo viability, and plays a supportive role in cell polarity by regulating the localization of polarity proteins and the positioning and stability of adherens junctions (Nam and Choi, 2006; Pénalva and Mirouse, 2012; Sen et al., 2012). Moreover, disrupted adhesion, defective cyst formation, and mis-localized polarity proteins and receptors are observed in PATJ-depleted mammalian epithelial cells (Latorre et al., 2005; Michel et al., 2005; Shin et al., 2005). Multiple PDZ domain protein 1 (Mupp1, also known as Mpdz), a paralog of PATJ, also binds Pals1 and several other tight junction proteins including claudins and JAMs (Hamazaki et al., 2002; Poliak et al., 2002). Although its function is not well understood, Mupp1 is expressed in brain cells, endothelial cells and numerous other cell types that lack PATJ expression and is frequently mutated in human disease (Al-Dosari et al., 2013; Shirley et al., 2004).
Synectin-binding RhoA exchange factor (Syx, also known as PLEKHG5), a Rho-specific GEF that is implicated to play a role in angiogenesis (Garnaas et al., 2008; Liu and Horowitz, 2006), interacts with PATJ and Mupp1 (Ernkvist et al., 2009; Estévez et al., 2008; Ngok et al., 2012) and colocalizes with members of the Crumbs complex in primary endothelial cells. Syx-mediated RhoA activation promotes endothelial junction integrity and barrier function in vitro and in vivo, at least in part by regulating the levels of VE-cadherin at the plasma membrane (Ngok et al., 2012). The role of Syx in epithelial cell polarity is unclear; however, it plays an important role in the directed cell migration and front–rear polarization of migrating brain and breast tumor cells (Dachsel et al., 2013), as well as in oligodendrocyte progenitor cells (OPCs) along white matter tracks (Binamé et al., 2013). Importantly, in the latter case, Crumbs and Par polarity complexes are recruited by OPCs that express the proteoglycan NG2 (also known as CSPG4), resulting in RhoA-to-Rac1 signaling through Tiam1 (Binamé et al., 2013). This study highlights the significance of functional interactions and crosstalk between different polarity complexes.
Two recent studies have shown that p114RhoGEF (also known as ARHGEF18) associates with two distinct protein complexes – one containing PATJ and Lulu2 (also known as EPB41L4B), the other composed of myosin II, cingulin and Rho kinase 2 (ROCK2) (Nakajima and Tanoue, 2011; Terry et al., 2011). It is unclear whether the interaction of p114RhoGEF with each complex is mutually exclusive, and whether the two complexes have cross-regulatory functions. However, the Lulu2–PATJ complex is involved in p114RhoGEF activation that is regulated by aPKC phosphorylation to control the circumferential actomyosin belt of polarized epithelial cells (Nakajima and Tanoue, 2011), whereas the other complex has been suggested to drive spatially restricted RhoA signaling to regulate epithelial junction formation and acini morphogenesis in three-dimensional culture (Terry et al., 2011). The identification of Syx and p114RhoGEF, both of which bind PATJ and promote cell–cell cohesion through RhoA, underscores the importance of RhoA activity in epithelial organization and suggests that the Crumbs complex defines the apical domain through RhoA signaling (Fig. 3).
In addition to exerting its function by activating RhoA, the Crumbs complex might facilitate and maintain apical–basal domain specification by excluding Rac1 and Cdc42 activities from the apical region through GAPs. Indeed, the Rac1 and Cdc42 GAP Rich1 (also known as ARHGAP17) is recruited to the Crumbs complex by angiomotin (Amot) to maintain tight junction stability by fine-tuning local Cdc42 activity (Wells et al., 2006). Alternatively, Rich1 is released from Amot by competitive binding of the Merlin/NF2 tumor suppressor, leading to a reduction of local Rac1 activity (Yi et al., 2011). Other means to suppress apical Rac1 activity are through the junctional male germ cell (Mgc) Rac GAP (also known as RacGAP1), which is part of the Ect2 complex, or through the Rac-specific filament A-binding GAP (FilGAP, also known as ARHGAP24), which is activated downstream of RhoA signaling and ROCK-mediated phosphorylation (Guillemot et al., 2014; Ohta et al., 2006; Ratheesh et al., 2012). Moreover, apical Rac GAP chimaerins CHN1 and CHN2 have been reported to inhibit Rac1 activity to control epithelial cyst morphogenesis (Yagi et al., 2012). Finally, additional Rho GEFs, i.e. TEM4 (also known as ARHGEF17) (Ngok et al., 2013), ARHGEF11 (Itoh et al., 2012) and GEF-H1 (also known as ARHGEF2) (Aijaz et al., 2005; Benais-Pont et al., 2003) localize apically and regulate junction integrity (TEM4 and ARHGEF11) and paracellular permeability (GEF-H1). These findings converge on RhoA signaling and exclusion of Rac1 and Cdc42 activity at the apical domain, suggesting that gradients of GTPase activities might exist in polarized cells to establish and maintain the epithelial architecture.
The Scribble complex – β-Pix and GIT1
The Scribble complex, which is composed of Scribble, Discs large (Dlg), and Lethal giant larvae (Lgl), is commonly found at the lateral domain of the plasma membrane and is the least well understood polarity complex in the context of mammalian cell–cell contacts. Studies of these proteins in Drosophila reveal mutant phenotypes, such as loss of epithelial cell polarity and disruption of tissue integrity, suggesting that functionally they belong to the same genetic pathway (Betschinger et al., 2003; Bilder et al., 2000; Bilder and Perrimon, 2000; Gateff, 1978; Mechler et al., 1985; Peng et al., 2000; Tanentzapf and Tepass, 2003; Woods et al., 1996). It is important to reiterate that although the three components are proposed to form a complex, evidence of their physical interaction is still limited, although emerging data support this possibility.
In mammalian epithelial cells, Scribble plays a role in hepatocyte growth factor (HGF)-induced epithelial tubulogenesis in three-dimensional culture (Eastburn et al., 2012), and its targeting to the cell membrane might depend on the interaction with ZO-1 and ZO-2 (also known as TJP1 and TJP2, respectively) (Ivanov et al., 2010; Métais et al., 2005) as well as β-catenin (Yates et al., 2013) and the presence of E-cadherin (Navarro et al., 2005). Scribble also stabilizes E-cadherin, α- and β-catenin at the cell border, and the depletion of Scribble increases E-cadherin endocytosis by decreasing E-cadherin–p120-catenin interactions, resulting in perturbed cell–cell adhesion (Lohia et al., 2012; Qin et al., 2005). Interestingly, Scribble has been shown to localize at both the apical mature junctions and at basolateral areas of cell–cell contact (Dow et al., 2003; Ivanov et al., 2010), suggesting that distinct Scribble complexes might exist and function in different membrane compartments to regulate epithelial monolayer organization. Moreover, Scribble knockdown in low cell density culture results in altered epithelial morphogenesis, whereas at higher cell density it influences adherens junction structure and delays tight junction formation without affecting the establishment and maintenance of apical–basal polarity (Qin et al., 2005). In vivo studies suggest that the function of Scribble in mammals might be different from that in Drosophila where it is crucial for epithelial cell polarity, and might be more relevant to planar cell polarity (PCP) and wound closure, processes related to organ patterning and front–rear polarity, respectively (Dow et al., 2007; Montcouquiol et al., 2003; Murdoch et al., 2003). Indeed, Scribble is required for proper lung morphogenesis through the regulation of the PCP protein Vangl2 (Yates et al., 2013).
Dlg has been shown to colocalize with Scribble (Dow et al., 2003). Although their direct physical interaction has not been confirmed (Fig. 1), it has been reported that Dlg and Scribble are linked by GUK-holder at Drosophila synapses (Mathew et al., 2002). In mammals, Dlg has further been found to colocalize with E-cadherin along the lateral membrane of polarized intestinal epithelial cells, and its expression is important for adherens junction and tight junction integrity (Laprise et al., 2004; Stucke et al., 2007). In mouse embryos, Dlg3 is needed for apical–basal polarity and PCP processes (Van Campenhout et al., 2011). Moreover, the localization and function of Dlg can be modulated by its phosphorylation as demonstrated in Drosophila neuromuscular junctions and other systems (Johnston et al., 2009; Koh et al., 1999; Mantovani and Banks, 2003; Narayan et al., 2009; Sabio et al., 2005; Wang et al., 2011; Zhang et al., 2007).
Lgl is thought to bind Scribble (Kallay et al., 2006), and its membrane localization is dependent on its phosphorylation status (Hutterer et al., 2004; Müsch et al., 2002; Wirtz-Peitz and Knoblich, 2006). Moreover, phosphorylation of Lgl2 allows its direct physical interaction with Dlg4 (Zhu et al., 2014) (see Fig. 1). Demonstrated in the Drosophila system, the most prominent role of Lgl in the establishment of apical–basal polarity comes from its interaction with Par6–aPKC in a Par3-exclusive manner (Betschinger et al., 2003; Yamanaka et al., 2003). Through the association with Par6, aPKC phosphorylates and displaces Lgl from Par6 (Hutterer et al., 2004; Yamanaka et al., 2003). Phosphorylated Lgl is then free to associate with the Scribble complex at the basolateral membrane, and Par6–aPKC can interact with Par3 to form the Par complex. Consistent with a key role for Lgl in the establishment of apical–basal polarity, downregulation of Lgl compromises tight junction disassembly and leads to the retention of apical protein accumulation upon cell depolarization, whereas Lgl overexpression results in loss of tight junctions in vertebrate epithelial cells (Chalmers et al., 2005; Yamanaka et al., 2006). Phosphorylation of Lgl by the Par6–aPKC complex is also important in front–rear polarization (Plant et al., 2003). Together with the observation that depletion of Lgl increases Par3 and Par6–aPKC association at the apical domain, these findings suggest an antagonistic relationship between Lgl and aPKC (Grifoni et al., 2007; Yamanaka et al., 2003). Furthermore, Par6–aPKC-associated Lgl is proposed to be inactive (Betschinger et al., 2005), whereas basolaterally localized Lgl is required for maintaining proper PATJ and Par6 localization at the apical region (Hutterer et al., 2004). Combined, the data suggest that mutual inhibition of the Crumbs–Scribble and Par–Scribble complexes is critical for defining apical and basolateral zones in epithelial cells.
