ABSTRACT
Cell polarity is characterised by differences in structure, composition and function between at least two poles of a cell. In epithelial cells, these spatial differences allow for the formation of defined apical and basal membranes. It has been increasingly recognised that cell–matrix interactions and integrins play an essential role in creating epithelial cell polarity, although key gaps in our knowledge remain. This Commentary will discuss the mounting evidence for the role of integrins in polarising epithelial cells. We build a model in which both inside-out signals to polarise basement membrane assembly at the basal surface, and outside-in signals to control microtubule apical–basal orientation and vesicular trafficking are required for establishing and maintaining the orientation of epithelial cell polarity. Finally, we discuss the relevance of the basal integrin polarity axis to cancer.
This article is part of a Minifocus on Establishing polarity. For further reading, please see related articles: ‘ERM proteins at a glance’ by Andrea McClatchey (J. Cell Sci. 127, 3199–3204). ‘Establishment of epithelial polarity – GEF who's minding the GAP?’ by Siu Ngok et al. (J. Cell Sci. 127, 3205–3215).
KEY WORDS: Epithelium, Mammary, Breast, Basement membrane, Laminin, Integrin, ILK, Polarity, Microtubule, +TIPS, Endocytosis
Introduction
Polarised epithelial cells form a continuous layer in which cells are connected by tight and adherens junctions, creating a barrier that separates the inside of our bodies from the outside environment. In many tissues, the apical surface of the cell faces towards the external environment whereas the basolateral surface sits adjacent to an internal-facing basement membrane. Deciphering mechanisms that control the orientation of polarity is crucial for understanding how epithelial barriers work, and how disrupted polarity causes carcinomas, lumenopathies and other epithelial diseases (Bryant and Mostov, 2008; Kao, 2013; Muthuswamy and Xue, 2012).
Tight and adherens junctions connect epithelial cells laterally, whilst at the basal surface, the epithelial monolayer interacts with the extracellular matrix (ECM) through integrin receptors. Tight junctions consist of peripheral and transmembrane proteins that form a strong seal between two cells at the apical surface; they control the selective diffusion of solutes, ions and proteins between the apical and basal tissue compartments (Etienne-Manneville, 2013). They also form a membrane boundary between the apical and basal surface of the cell, thereby preventing the lateral diffusion of integral membrane proteins between poles and maintaining the identity of each cellular surface (Harris and Tepass, 2010). Adherens junctions classically consist of transmembrane cadherins and peripheral catenins. These junctions form homophilic intercellular interactions and connect intracellularly to the actin cytoskeleton.
Epithelial cell polarity is established by a series of complex but coordinated events involving three multiprotein complexes, each named after their founding members, the Par, Crumbs and Scribble proteins (Macara, 2004). Mutual inhibition between individual proteins within these complexes generates spatially separate domains at the apical surface, the tight junction, and the lateral and basal surfaces (St Johnston and Ahringer, 2010). Distinct membrane targeting and endocytic recycling pathways direct apical and basolateral proteins to the correct membrane surface (Apodaca et al., 2012). Together, these mechanisms create a fully polarised epithelial cell. However, the orientation of epithelial polarity requires extrinsic signals, which originate within the ECM.
In this Commentary, we will present the recently emerging role of integrins in epithelial polarity. As discussed below, integrins are involved in establishing an ‘outside-in’ and ‘inside-out’ signalling cascade that ultimately controls the orientation of the microtubule network and thus the vesicular transport of a polarised cell. Finally, we will also consider the increasing evidence that integrin-mediated polarity is implicated in cancer.
The ECM orientates polarity in epithelial cells
The basement membrane is a 100-nm thin ECM that contains a meshwork of laminins, collagen IV, proteoglycans and nidogen. Cells can interact with the basement membrane by binding basement membrane components through cell surface integrin receptors. These interactions allow the basement membrane to provide epithelia with survival, proliferation and differentiation signals, as well as directional cues to establish polarity. Early evidence for the role of basement membrane in epithelial polarity came from studies on kidney glomeruli, where the use of function-blocking antibodies established a requirement for laminin (Klein et al., 1988). This basement membrane protein is also involved in setting up polarity in Madin–Darby canine kidney (MDCK) cells (O'Brien et al., 2001). Genetic studies in Caenorhabditis elegans have revealed that laminin is required to localise Par3 at the opposite apical surface of epithelia during the development of pharyngeal cysts (Rasmussen et al., 2012). This results in the constriction of the apical surface to form a lumen in the middle of the cyst, but in the absence of laminin, constriction occurs at the peripheral surface, leading to multi-lumen cysts and perturbed morphogenesis.