In mammalian cells, Scribble associates with the Rac and Cdc42 GEF β-Pix (also known as ARHGEF7) and the Arf GAP G-protein-coupled receptor kinase-interacting protein 1 (GIT1) (Audebert et al., 2004), and depletion of either Scribble or β-Pix inhibits three-dimensional epithelial remodeling (Eastburn et al., 2012). Moreover, Scribble regulates the polarization of migrating astrocytes by targeting β-Pix and Cdc42 activation to the leading edge to mediate directed cell migration in vitro (Osmani et al., 2006).
Given the distinct functions that polarity complexes exert to generate polarization in different cell types (Iden and Collard, 2008), it is conceivable that Scribble might also act independently of Cdc42 in some contexts. GIT1 is a β-Pix-binding GAP that inactivates ARF6 (Vitale et al., 2000; Zhao et al., 2000), an ADP-ribosylation factor GTPase that negatively regulates both adherens junction and tight junction assembly and dynamics through endocytosis of junctional components (Klein et al., 2008; Luton et al., 2004; Palacios et al., 2001; Palacios et al., 2002). Scribble-associated GIT1 might therefore suppress the activation of ARF6 at the membrane (Macia et al., 2004; Miura et al., 2009) to reduce recycling of junctional proteins and preserve cell–cell cohesion as well as apical–basal polarity.
Conclusions and perspectives
Through their effects on the cytoskeleton, Rho GTPases are key players in cell adhesion, directed migration and cell polarity. The link between the conserved polarity complexes and RhoA, Rac1, and Cdc42 provides the means for the specification of distinct sub-domains at the plasma membrane and the establishment of cellular asymmetry. Through the regulation of GEFs and GAPs, the Crumbs complex is coupled with RhoA signaling at the most apical domain, whereas a gradually increasing gradient of Rac1 and possibly Cdc42 activities is directed towards the sub-apical and lateral zones along the axis of epithelial cell–cell contact. This functional differentiation of the apical and basolateral membrane domains is further potentiated by the well-established negative crosstalk between RhoA and Rac1, which results in mutual exclusion of RhoA and Rac1 activities. Indeed, by acting through distinct Rho GTPases, polarity complexes differentially affect cytoskeletal organization in apical and basolateral membrane subdomains. Basolateral activation of Rac1 and Cdc42 results in actin polymerization and membrane protrusive activity that is crucial for directed cell migration, for example, upon cell wounding, and for the formation of nascent cell–cell junctions. Apical activation of RhoA, by contrast, leads to formin-mediated formation of the circumferential actin ring and Rho kinase-induced contractility, key events in the organization of epithelial monolayers. It is important, however, to note that the picture is likely to be significantly more complex. This probably accounts for the large number of Rho GTPase regulators at the cell–cell junctions. Polarity proteins exist in multiple complexes that could significantly affect local Rho GTPase signaling. For example, apical Cdc42 activation is required for brush border formation (Zihni et al., 2014), but suppression of Cdc42 activity at the apical mature junctions is important for suppression of endocytic degradation of key junctional components leading to increased junctional integrity. Importantly, polarity complexes might also affect microtubule organization, exocyst function, endocytosis, apoptosis and nuclear signaling through Rho GTPases, all of which play important roles in epithelial organization and apical–basal polarity.
Disrupting the balance of Rho GTPase signaling in quiescent polarized epithelial cells might rewire a number of signaling pathways, such as pro-migratory and proliferative signaling, in the case of epithelial–mesenchymal transition (EMT) as well as tumorigenesis, or senescence and apoptotic pathways, in a normal physiological context. Despite the recent progress on elucidating the role of Rho GTPases in cell polarization, our current understanding of the physical and functional composition of polarity complexes, their crosstalk, and the role of specific GEFs and GAPs on cell polarity is very limited. How subgroups of related GEFs and GAPs (and perhaps GDIs) cooperate or antagonize each other to regulate Rho GTPase signaling at distinct locations in epithelial cells is also unclear.
Despite the close relationship between polarity complexes and Rho family GTPases, polarity proteins can also function in a GTPase-independent manner, for example, through regulation of protein phosphorylation, their own scaffolding properties and the recruitment of key polarity effectors. All three polarity complexes can regulate the Hippo pathway to control cell growth (Chen et al., 2010; Grzeschik et al., 2010; Robinson et al., 2010; Varelas et al., 2010; Skouloudaki et al., 2009). Moreover, both aPKC and Drosophila Crumbs can modulate Notch signaling, with important implications in cell proliferation and the specification of tissue boundaries (Herranz et al., 2006; Richardson and Pichaud, 2010; Smith et al., 2007). Par3 can directly or indirectly modulate the dynamics and organization of microtubules (Chen et al., 2013; Schmoranzer et al., 2009). Finally, polarity proteins interact with adaptor or scaffolding proteins as well as transmembrane receptors, independently of Rho GTPases, to modulate cell polarity (Hayase et al., 2013; Qin et al., 2005; Yang et al., 2012).
There are many gaps in knowledge that need to be filled in order to gain a better understanding of the normal function of polarity complexes and their deregulation in human disease, such as cancer. Furthermore, integrating crosstalk and connecting antagonistic signaling with specific cellular responses should be one of the major long-term goals in the field.
Supplementary Material
Footnotes
Competing interests
The authors declare no competing interests.
Funding
The work of our laboratories was supported by National Institutes of Health (to P.Z.A.); and the Mayo Graduate School (to S.P.N.). Deposited in PMC for release after 12 months.
References
- Adachi M., Hamazaki Y., Kobayashi Y., Itoh M., Tsukita S., Furuse M., Tsukita S. (2009). Similar and distinct properties of MUPP1 and Patj, two homologous PDZ domain-containing tight-junction proteins. Mol. Cell. Biol. 29, 2372–2389 10.1128/MCB.01505-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams C. L., Nelson W. J. (1998). Cytomechanics of cadherin-mediated cell-cell adhesion. Curr. Opin. Cell Biol. 10, 572–577 10.1016/S0955-0674(98)80031-8 [DOI] [PubMed] [Google Scholar]
- Adams C. L., Chen Y. T., Smith S. J., Nelson W. J. (1998). Mechanisms of epithelial cell-cell adhesion and cell compaction revealed by high-resolution tracking of E-cadherin-green fluorescent protein. J. Cell Biol. 142, 1105–1119 10.1083/jcb.142.4.1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aijaz S., D'Atri F., Citi S., Balda M. S., Matter K. (2005). Binding of GEF-H1 to the tight junction-associated adaptor cingulin results in inhibition of Rho signaling and G1/S phase transition. Dev. Cell 8, 777–786 10.1016/j.devcel.2005.03.003 [DOI] [PubMed] [Google Scholar]
- Al-Dosari M. S., Al-Owain M., Tulbah M., Kurdi W., Adly N., Al-Hemidan A., Masoodi T. A., Albash B., Alkuraya F. S. (2013). Mutation in MPDZ causes severe congenital hydrocephalus. J. Med. Genet. 50, 54–58 10.1136/jmedgenet-2012-101294 [DOI] [PubMed] [Google Scholar]
- Anderson D. C., Gill J. S., Cinalli R. M., Nance J. (2008). Polarization of the C. elegans embryo by RhoGAP-mediated exclusion of PAR-6 from cell contacts. Science 320, 1771–1774 10.1126/science.1156063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apodaca G., Gallo L. I., Bryant D. M. (2012). Role of membrane traffic in the generation of epithelial cell asymmetry. Nat. Cell Biol. 14, 1235–1243 10.1038/ncb2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audebert S., Navarro C., Nourry C., Chasserot-Golaz S., Lécine P., Bellaiche Y., Dupont J. L., Premont R. T., Sempéré C., Strub J. M. et al. (2004). Mammalian Scribble forms a tight complex with the betaPIX exchange factor. Curr. Biol. 14, 987–995 10.1016/j.cub.2004.05.051 [DOI] [PubMed] [Google Scholar]
- Baas A. F., Kuipers J., van der Wel N. N., Batlle E., Koerten H. K., Peters P. J., Clevers H. C. (2004). Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell 116, 457–466 10.1016/S0092-8674(04)00114-X [DOI] [PubMed] [Google Scholar]
- Benais-Pont G., Punn A., Flores-Maldonado C., Eckert J., Raposo G., Fleming T. P., Cereijido M., Balda M. S., Matter K. (2003). Identification of a tight junction-associated guanine nucleotide exchange factor that activates Rho and regulates paracellular permeability. J. Cell Biol. 160, 729–740 10.1083/jcb.200211047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betschinger J., Mechtler K., Knoblich J. A. (2003). The Par complex directs asymmetric cell division by phosphorylating the cytoskeletal protein Lgl. Nature 422, 326–330 10.1038/nature01486 [DOI] [PubMed] [Google Scholar]
- Betschinger J., Eisenhaber F., Knoblich J. A. (2005). Phosphorylation-induced autoinhibition regulates the cytoskeletal protein Lethal (2) giant larvae. Curr. Biol. 15, 276–282 10.1016/j.cub.2005.01.012 [DOI] [PubMed] [Google Scholar]
- Bilder D., Perrimon N. (2000). Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature 403, 676–680 10.1038/35001108 [DOI] [PubMed] [Google Scholar]
- Bilder D., Li M., Perrimon N. (2000). Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science 289, 113–116 10.1126/science.289.5476.113 [DOI] [PubMed] [Google Scholar]
- Bilder D., Schober M., Perrimon N. (2003). Integrated activity of PDZ protein complexes regulates epithelial polarity. Nat. Cell Biol. 5, 53–58 10.1038/ncb897 [DOI] [PubMed] [Google Scholar]
- Binamé F., Sakry D., Dimou L., Jolivel V., Trotter J. (2013). NG2 regulates directional migration of oligodendrocyte precursor cells via Rho GTPases and polarity complex proteins. J. Neurosci. 33, 10858–10874 10.1523/JNEUROSCI.5010-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray K., Brakebusch C., Vargo-Gogola T. (2011). The Rho GTPase Cdc42 is required for primary mammary epithelial cell morphogenesis in vitro. Small GTPases 2, 247–258 10.4161/sgtp.2.5.18163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant D. M., Datta A., Rodríguez-Fraticelli A. E., Peränen J., Martín-Belmonte F., Mostov K. E. (2010). A molecular network for de novo generation of the apical surface and lumen. Nat. Cell Biol. 12, 1035–1045 10.1038/ncb2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers A. D., Pambos M., Mason J., Lang S., Wylie C., Papalopulu N. (2005). aPKC, Crumbs3 and Lgl2 control apicobasal polarity in early vertebrate development. Development 132, 977–986 10.1242/dev.01645 [DOI] [PubMed] [Google Scholar]
- Chen X., Macara I. G. (2005). Par-3 controls tight junction assembly through the Rac exchange factor Tiam1. Nat. Cell Biol. 7, 262–269 10.1038/ncb1226 [DOI] [PubMed] [Google Scholar]
- Chen C. L., Gajewski K. M., Hamaratoglu F., Bossuyt W., Sansores-Garcia L., Tao C., Halder G. (2010). The apical-basal cell polarity determinant Crumbs regulates Hippo signaling in Drosophila. Proc. Natl. Acad. Sci. USA 107, 15810–15815 10.1073/pnas.1004060107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Chen J., Shi H., Wei M., Castaneda-Castellanos D. R., Bultje R. S., Pei X., Kriegstein A. R., Zhang M., Shi S. H. (2013). Regulation of microtubule stability and organization by mammalian Par3 in specifying neuronal polarity. Dev. Cell 24, 26–40 10.1016/j.devcel.2012.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook D. R., Rossman K. L., Der C. J. (2013). Rho guanine nucleotide exchange factors: regulators of Rho GTPase activity in development and disease. Oncogene 10.1038/onc.2013.362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dachsel J. C., Ngok S. P., Lewis-Tuffin L. J., Kourtidis A., Geyer R., Johnston L., Feathers R., Anastasiadis P. Z. (2013). The Rho guanine nucleotide exchange factor Syx regulates the balance of dia and ROCK activities to promote polarized-cancer-cell migration. Mol. Cell. Biol. 33, 4909–4918 10.1128/MCB.00565-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hollander A. I., ten Brink J. B., de Kok Y. J., van Soest S., van den Born L. I., van Driel M. A., van de Pol D. J., Payne A. M., Bhattacharya S. S., Kellner U. et al. (1999). Mutations in a human homologue of Drosophila crumbs cause retinitis pigmentosa (RP12). Nat. Genet. 23, 217–221 10.1038/13848 [DOI] [PubMed] [Google Scholar]
- den Hollander A. I., Ghiani M., de Kok Y. J., Wijnholds J., Ballabio A., Cremers F. P., Broccoli V. (2002). Isolation of Crb1, a mouse homologue of Drosophila crumbs, and analysis of its expression pattern in eye and brain. Mech. Dev. 110, 203–207 10.1016/S0925-4773(01)00568-8 [DOI] [PubMed] [Google Scholar]
- Doerflinger H., Vogt N., Torres I. L., Mirouse V., Koch I., Nüsslein-Volhard C., St Johnston D. (2010). Bazooka is required for polarisation of the Drosophila anterior-posterior axis. Development 137, 1765–1773 10.1242/dev.045807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow L. E., Brumby A. M., Muratore R., Coombe M. L., Sedelies K. A., Trapani J. A., Russell S. M., Richardson H. E., Humbert P. O. (2003). hScrib is a functional homologue of the Drosophila tumour suppressor Scribble. Oncogene 22, 9225–9230 10.1038/sj.onc.1207154 [DOI] [PubMed] [Google Scholar]
- Dow L. E., Kauffman J. S., Caddy J., Zarbalis K., Peterson A. S., Jane S. M., Russell S. M., Humbert P. O. (2007). The tumour-suppressor Scribble dictates cell polarity during directed epithelial migration: regulation of Rho GTPase recruitment to the leading edge. Oncogene 26, 2272–2282 10.1038/sj.onc.1210016 [DOI] [PubMed] [Google Scholar]
- Eastburn D. J., Zegers M. M., Mostov K. E. (2012). Scrib regulates HGF-mediated epithelial morphogenesis and is stabilized by Sgt1-HSP90. J. Cell Sci. 125, 4147–4157 10.1242/jcs.108670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebnet K., Suzuki A., Horikoshi Y., Hirose T., Meyer Zu Brickwedde M. K., Ohno S., Vestweber D. (2001). The cell polarity protein ASIP/PAR-3 directly associates with junctional adhesion molecule (JAM). EMBO J. 20, 3738–3748 10.1093/emboj/20.14.3738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebnet K., Aurrand-Lions M., Kuhn A., Kiefer F., Butz S., Zander K., Meyer zu Brickwedde M. K., Suzuki A., Imhof B. A., Vestweber D. (2003). The junctional adhesion molecule (JAM) family members JAM-2 and JAM-3 associate with the cell polarity protein PAR-3: a possible role for JAMs in endothelial cell polarity. J. Cell Sci. 116, 3879–3891 10.1242/jcs.00704 [DOI] [PubMed] [Google Scholar]
- Elbert M., Cohen D., Müsch A. (2006). PAR1b promotes cell-cell adhesion and inhibits dishevelled-mediated transformation of Madin-Darby canine kidney cells. Mol. Biol. Cell 17, 3345–3355 10.1091/mbc.E06-03-0193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernkvist M., Luna Persson N., Audebert S., Lecine P., Sinha I., Liu M., Schlueter M., Horowitz A., Aase K., Weide T. et al. (2009). The Amot/Patj/Syx signaling complex spatially controls RhoA GTPase activity in migrating endothelial cells. Blood 113, 244–253 10.1182/blood-2008-04-153874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estévez M. A., Henderson J. A., Ahn D., Zhu X. R., Poschmann G., Lübbert H., Marx R., Baraban J. M. (2008). The neuronal RhoA GEF, Tech, interacts with the synaptic multi-PDZ-domain-containing protein, MUPP1. J. Neurochem. 106, 1287–1297 10.1111/j.1471-4159.2008.05472.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etemad-Moghadam B., Guo S., Kemphues K. J. (1995). Asymmetrically distributed PAR-3 protein contributes to cell polarity and spindle alignment in early C. elegans embryos. Cell 83, 743–752 10.1016/0092-8674(95)90187-6 [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S., Hall A. (2002). Rho GTPases in cell biology. Nature 420, 629–635 10.1038/nature01148 [DOI] [PubMed] [Google Scholar]
- Fletcher G. C., Lucas E. P., Brain R., Tournier A., Thompson B. J. (2012). Positive feedback and mutual antagonism combine to polarize Crumbs in the Drosophila follicle cell epithelium. Curr. Biol. 22, 1116–1122 10.1016/j.cub.2012.04.020 [DOI] [PubMed] [Google Scholar]
- Fogg V. C., Liu C. J., Margolis B. (2005). Multiple regions of Crumbs3 are required for tight junction formation in MCF10A cells. J. Cell Sci. 118, 2859–2869 10.1242/jcs.02412 [DOI] [PubMed] [Google Scholar]
- Fukuhara A., Shimizu K., Kawakatsu T., Fukuhara T., Takai Y. (2003). Involvement of nectin-activated Cdc42 small G protein in organization of adherens and tight junctions in Madin-Darby canine kidney cells. J. Biol. Chem. 278, 51885–51893 10.1074/jbc.M308015200 [DOI] [PubMed] [Google Scholar]
- Gao L., Joberty G., Macara I. G. (2002). Assembly of epithelial tight junctions is negatively regulated by Par6. Curr. Biol. 12, 221–225 10.1016/S0960-9822(01)00663-7 [DOI] [PubMed] [Google Scholar]
- García-Mata R., Burridge K. (2007). Catching a GEF by its tail. Trends Cell Biol. 17, 36–43 10.1016/j.tcb.2006.11.004 [DOI] [PubMed] [Google Scholar]
- Garcia-Mata R., Boulter E., Burridge K. (2011). The ‘invisible hand’: regulation of RHO GTPases by RHOGDIs. Nat. Rev. Mol. Cell Biol. 12, 493–504 10.1038/nrm3153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnaas M. K., Moodie K. L., Liu M. L., Samant G. V., Li K., Marx R., Baraban J. M., Horowitz A., Ramchandran R. (2008). Syx, a RhoA guanine exchange factor, is essential for angiogenesis in vivo. Circ. Res. 103, 710–716 10.1161/CIRCRESAHA.108.181388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gateff E. (1978). Malignant neoplasms of genetic origin in Drosophila melanogaster. Science 200, 1448–1459 10.1126/science.96525 [DOI] [PubMed] [Google Scholar]
- Georgiou M., Marinari E., Burden J., Baum B. (2008). Cdc42, Par6, and aPKC regulate Arp2/3-mediated endocytosis to control local adherens junction stability. Curr. Biol. 18, 1631–1638 10.1016/j.cub.2008.09.029 [DOI] [PubMed] [Google Scholar]
- Gloerich M., ten Klooster J. P., Vliem M. J., Koorman T., Zwartkruis F. J., Clevers H., Bos J. L. (2012). Rap2A links intestinal cell polarity to brush border formation. Nat. Cell Biol. 14, 793–801 10.1038/ncb2537 [DOI] [PubMed] [Google Scholar]
- Grawe F., Wodarz A., Lee B., Knust E., Skaer H. (1996). The Drosophila genes crumbs and stardust are involved in the biogenesis of adherens junctions. Development 122, 951–959 [DOI] [PubMed] [Google Scholar]
- Grifoni D., Garoia F., Bellosta P., Parisi F., De Biase D., Collina G., Strand D., Cavicchi S., Pession A. (2007). aPKCzeta cortical loading is associated with Lgl cytoplasmic release and tumor growth in Drosophila and human epithelia. Oncogene 26, 5960–5965 10.1038/sj.onc.1210389 [DOI] [PubMed] [Google Scholar]
- Grise F., Bidaud A., Moreau V. (2009). Rho GTPases in hepatocellular carcinoma. Biochim. Biophys. Acta 1795, 137–151 [DOI] [PubMed] [Google Scholar]
- Grzeschik N. A., Parsons L. M., Allott M. L., Harvey K. F., Richardson H. E. (2010). Lgl, aPKC, and Crumbs regulate the Salvador/Warts/Hippo pathway through two distinct mechanisms. Curr. Biol. 20, 573–581 10.1016/j.cub.2010.01.055 [DOI] [PubMed] [Google Scholar]
- Guillemot L., Paschoud S., Jond L., Foglia A., Citi S. (2008). Paracingulin regulates the activity of Rac1 and RhoA GTPases by recruiting Tiam1 and GEF-H1 to epithelial junctions. Mol. Biol. Cell 19, 4442–4453 10.1091/mbc.E08-06-0558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemot L., Guerrera D., Spadaro D., Tapia R., Jond L., Citi S. (2014). MgcRacGAP interacts with cingulin and paracingulin to regulate rac1 activation and development of the tight junction barrier during epithelial junction assembly. Mol. Biol. Cell 10.1091/mbc.E13-11-0680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S., Kemphues K. J. (1995). par-1, a gene required for establishing polarity in C. elegans embryos, encodes a putative Ser/Thr kinase that is asymmetrically distributed. Cell 81, 611–620 10.1016/0092-8674(95)90082-9 [DOI] [PubMed] [Google Scholar]
- Hamazaki Y., Itoh M., Sasaki H., Furuse M., Tsukita S. (2002). Multi-PDZ domain protein 1 (MUPP1) is concentrated at tight junctions through its possible interaction with claudin-1 and junctional adhesion molecule. J. Biol. Chem. 277, 455–461 10.1074/jbc.M109005200 [DOI] [PubMed] [Google Scholar]
- Hayase J., Kamakura S., Iwakiri Y., Yamaguchi Y., Izaki T., Ito T., Sumimoto H. (2013). The WD40 protein Morg1 facilitates Par6-aPKC binding to Crb3 for apical identity in epithelial cells. J. Cell Biol. 200, 635–650 10.1083/jcb.201208150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrique D., Schweisguth F. (2003). Cell polarity: the ups and downs of the Par6/aPKC complex. Curr. Opin. Genet. Dev. 13, 341–350 10.1016/S0959-437X(03)00077-7 [DOI] [PubMed] [Google Scholar]
- Herranz H., Stamataki E., Feiguin F., Milán M. (2006). Self-refinement of Notch activity through the transmembrane protein Crumbs: modulation of gamma-secretase activity. EMBO Rep. 7, 297–302 10.1038/sj.embor.7400617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose T., Izumi Y., Nagashima Y., Tamai-Nagai Y., Kurihara H., Sakai T., Suzuki Y., Yamanaka T., Suzuki A., Mizuno K. et al. (2002). Involvement of ASIP/PAR-3 in the promotion of epithelial tight junction formation. J. Cell Sci. 115, 2485–2495 [DOI] [PubMed] [Google Scholar]
- Horikoshi Y., Suzuki A., Yamanaka T., Sasaki K., Mizuno K., Sawada H., Yonemura S., Ohno S. (2009). Interaction between PAR-3 and the aPKC-PAR-6 complex is indispensable for apical domain development of epithelial cells. J. Cell Sci. 122, 1595–1606 10.1242/jcs.043174 [DOI] [PubMed] [Google Scholar]
- Hung T. J., Kemphues K. J. (1999). PAR-6 is a conserved PDZ domain-containing protein that colocalizes with PAR-3 in Caenorhabditis elegans embryos. Development 126, 127–135 [DOI] [PubMed] [Google Scholar]
- Hurd T. W., Gao L., Roh M. H., Macara I. G., Margolis B. (2003). Direct interaction of two polarity complexes implicated in epithelial tight junction assembly. Nat. Cell Biol. 5, 137–142 10.1038/ncb923 [DOI] [PubMed] [Google Scholar]
- Hurov J. B., Watkins J. L., Piwnica-Worms H. (2004). Atypical PKC phosphorylates PAR-1 kinases to regulate localization and activity. Curr. Biol. 14, 736–741 10.1016/j.cub.2004.04.007 [DOI] [PubMed] [Google Scholar]
- Hutterer A., Betschinger J., Petronczki M., Knoblich J. A. (2004). Sequential roles of Cdc42, Par-6, aPKC, and Lgl in the establishment of epithelial polarity during Drosophila embryogenesis. Dev. Cell 6, 845–854 10.1016/j.devcel.2004.05.003 [DOI] [PubMed] [Google Scholar]
- Iden S., Collard J. G. (2008). Crosstalk between small GTPases and polarity proteins in cell polarization. Nat. Rev. Mol. Cell Biol. 9, 846–859 10.1038/nrm2521 [DOI] [PubMed] [Google Scholar]
- Iden S., Misselwitz S., Peddibhotla S. S., Tuncay H., Rehder D., Gerke V., Robenek H., Suzuki A., Ebnet K. (2012). aPKC phosphorylates JAM-A at Ser285 to promote cell contact maturation and tight junction formation. J. Cell Biol. 196, 623–639 10.1083/jcb.201104143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiuchi T., Takeichi M. (2011). Willin and Par3 cooperatively regulate epithelial apical constriction through aPKC-mediated ROCK phosphorylation. Nat. Cell Biol. 13, 860–866 10.1038/ncb2274 [DOI] [PubMed] [Google Scholar]
- Itoh M., Sasaki H., Furuse M., Ozaki H., Kita T., Tsukita S. (2001). Junctional adhesion molecule (JAM) binds to PAR-3: a possible mechanism for the recruitment of PAR-3 to tight junctions. J. Cell Biol. 154, 491–498 10.1083/jcb.200103047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M., Tsukita S., Yamazaki Y., Sugimoto H. (2012). Rho GTP exchange factor ARHGEF11 regulates the integrity of epithelial junctions by connecting ZO-1 and RhoA-myosin II signaling. Proc. Natl. Acad. Sci. USA 109, 9905–9910 10.1073/pnas.1115063109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A. I., Hunt D., Utech M., Nusrat A., Parkos C. A. (2005). Differential roles for actin polymerization and a myosin II motor in assembly of the epithelial apical junctional complex. Mol. Biol. Cell 16, 2636–2650 10.1091/mbc.E05-01-0043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A. I., Young C., Den Beste K., Capaldo C. T., Humbert P. O., Brennwald P., Parkos C. A., Nusrat A. (2010). Tumor suppressor scribble regulates assembly of tight junctions in the intestinal epithelium. Am. J. Pathol. 176, 134–145 10.2353/ajpath.2010.090220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y., Hirose T., Tamai Y., Hirai S., Nagashima Y., Fujimoto T., Tabuse Y., Kemphues K. J., Ohno S. (1998). An atypical PKC directly associates and colocalizes at the epithelial tight junction with ASIP, a mammalian homologue of Caenorhabditis elegans polarity protein PAR-3. J. Cell Biol. 143, 95–106 10.1083/jcb.143.1.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joberty G., Petersen C., Gao L., Macara I. G. (2000). The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat. Cell Biol. 2, 531–539 10.1038/35019573 [DOI] [PubMed] [Google Scholar]
- Johansson A., Driessens M., Aspenström P. (2000). The mammalian homologue of the Caenorhabditis elegans polarity protein PAR-6 is a binding partner for the Rho GTPases Cdc42 and Rac1. J. Cell Sci. 113, 3267–3275 [DOI] [PubMed] [Google Scholar]
- Johnston C. A., Hirono K., Prehoda K. E., Doe C. Q. (2009). Identification of an Aurora-A/PinsLINKER/Dlg spindle orientation pathway using induced cell polarity in S2 cells. Cell 138, 1150–1163 10.1016/j.cell.2009.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou T. S., Nelson W. J. (1998). Effects of regulated expression of mutant RhoA and Rac1 small GTPases on the development of epithelial (MDCK) cell polarity. J. Cell Biol. 142, 85–100 10.1083/jcb.142.1.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou T. S., Schneeberger E. E., Nelson W. J. (1998). Structural and functional regulation of tight junctions by RhoA and Rac1 small GTPases. J. Cell Biol. 142, 101–115 10.1083/jcb.142.1.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgens G., Wieschaus E., Nusslein-Volhard C., Kluding H. (1984). Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster. Roux's Arch. Develop. Biol. 193, 283–295 10.1007/BF00848157 [DOI] [PubMed] [Google Scholar]
- Kallay L. M., McNickle A., Brennwald P. J., Hubbard A. L., Braiterman L. T. (2006). Scribble associates with two polarity proteins, Lgl2 and Vangl2, via distinct molecular domains. J. Cell. Biochem. 99, 647–664 10.1002/jcb.20992 [DOI] [PubMed] [Google Scholar]
- Kawakatsu T., Shimizu K., Honda T., Fukuhara T., Hoshino T., Takai Y. (2002). Trans-interactions of nectins induce formation of filopodia and Lamellipodia through the respective activation of Cdc42 and Rac small G proteins. J. Biol. Chem. 277, 50749–50755 10.1074/jbc.M209846200 [DOI] [PubMed] [Google Scholar]
- Kawazu M., Ueno T., Kontani K., Ogita Y., Ando M., Fukumura K., Yamato A., Soda M., Takeuchi K., Miki Y. et al. (2013). Transforming mutations of RAC guanosine triphosphatases in human cancers. Proc. Natl. Acad. Sci. USA 110, 3029–3034 10.1073/pnas.1216141110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemphues K. (2000). PARsing embryonic polarity. Cell 101, 345–348 10.1016/S0092-8674(00)80844-2 [DOI] [PubMed] [Google Scholar]
- Kemphues K. J., Priess J. R., Morton D. G., Cheng N. S. (1988). Identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell 52, 311–320 10.1016/S0092-8674(88)80024-2 [DOI] [PubMed] [Google Scholar]
- Kempkens O., Médina E., Fernandez-Ballester G., Ozüyaman S., Le Bivic A., Serrano L., Knust E. (2006). Computer modelling in combination with in vitro studies reveals similar binding affinities of Drosophila Crumbs for the PDZ domains of Stardust and DmPar-6. Eur. J. Cell Biol. 85, 753–767 10.1016/j.ejcb.2006.03.003 [DOI] [PubMed] [Google Scholar]
- Kim S. H., Li Z., Sacks D. B. (2000). E-cadherin-mediated cell-cell attachment activates Cdc42. J. Biol. Chem. 275, 36999–37005 10.1074/jbc.M003430200 [DOI] [PubMed] [Google Scholar]
- Klebes A., Knust E. (2000). A conserved motif in Crumbs is required for E-cadherin localisation and zonula adherens formation in Drosophila. Curr. Biol. 10, 76–85 10.1016/S0960-9822(99)00277-8 [DOI] [PubMed] [Google Scholar]
- Klein S., Partisani M., Franco M., Luton F. (2008). EFA6 facilitates the assembly of the tight junction by coordinating an Arf6-dependent and -independent pathway. J. Biol. Chem. 283, 30129–30138 10.1074/jbc.M803375200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knust E., Bossinger O. (2002). Composition and formation of intercellular junctions in epithelial cells. Science 298, 1955–1959 10.1126/science.1072161 [DOI] [PubMed] [Google Scholar]
- Kodama A., Takaishi K., Nakano K., Nishioka H., Takai Y. (1999). Involvement of Cdc42 small G protein in cell-cell adhesion, migration and morphology of MDCK cells. Oncogene 18, 3996–4006 10.1038/sj.onc.1202773 [DOI] [PubMed] [Google Scholar]
- Koh Y. H., Popova E., Thomas U., Griffith L. C., Budnik V. (1999). Regulation of DLG localization at synapses by CaMKII-dependent phosphorylation. Cell 98, 353–363 10.1016/S0092-8674(00)81964-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahn M. P., Bückers J., Kastrup L., Wodarz A. (2010). Formation of a Bazooka-Stardust complex is essential for plasma membrane polarity in epithelia. J. Cell Biol. 190, 751–760 10.1083/jcb.201006029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarche N., Hall A. (1994). GAPs for rho-related GTPases. Trends Genet. 10, 436–440 10.1016/0168-9525(94)90114-7 [DOI] [PubMed] [Google Scholar]
- Lambert J. M., Lambert Q. T., Reuther G. W., Malliri A., Siderovski D. P., Sondek J., Collard J. G., Der C. J. (2002). Tiam1 mediates Ras activation of Rac by a PI(3)K-independent mechanism. Nat. Cell Biol. 4, 621–625 [DOI] [PubMed] [Google Scholar]
- Laprise P., Viel A., Rivard N. (2004). Human homolog of disc-large is required for adherens junction assembly and differentiation of human intestinal epithelial cells. J. Biol. Chem. 279, 10157–10166 10.1074/jbc.M309843200 [DOI] [PubMed] [Google Scholar]
- Latorre I. J., Roh M. H., Frese K. K., Weiss R. S., Margolis B., Javier R. T. (2005). Viral oncoprotein-induced mislocalization of select PDZ proteins disrupts tight junctions and causes polarity defects in epithelial cells. J. Cell Sci. 118, 4283–4293 10.1242/jcs.02560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. L., Streuli C. H. (2014). Integrins and epithelial cell polarity. J. Cell Sci. 127, 3217–3225 10.1242/jcs.146142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmers C., Médina E., Delgrossi M. H., Michel D., Arsanto J. P., Le Bivic A. (2002). hINADl/PATJ, a homolog of discs lost, interacts with crumbs and localizes to tight junctions in human epithelial cells. J. Biol. Chem. 277, 25408–25415 10.1074/jbc.M202196200 [DOI] [PubMed] [Google Scholar]
- Lemmers C., Michel D., Lane-Guermonprez L., Delgrossi M. H., Médina E., Arsanto J. P., Le Bivic A. (2004). CRB3 binds directly to Par6 and regulates the morphogenesis of the tight junctions in mammalian epithelial cells. Mol. Biol. Cell 15, 1324–1333 10.1091/mbc.E03-04-0235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D., Edwards A. S., Fawcett J. P., Mbamalu G., Scott J. D., Pawson T. (2000). A mammalian PAR-3-PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nat. Cell Biol. 2, 549–552 10.1038/35019592 [DOI] [PubMed] [Google Scholar]
- Ling C., Zuo D., Xue B., Muthuswamy S., Muller W. J. (2010). A novel role for 14-3-3sigma in regulating epithelial cell polarity. Genes Dev. 24, 947–956 10.1101/gad.1896810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Horowitz A. (2006). A PDZ-binding motif as a critical determinant of Rho guanine exchange factor function and cell phenotype. Mol. Biol. Cell 17, 1880–1887 10.1091/mbc.E06-01-0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. F., Ishida H., Raziuddin R., Miki T. (2004). Nucleotide exchange factor ECT2 interacts with the polarity protein complex Par6/Par3/protein kinase Czeta (PKCzeta) and regulates PKCzeta activity. Mol. Cell. Biol. 24, 6665–6675 10.1128/MCB.24.15.6665-6675.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. F., Ohno S., Miki T. (2006). Nucleotide exchange factor ECT2 regulates epithelial cell polarity. Cell. Signal. 18, 1604–1615 10.1016/j.cellsig.2006.01.007 [DOI] [PubMed] [Google Scholar]
- Lohia M., Qin Y., Macara I. G. (2012). The Scribble polarity protein stabilizes E-cadherin/p120-catenin binding and blocks retrieval of E-cadherin to the Golgi. PLoS ONE 7, e51130 10.1371/journal.pone.0051130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luton F., Klein S., Chauvin J. P., Le Bivic A., Bourgoin S., Franco M., Chardin P. (2004). EFA6, exchange factor for ARF6, regulates the actin cytoskeleton and associated tight junction in response to E-cadherin engagement. Mol. Biol. Cell 15, 1134–1145 10.1091/mbc.E03-10-0751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macara I. G. (2004). Parsing the polarity code. Nat. Rev. Mol. Cell Biol. 5, 220–231 10.1038/nrm1332 [DOI] [PubMed] [Google Scholar]
- Macia E., Luton F., Partisani M., Cherfils J., Chardin P., Franco M. (2004). The GDP-bound form of Arf6 is located at the plasma membrane. J. Cell Sci. 117, 2389–2398 10.1242/jcs.01090 [DOI] [PubMed] [Google Scholar]
- Mack N. A., Porter A. P., Whalley H. J., Schwarz J. P., Jones R. C., Khaja A. S., Bjartell A., Anderson K. I., Malliri A. (2012). β2-syntrophin and Par-3 promote an apicobasal Rac activity gradient at cell-cell junctions by differentially regulating Tiam1 activity. Nat. Cell Biol. 14, 1169–1180 10.1038/ncb2608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarova O., Roh M. H., Liu C. J., Laurinec S., Margolis B. (2003). Mammalian Crumbs3 is a small transmembrane protein linked to protein associated with Lin-7 (Pals1). Gene 302, 21–29 10.1016/S0378111902010843 [DOI] [PubMed] [Google Scholar]
- Mantovani F., Banks L. (2003). Regulation of the discs large tumor suppressor by a phosphorylation-dependent interaction with the beta-TrCP ubiquitin ligase receptor. J. Biol. Chem. 278, 42477–42486 10.1074/jbc.M302799200 [DOI] [PubMed] [Google Scholar]
- Martin-Belmonte F., Gassama A., Datta A., Yu W., Rescher U., Gerke V., Mostov K. (2007). PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell 128, 383–397 10.1016/j.cell.2006.11.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew D., Gramates L. S., Packard M., Thomas U., Bilder D., Perrimon N., Gorczyca M., Budnik V. (2002). Recruitment of scribble to the synaptic scaffolding complex requires GUK-holder, a novel DLG binding protein. Curr. Biol. 12, 531–539 10.1016/S0960-9822(02)00758-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey L. M., Macara I. G. (2009). Widely conserved signaling pathways in the establishment of cell polarity. Cold Spring Harb. Perspect. Biol. 1, a001370 10.1101/cshperspect.a001370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClatchey A. I. (2014). ERM proteins at a glance. J. Cell Sci. 127, 3199–3204. 10.1242/jcs.098343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechler B. M., McGinnis W., Gehring W. J. (1985). Molecular cloning of lethal(2)giant larvae, a recessive oncogene of Drosophila melanogaster. EMBO J. 4, 1551–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller N., Merlot S., Guda C. (2005). CZH proteins: a new family of Rho-GEFs. J. Cell Sci. 118, 4937–4946 10.1242/jcs.02671 [DOI] [PubMed] [Google Scholar]
- Mertens A. E., Rygiel T. P., Olivo C., van der Kammen R., Collard J. G. (2005). The Rac activator Tiam1 controls tight junction biogenesis in keratinocytes through binding to and activation of the Par polarity complex. J. Cell Biol. 170, 1029–1037 10.1083/jcb.200502129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens A. E., Pegtel D. M., Collard J. G. (2006). Tiam1 takes PARt in cell polarity. Trends Cell Biol. 16, 308–316 10.1016/j.tcb.2006.04.001 [DOI] [PubMed] [Google Scholar]
- Métais J. Y., Navarro C., Santoni M. J., Audebert S., Borg J. P. (2005). hScrib interacts with ZO-2 at the cell-cell junctions of epithelial cells. FEBS Lett. 579, 3725–3730 10.1016/j.febslet.2005.05.062 [DOI] [PubMed] [Google Scholar]
- Michel D., Arsanto J. P., Massey-Harroche D., Béclin C., Wijnholds J., Le Bivic A. (2005). PATJ connects and stabilizes apical and lateral components of tight junctions in human intestinal cells. J. Cell Sci. 118, 4049–4057 10.1242/jcs.02528 [DOI] [PubMed] [Google Scholar]
- Miura K., Nam J. M., Kojima C., Mochizuki N., Sabe H. (2009). EphA2 engages Git1 to suppress Arf6 activity modulating epithelial cell-cell contacts. Mol. Biol. Cell 20, 1949–1959 10.1091/mbc.E08-06-0549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno K., Suzuki A., Hirose T., Kitamura K., Kutsuzawa K., Futaki M., Amano Y., Ohno S. (2003). Self-association of PAR-3-mediated by the conserved N-terminal domain contributes to the development of epithelial tight junctions. J. Biol. Chem. 278, 31240–31250 10.1074/jbc.M303593200 [DOI] [PubMed] [Google Scholar]
- Montcouquiol M., Rachel R. A., Lanford P. J., Copeland N. G., Jenkins N. A., Kelley M. W. (2003). Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature 423, 173–177 10.1038/nature01618 [DOI] [PubMed] [Google Scholar]
- Moon S. Y., Zheng Y. (2003). Rho GTPase-activating proteins in cell regulation. Trends Cell Biol. 13, 13–22 10.1016/S0962-8924(02)00004-1 [DOI] [PubMed] [Google Scholar]
- Morais-de-Sá E., Mirouse V., St Johnston D. (2010). aPKC phosphorylation of Bazooka defines the apical/lateral border in Drosophila epithelial cells. Cell 141, 509–523 10.1016/j.cell.2010.02.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller H. A., Wieschaus E. (1996). armadillo, bazooka, and stardust are critical for early stages in formation of the zonula adherens and maintenance of the polarized blastoderm epithelium in Drosophila. J. Cell Biol. 134, 149–163 10.1083/jcb.134.1.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch J. N., Henderson D. J., Doudney K., Gaston-Massuet C., Phillips H. M., Paternotte C., Arkell R., Stanier P., Copp A. J. (2003). Disruption of scribble (Scrb1) causes severe neural tube defects in the circletail mouse. Hum. Mol. Genet. 12, 87–98 10.1093/hmg/ddg014 [DOI] [PubMed] [Google Scholar]
- Müsch A., Cohen D., Yeaman C., Nelson W. J., Rodriguez-Boulan E., Brennwald P. J. (2002). Mammalian homolog of Drosophila tumor suppressor lethal (2) giant larvae interacts with basolateral exocytic machinery in Madin-Darby canine kidney cells. Mol. Biol. Cell 13, 158–168 10.1091/mbc.01-10-0496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai-Tamai Y., Mizuno K., Hirose T., Suzuki A., Ohno S. (2002). Regulated protein-protein interaction between aPKC and PAR-3 plays an essential role in the polarization of epithelial cells. Genes Cells 7, 1161–1171 10.1046/j.1365-2443.2002.00590.x [DOI] [PubMed] [Google Scholar]
- Nakagawa M., Fukata M., Yamaga M., Itoh N., Kaibuchi K. (2001). Recruitment and activation of Rac1 by the formation of E-cadherin-mediated cell-cell adhesion sites. J. Cell Sci. 114, 1829–1838 [DOI] [PubMed] [Google Scholar]
- Nakajima H., Tanoue T. (2011). Lulu2 regulates the circumferential actomyosin tensile system in epithelial cells through p114RhoGEF. J. Cell Biol. 195, 245–261 10.1083/jcb.201104118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam S. C., Choi K. W. (2003). Interaction of Par-6 and Crumbs complexes is essential for photoreceptor morphogenesis in Drosophila. Development 130, 4363–4372 10.1242/dev.00648 [DOI] [PubMed] [Google Scholar]
- Nam S. C., Choi K. W. (2006). Domain-specific early and late function of Dpatj in Drosophila photoreceptor cells. Dev. Dyn. 235, 1501–1507 10.1002/dvdy.20726 [DOI] [PubMed] [Google Scholar]
- Narayan N., Massimi P., Banks L. (2009). CDK phosphorylation of the discs large tumour suppressor controls its localisation and stability. J. Cell Sci. 122, 65–74 10.1242/jcs.024554 [DOI] [PubMed] [Google Scholar]
- Navarro C., Nola S., Audebert S., Santoni M. J., Arsanto J. P., Ginestier C., Marchetto S., Jacquemier J., Isnardon D., Le Bivic A. et al. (2005). Junctional recruitment of mammalian Scribble relies on E-cadherin engagement. Oncogene 24, 4330–4339 10.1038/sj.onc.1208632 [DOI] [PubMed] [Google Scholar]
- Ngok S. P., Geyer R., Liu M., Kourtidis A., Agrawal S., Wu C., Seerapu H. R., Lewis-Tuffin L. J., Moodie K. L., Huveldt D. et al. (2012). VEGF and Angiopoietin-1 exert opposing effects on cell junctions by regulating the Rho GEF Syx. J. Cell Biol. 199, 1103–1115 10.1083/jcb.201207009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngok S. P., Geyer R., Kourtidis A., Mitin N., Feathers R., Der C., Anastasiadis P. Z. (2013). TEM4 is a junctional Rho GEF required for cell-cell adhesion, monolayer integrity and barrier function. J. Cell Sci. 126, 3271–3277 10.1242/jcs.123869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimnual A. S., Taylor L. J., Bar-Sagi D. (2003). Redox-dependent downregulation of Rho by Rac. Nat. Cell Biol. 5, 236–241 10.1038/ncb938 [DOI] [PubMed] [Google Scholar]
- Nishimura T., Yamaguchi T., Kato K., Yoshizawa M., Nabeshima Y., Ohno S., Hoshino M., Kaibuchi K. (2005). PAR-6-PAR-3 mediates Cdc42-induced Rac activation through the Rac GEFs STEF/Tiam1. Nat. Cell Biol. 7, 270–277 10.1038/ncb1227 [DOI] [PubMed] [Google Scholar]
- Nobes C., Hall A. (1994). Regulation and function of the Rho subfamily of small GTPases. Curr. Opin. Genet. Dev. 4, 77–81 10.1016/0959-437X(94)90094-9 [DOI] [PubMed] [Google Scholar]
- Noda Y., Takeya R., Ohno S., Naito S., Ito T., Sumimoto H. (2001). Human homologues of the Caenorhabditis elegans cell polarity protein PAR6 as an adaptor that links the small GTPases Rac and Cdc42 to atypical protein kinase C. Genes Cells 6, 107–119 10.1046/j.1365-2443.2001.00404.x [DOI] [PubMed] [Google Scholar]
- Ohta Y., Hartwig J. H., Stossel T. P. (2006). FilGAP, a Rho- and ROCK-regulated GAP for Rac binds filamin A to control actin remodelling. Nat. Cell Biol. 8, 803–814 10.1038/ncb1437 [DOI] [PubMed] [Google Scholar]
- Olofsson B. (1999). Rho guanine dissociation inhibitors: pivotal molecules in cellular signalling. Cell. Signal. 11, 545–554 10.1016/S0898-6568(98)00063-1 [DOI] [PubMed] [Google Scholar]
- Ooshio T., Fujita N., Yamada A., Sato T., Kitagawa Y., Okamoto R., Nakata S., Miki A., Irie K., Takai Y. (2007). Cooperative roles of Par-3 and afadin in the formation of adherens and tight junctions. J. Cell Sci. 120, 2352–2365 10.1242/jcs.03470 [DOI] [PubMed] [Google Scholar]
- Osmani N., Vitale N., Borg J. P., Etienne-Manneville S. (2006). Scrib controls Cdc42 localization and activity to promote cell polarization during astrocyte migration. Curr. Biol. 16, 2395–2405 10.1016/j.cub.2006.10.026 [DOI] [PubMed] [Google Scholar]
- Ossipova O., Tabler J., Green J. B., Sokol S. Y. (2007). PAR1 specifies ciliated cells in vertebrate ectoderm downstream of aPKC. Development 134, 4297–4306 10.1242/dev.009282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani T., Ichii T., Aono S., Takeichi M. (2006). Cdc42 GEF Tuba regulates the junctional configuration of simple epithelial cells. J. Cell Biol. 175, 135–146 10.1083/jcb.200605012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdamar B., Bose R., Barrios-Rodiles M., Wang H. R., Zhang Y., Wrana J. L. (2005). Regulation of the polarity protein Par6 by TGFbeta receptors controls epithelial cell plasticity. Science 307, 1603–1609 10.1126/science.1105718 [DOI] [PubMed] [Google Scholar]
- Palacios F., Price L., Schweitzer J., Collard J. G., D'Souza-Schorey C. (2001). An essential role for ARF6-regulated membrane traffic in adherens junction turnover and epithelial cell migration. EMBO J. 20, 4973–4986 10.1093/emboj/20.17.4973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios F., Schweitzer J. K., Boshans R. L., D'Souza-Schorey C. (2002). ARF6-GTP recruits Nm23-H1 to facilitate dynamin-mediated endocytosis during adherens junctions disassembly. Nat. Cell Biol. 4, 929–936 10.1038/ncb881 [DOI] [PubMed] [Google Scholar]
- Partanen J. I., Nieminen A. I., Mäkelä T. P., Klefstrom J. (2007). Suppression of oncogenic properties of c-Myc by LKB1-controlled epithelial organization. Proc. Natl. Acad. Sci. USA 104, 14694–14699 10.1073/pnas.0704677104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck J., Douglas G., 4th W. u., C. H. and Burbelo P. D. (2002). Human RhoGAP domain-containing proteins: structure, function and evolutionary relationships. FEBS Lett. 528, 27–34 10.1016/S0014-5793(02)03331-8 [DOI] [PubMed] [Google Scholar]
- Pénalva C., Mirouse V. (2012). Tissue-specific function of Patj in regulating the Crumbs complex and epithelial polarity. Development 139, 4549–4554 10.1242/dev.085449 [DOI] [PubMed] [Google Scholar]
- Peng C. Y., Manning L., Albertson R., Doe C. Q. (2000). The tumour-suppressor genes lgl and dlg regulate basal protein targeting in Drosophila neuroblasts. Nature 408, 596–600 10.1038/35046094 [DOI] [PubMed] [Google Scholar]
- Petronczki M., Knoblich J. A. (2001). DmPAR-6 directs epithelial polarity and asymmetric cell division of neuroblasts in Drosophila. Nat. Cell Biol. 3, 43–49 [DOI] [PubMed] [Google Scholar]
- Plant P. J., Fawcett J. P., Lin D. C., Holdorf A. D., Binns K., Kulkarni S., Pawson T. (2003). A polarity complex of mPar-6 and atypical PKC binds, phosphorylates and regulates mammalian Lgl. Nat. Cell Biol. 5, 301–308 10.1038/ncb948 [DOI] [PubMed] [Google Scholar]
- Poliak S., Matlis S., Ullmer C., Scherer S. S., Peles E. (2002). Distinct claudins and associated PDZ proteins form different autotypic tight junctions in myelinating Schwann cells. J. Cell Biol. 159, 361–372 10.1083/jcb.200207050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y., Capaldo C., Gumbiner B. M., Macara I. G. (2005). The mammalian Scribble polarity protein regulates epithelial cell adhesion and migration through E-cadherin. J. Cell Biol. 171, 1061–1071 10.1083/jcb.200506094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y., Meisen W. H., Hao Y., Macara I. G. (2010). Tuba, a Cdc42 GEF, is required for polarized spindle orientation during epithelial cyst formation. J. Cell Biol. 189, 661–669 10.1083/jcb.201002097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu R. G., Abo A., Steven Martin G. (2000). A human homolog of the C. elegans polarity determinant Par-6 links Rac and Cdc42 to PKCzeta signaling and cell transformation. Curr. Biol. 10, 697–707 10.1016/S0960-9822(00)00535-2 [DOI] [PubMed] [Google Scholar]
- Ratheesh A., Gomez G. A., Priya R., Verma S., Kovacs E. M., Jiang K., Brown N. H., Akhmanova A., Stehbens S. J., Yap A. S. (2012). Centralspindlin and α-catenin regulate Rho signalling at the epithelial zonula adherens. Nat. Cell Biol. 14, 818–828 10.1038/ncb2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson E. C., Pichaud F. (2010). Crumbs is required to achieve proper organ size control during Drosophila head development. Development 137, 641–650 10.1242/dev.041913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson B. S., Huang J., Hong Y., Moberg K. H. (2010). Crumbs regulates Salvador/Warts/Hippo signaling in Drosophila via the FERM-domain protein Expanded. Curr. Biol. 20, 582–590 10.1016/j.cub.2010.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh M. H., Liu C. J., Laurinec S., Margolis B. (2002a). The carboxyl terminus of zona occludens-3 binds and recruits a mammalian homologue of discs lost to tight junctions. J. Biol. Chem. 277, 27501–27509 10.1074/jbc.M201177200 [DOI] [PubMed] [Google Scholar]
- Roh M. H., Makarova O., Liu C. J., Shin K., Lee S., Laurinec S., Goyal M., Wiggins R., Margolis B. (2002b). The Maguk protein, Pals1, functions as an adapter, linking mammalian homologues of Crumbs and Discs Lost. J. Cell Biol. 157, 161–172 10.1083/jcb.200109010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh M. H., Fan S., Liu C. J., Margolis B. (2003). The Crumbs3-Pals1 complex participates in the establishment of polarity in mammalian epithelial cells. J. Cell Sci. 116, 2895–2906 10.1242/jcs.00500 [DOI] [PubMed] [Google Scholar]
- Rossman K. L., Der C. J., Sondek J. (2005). GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat. Rev. Mol. Cell Biol. 6, 167–180 10.1038/nrm1587 [DOI] [PubMed] [Google Scholar]
- Sabio G., Arthur J. S., Kuma Y., Peggie M., Carr J., Murray-Tait V., Centeno F., Goedert M., Morrice N. A., Cuenda A. (2005). p38gamma regulates the localisation of SAP97 in the cytoskeleton by modulating its interaction with GKAP. EMBO J. 24, 1134–1145 10.1038/sj.emboj.7600578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarin S. N., Ivanov A. I., Flatau G., Parkos C. A., Nusrat A. (2007). Rho/Rho-associated kinase-II signaling mediates disassembly of epithelial apical junctions. Mol. Biol. Cell 18, 3429–3439 10.1091/mbc.E07-04-0315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A., Hall A. (2002). Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 16, 1587–1609 10.1101/gad.1003302 [DOI] [PubMed] [Google Scholar]
- Schmoranzer J., Fawcett J. P., Segura M., Tan S., Vallee R. B., Pawson T., Gundersen G. G. (2009). Par3 and dynein associate to regulate local microtubule dynamics and centrosome orientation during migration. Curr. Biol. 19, 1065–1074 10.1016/j.cub.2009.05.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen A., Nagy-Zsvér-Vadas Z., Krahn M. P. (2012). Drosophila PATJ supports adherens junction stability by modulating Myosin light chain activity. J. Cell Biol. 199, 685–698 10.1083/jcb.201206064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin K., Straight S., Margolis B. (2005). PATJ regulates tight junction formation and polarity in mammalian epithelial cells. J. Cell Biol. 168, 705–711 10.1083/jcb.200408064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley R. L., Walter N. A., Reilly M. T., Fehr C., Buck K. J. (2004). Mpdz is a quantitative trait gene for drug withdrawal seizures. Nat. Neurosci. 7, 699–700 10.1038/nn1271 [DOI] [PubMed] [Google Scholar]
- Skouloudaki K., Puetz M., Simons M., Courbard J. R., Boehlke C., Hartleben B., Engel C., Moeller M. J., Englert C., Bollig F. et al. (2009). Scribble participates in Hippo signaling and is required for normal zebrafish pronephros development. Proc. Natl. Acad. Sci. USA 106, 8579–8584 10.1073/pnas.0811691106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. A., Lau K. M., Rahmani Z., Dho S. E., Brothers G., She Y. M., Berry D. M., Bonneil E., Thibault P., Schweisguth F. et al. (2007). aPKC-mediated phosphorylation regulates asymmetric membrane localization of the cell fate determinant Numb. EMBO J. 26, 468–480 10.1038/sj.emboj.7601495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotillos S., Díaz-Meco M. T., Caminero E., Moscat J., Campuzano S. (2004). DaPKC-dependent phosphorylation of Crumbs is required for epithelial cell polarity in Drosophila. J. Cell Biol. 166, 549–557 10.1083/jcb.200311031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Johnston D., Ahringer J. (2010). Cell polarity in eggs and epithelia: parallels and diversity. Cell 141, 757–774 10.1016/j.cell.2010.05.011 [DOI] [PubMed] [Google Scholar]
- Straight S. W., Shin K., Fogg V. C., Fan S., Liu C. J., Roh M., Margolis B. (2004). Loss of PALS1 expression leads to tight junction and polarity defects. Mol. Biol. Cell 15, 1981–1990 10.1091/mbc.E03-08-0620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucke V. M., Timmerman E., Vandekerckhove J., Gevaert K., Hall A. (2007). The MAGUK protein MPP7 binds to the polarity protein hDlg1 and facilitates epithelial tight junction formation. Mol. Biol. Cell 18, 1744–1755 10.1091/mbc.E06-11-0980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Ishiyama C., Hashiba K., Shimizu M., Ebnet K., Ohno S. (2002). aPKC kinase activity is required for the asymmetric differentiation of the premature junctional complex during epithelial cell polarization. J. Cell Sci. 115, 3565–3573 10.1242/jcs.00032 [DOI] [PubMed] [Google Scholar]
- Suzuki A., Hirata M., Kamimura K., Maniwa R., Yamanaka T., Mizuno K., Kishikawa M., Hirose H., Amano Y., Izumi N. et al. (2004). aPKC acts upstream of PAR-1b in both the establishment and maintenance of mammalian epithelial polarity. Curr. Biol. 14, 1425–1435 10.1016/j.cub.2004.08.021 [DOI] [PubMed] [Google Scholar]
- Tanentzapf G., Tepass U. (2003). Interactions between the crumbs, lethal giant larvae and bazooka pathways in epithelial polarization. Nat. Cell Biol. 5, 46–52 10.1038/ncb896 [DOI] [PubMed] [Google Scholar]
- Tanwar P. S., Kaneko-Tarui T., Zhang L., Teixeira J. M. (2012). Altered LKB1/AMPK/TSC1/TSC2/mTOR signaling causes disruption of Sertoli cell polarity and spermatogenesis. Hum. Mol. Genet. 21, 4394–4405 10.1093/hmg/dds272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Klooster J. P., Jansen M., Yuan J., Oorschot V., Begthel H., Di Giacomo V., Colland F., de Koning J., Maurice M. M., Hornbeck P. et al. (2009). Mst4 and Ezrin induce brush borders downstream of the Lkb1/Strad/Mo25 polarization complex. Dev. Cell 16, 551–562 10.1016/j.devcel.2009.01.016 [DOI] [PubMed] [Google Scholar]
- Tepass U., Theres C., Knust E. (1990). crumbs encodes an EGF-like protein expressed on apical membranes of Drosophila epithelial cells and required for organization of epithelia. Cell 61, 787–799 10.1016/0092-8674(90)90189-L [DOI] [PubMed] [Google Scholar]
- Terry S. J., Zihni C., Elbediwy A., Vitiello E., Leefa Chong San I. V., Balda M. S., Matter K. (2011). Spatially restricted activation of RhoA signalling at epithelial junctions by p114RhoGEF drives junction formation and morphogenesis. Nat. Cell Biol. 13, 159–166 10.1038/ncb2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaezi A., Bauer C., Vasioukhin V., Fuchs E. (2002). Actin cable dynamics and Rho/Rock orchestrate a polarized cytoskeletal architecture in the early steps of assembling a stratified epithelium. Dev. Cell 3, 367–381 10.1016/S1534-5807(02)00259-9 [DOI] [PubMed] [Google Scholar]
- Van Campenhout C. A., Eitelhuber A., Gloeckner C. J., Giallonardo P., Gegg M., Oller H., Grant S. G., Krappmann D., Ueffing M., Lickert H. (2011). Dlg3 trafficking and apical tight junction formation is regulated by nedd4 and nedd4-2 e3 ubiquitin ligases. Dev. Cell 21, 479–491 10.1016/j.devcel.2011.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hurk J. A., Rashbass P., Roepman R., Davis J., Voesenek K. E., Arends M. L., Zonneveld M. N., van Roekel M. H., Cameron K., Rohrschneider K. et al. (2005). Characterization of the Crumbs homolog 2 (CRB2) gene and analysis of its role in retinitis pigmentosa and Leber congenital amaurosis. Mol. Vis. 11, 263–273 [PubMed] [Google Scholar]
- Varelas X., Samavarchi-Tehrani P., Narimatsu M., Weiss A., Cockburn K., Larsen B. G., Rossant J., Wrana J. L. (2010). The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-β-SMAD pathway. Dev. Cell 19, 831–844 10.1016/j.devcel.2010.11.012 [DOI] [PubMed] [Google Scholar]
- Vitale N., Patton W. A., Moss J., Vaughan M., Lefkowitz R. J., Premont R. T. (2000). GIT proteins, A novel family of phosphatidylinositol 3,4, 5-trisphosphate-stimulated GTPase-activating proteins for ARF6. J. Biol. Chem. 275, 13901–13906 10.1074/jbc.275.18.13901 [DOI] [PubMed] [Google Scholar]
- Walther R. F., Pichaud F. (2010). Crumbs/DaPKC-dependent apical exclusion of Bazooka promotes photoreceptor polarity remodeling. Curr. Biol. 20, 1065–1074 10.1016/j.cub.2010.04.049 [DOI] [PubMed] [Google Scholar]
- Wang Q., Hurd T. W., Margolis B. (2004). Tight junction protein Par6 interacts with an evolutionarily conserved region in the amino terminus of PALS1/stardust. J. Biol. Chem. 279, 30715–30721 10.1074/jbc.M401930200 [DOI] [PubMed] [Google Scholar]
- Wang Q., Chen X. W., Margolis B. (2007). PALS1 regulates E-cadherin trafficking in mammalian epithelial cells. Mol. Biol. Cell 18, 874–885 10.1091/mbc.E06-07-0651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Yang J., Tsai A., Kuca T., Sanny J., Lee J., Dong K., Harden N., Krieger C. (2011). Drosophila adducin regulates Dlg phosphorylation and targeting of Dlg to the synapse and epithelial membrane. Dev. Biol. 357, 392–403 10.1016/j.ydbio.2011.07.010 [DOI] [PubMed] [Google Scholar]
- Watkins J. L., Lewandowski K. T., Meek S. E., Storz P., Toker A., Piwnica-Worms H. (2008). Phosphorylation of the Par-1 polarity kinase by protein kinase D regulates 14-3-3 binding and membrane association. Proc. Natl. Acad. Sci. USA 105, 18378–18383 10.1073/pnas.0809661105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells C. D., Fawcett J. P., Traweger A., Yamanaka Y., Goudreault M., Elder K., Kulkarni S., Gish G., Virag C., Lim C. et al. (2006). A Rich1/Amot complex regulates the Cdc42 GTPase and apical-polarity proteins in epithelial cells. Cell 125, 535–548 10.1016/j.cell.2006.02.045 [DOI] [PubMed] [Google Scholar]
- Whiteman E. L., Liu C. J., Fearon E. R., Margolis B. (2008). The transcription factor snail represses Crumbs3 expression and disrupts apico-basal polarity complexes. Oncogene 27, 3875–3879 10.1038/onc.2008.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman E. L., Fan S., Harder J. L., Walton K. D., Liu C. J., Soofi A., Fogg V. C., Hershenson M. B., Dressler G. R., Deutsch G. H. et al. (2014). Crumbs3 is essential for proper epithelial development and viability. Mol. Cell. Biol. 34, 43–56 10.1128/MCB.00999-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildenberg G. A., Dohn M. R., Carnahan R. H., Davis M. A., Lobdell N. A., Settleman J., Reynolds A. B. (2006). p120-catenin and p190RhoGAP regulate cell-cell adhesion by coordinating antagonism between Rac and Rho. Cell 127, 1027–1039 10.1016/j.cell.2006.09.046 [DOI] [PubMed] [Google Scholar]
- Winter J. F., Höpfner S., Korn K., Farnung B. O., Bradshaw C. R., Marsico G., Volkmer M., Habermann B., Zerial M. (2012). Caenorhabditis elegans screen reveals role of PAR-5 in RAB-11-recycling endosome positioning and apicobasal cell polarity. Nat. Cell Biol. 14, 666–676 10.1038/ncb2508 [DOI] [PubMed] [Google Scholar]
- Wirtz-Peitz F., Knoblich J. A. (2006). Lethal giant larvae take on a life of their own. Trends Cell Biol. 16, 234–241 10.1016/j.tcb.2006.03.006 [DOI] [PubMed] [Google Scholar]
- Wodarz A., Grawe F., Knust E. (1993). CRUMBS is involved in the control of apical protein targeting during Drosophila epithelial development. Mech. Dev. 44, 175–187 10.1016/0925-4773(93)90066-7 [DOI] [PubMed] [Google Scholar]
- Wodarz A., Hinz U., Engelbert M., Knust E. (1995). Expression of crumbs confers apical character on plasma membrane domains of ectodermal epithelia of Drosophila. Cell 82, 67–76 10.1016/0092-8674(95)90053-5 [DOI] [PubMed] [Google Scholar]
- Wodarz A., Ramrath A., Grimm A., Knust E. (2000). Drosophila atypical protein kinase C associates with Bazooka and controls polarity of epithelia and neuroblasts. J. Cell Biol. 150, 1361–1374 10.1083/jcb.150.6.1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. F., Hough C., Peel D., Callaini G., Bryant P. J. (1996). Dlg protein is required for junction structure, cell polarity, and proliferation control in Drosophila epithelia. J. Cell Biol. 134, 1469–1482 10.1083/jcb.134.6.1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi S., Matsuda M., Kiyokawa E. (2012). Chimaerin suppresses Rac1 activation at the apical membrane to maintain the cyst structure. PLoS ONE 7, e52258 10.1371/journal.pone.0052258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka T., Horikoshi Y., Suzuki A., Sugiyama Y., Kitamura K., Maniwa R., Nagai Y., Yamashita A., Hirose T., Ishikawa H. et al. (2001). PAR-6 regulates aPKC activity in a novel way and mediates cell-cell contact-induced formation of the epithelial junctional complex. Genes Cells 6, 721–731 10.1046/j.1365-2443.2001.00453.x [DOI] [PubMed] [Google Scholar]
- Yamanaka T., Horikoshi Y., Sugiyama Y., Ishiyama C., Suzuki A., Hirose T., Iwamatsu A., Shinohara A., Ohno S. (2003). Mammalian Lgl forms a protein complex with PAR-6 and aPKC independently of PAR-3 to regulate epithelial cell polarity. Curr. Biol. 13, 734–743 10.1016/S0960-9822(03)00244-6 [DOI] [PubMed] [Google Scholar]
- Yamanaka T., Horikoshi Y., Izumi N., Suzuki A., Mizuno K., Ohno S. (2006). Lgl mediates apical domain disassembly by suppressing the PAR-3-aPKC-PAR-6 complex to orient apical membrane polarity. J. Cell Sci. 119, 2107–2118 10.1242/jcs.02938 [DOI] [PubMed] [Google Scholar]
- Yang Z., Xue B., Umitsu M., Ikura M., Muthuswamy S. K., Neel B. G. (2012). The signaling adaptor GAB1 regulates cell polarity by acting as a PAR protein scaffold. Mol. Cell 47, 469–483 10.1016/j.molcel.2012.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates L. L., Schnatwinkel C., Hazelwood L., Chessum L., Paudyal A., Hilton H., Romero M. R., Wilde J., Bogani D., Sanderson J. et al. (2013). Scribble is required for normal epithelial cell-cell contacts and lumen morphogenesis in the mammalian lung. Dev. Biol. 373, 267–280 10.1016/j.ydbio.2012.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi C., Troutman S., Fera D., Stemmer-Rachamimov A., Avila J. L., Christian N., Persson N. L., Shimono A., Speicher D. W., Marmorstein R. et al. (2011). A tight junction-associated Merlin-angiomotin complex mediates Merlin's regulation of mitogenic signaling and tumor suppressive functions. Cancer Cell 19, 527–540 10.1016/j.ccr.2011.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Macara I. G. (2008). The PAR-6 polarity protein regulates dendritic spine morphogenesis through p190 RhoGAP and the Rho GTPase. Dev. Cell 14, 216–226 10.1016/j.devcel.2007.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Guo H., Kwan H., Wang J. W., Kosek J., Lu B. (2007). PAR-1 kinase phosphorylates Dlg and regulates its postsynaptic targeting at the Drosophila neuromuscular junction. Neuron 53, 201–215 10.1016/j.neuron.2006.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z. S., Manser E., Loo T. H., Lim L. (2000). Coupling of PAK-interacting exchange factor PIX to GIT1 promotes focal complex disassembly. Mol. Cell. Biol. 20, 6354–6363 10.1128/MCB.20.17.6354-6363.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Shang Y., Wan Q., Xia Y., Chen J., Du Q., Zhang M. (2014). Phosphorylatiohn-dependent interaction between tumor suppressors Dlg and Lgl. Cell Res. 24, 451–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zihni C., Munro P. M., Elbediwy A., Keep N. H., Terry S. J., Harris J., Balda M. S., Matter K. (2014). Dbl3 drives Cdc42 signaling at the apical margin to regulate junction position and apical differentiation. J. Cell Biol. 204, 111–127 10.1083/jcb.201304064 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