Basement membranes are synthesised by collaboration between epithelia and other cells, for example, fibroblasts in skin and endothelial cells in the glomerulus, both of which secrete basement membrane components and organise them into an ECM at the cell–cell interface. Getting the epithelially derived basement membrane proteins to the right place requires secretion from the basal surface. Therefore, forming the extrinsic polarity cue (i.e. the basement membrane) and setting up intracellular polarity at the basal cell surface must occur simultaneously. Studies in the Drosophila egg chamber have revealed that the spatial control of basement membrane production at the basal surface requires exocytosis and basement membrane remodelling; the cargo receptor Tango1 contributes to basement membrane secretion at basal endoplasmic reticulum exit sites, the vesicle trafficking GTPase Rab10 and its guanine-nucleotide-exchange factor (GEF) Crag restrict vesicle delivery to the basal surface (Lerner et al., 2013). This might prevent basement membrane proteins from taking a Rab11-mediated trafficking route to the apical surface. However, Rab10 is not essential for lumen formation in MDCK cells, so it is not clear yet whether this mechanism is restricted to lumenogenesis in Drosophila (Bryant et al., 2010). A secreted serine-protease-like protein, Scarface, also contributes to the orientation of basement membrane secretion (Sorrosal et al., 2010). In polarised intestinal epithelia, the secretion of ECM components such as collagens relies on the formation of stabilised coat protein complex II (COPII) vesicles together with the cargo selection module Sec13–Sec31 (Townley et al., 2012).
In the Drosophila egg chamber, Rab10 is required for basal basement membrane secretion during rotational morphogenesis, which sets up the additional axis of planar polarity. Collective rotation of the follicle cells is required for ECM assembly (Haigo and Bilder, 2011). Rotation also participates in the establishment of other epithelia. In three-dimensional (3D) cultures, mammary epithelial cells (MECs) rotate to form acini (Tanner et al., 2012). This process is needed to assemble laminin into a discrete basement membrane; interestingly though, rotation is not required to construct ECMs that contain stromal proteins such as fibronectin (Wang et al., 2013). Taken together, the basement membrane is an essential extrinsic cue that orientates epithelial polarity. However the mechanisms of positioning and assembling basement membrane are poorly understood.
Trafficking basement membrane components to the basal epithelial surface is essential, and it is not known if the Rab10 system has a similar role in vertebrates to that in Drospohila. Much is now understood about integrin trafficking, particularly during migration, although we do not know whether similar or different Rabs regulate integrin targeting to the basal surface of stationary epithelia (Bridgewater et al., 2012). It is also not yet clear whether integrin-regulated exocytic pathways contribute to epithelial polarity. In neurons, such a β1 integrin signalling pathway exists, in which actin remodelling by the Arp2/3 complex results in the exocytosis of secretory vesicles (Gupton and Gertler, 2010). It will be important to determine whether similar integrin pathways have a role in the exocytosis of basement membrane proteins during epithelial polarity establishment.
Although rotational basement membrane deposition might occur in an isolated cell system, such as the egg cylinder, there is no evidence yet that rotation is required for vertebrate epithelial polarity in vivo. Four-dimensional (4D)-imaging has revealed that some cells of epithelial ducts have extremely dynamic migration in organotypic models, and it will be interesting to determine whether cell movement has a wider role in laying down basement membranes during the developmental programming of epithelial morphogenesis and polarity (Ewald et al., 2008).
A cascade of ‘outside-in’ and ‘inside-out’ signalling establishes polarity
Adhesion to the basement membrane is mediated by integrins and the transmembrane proteoglycan dystroglycan, which are integral membrane proteins that bind to ECM components as well as intracellular proteins (Hohenester and Yurchenco, 2013). Integrins regulate diverse cellular behaviours, which stems from their ability to initiate a range of signalling pathways (Streuli and Akhtar, 2009). After binding to ECM, the intracellular domain of the integrin dimer recruits an array of cytoskeletal adaptors, such as talin and vinculin, as well as signalling platforms, for example the integrin linked kinase (ILK)–PINCH–Parvin complex, and enzymes such as focal adhesion kinase. Together, these proteins form focal adhesion complexes that control cytoskeletal organisation and intracellular signalling (Rooney and Streuli, 2011).
β1 integrins are widely expressed in epithelial cells, including keratinocytes, kidney, pancreas and glandular epithelia, and genetic deletion approaches have revealed that they have a central role in establishing their polarity, as well as in other cell types, such as endothelia (Table 1). Integrins control both basement membrane deposition and intracellular apical–basal orientation. MDCK cells in suspension without exogenous ECM proteins form hollow cysts in which the apical surface faces outwards, but after transfer to a collagen I gel, polarity becomes inverted in a β1-integrin-dependent mechanism (Ojakian and Schwimmer, 1994; Wang et al., 1990).
Table 1. Phenotypic consequences of ablation of the β1-integrin-encoding gene on polarity in mouse and cell culture models.
Rac1 has been linked to the induction of epithelial polarity in cells adhering to collagenous ECM. This small GTPase is an integrin-regulated switch that has a central role in a variety of actin-dependent processes, including the formation of actin-dense lamellipodia at the laterally polarised leading edge of migrating cells (Heasman and Ridley, 2008; Mack et al., 2011). ECM overlay models have been used to investigate mechanisms of establishing epithelial apical–basal polarity. In these experiments, ECM components are added to the medium of cultured cell monolayers to generate a matrix on the apical cell surface, thereby inverting polarity. Such assays have revealed that, in MDCK cells, β1 integrin binding to extracellular collagen activates Rac1, which in turn induces a reorganisation of laminin at the apical surface of the cell (Yu et al., 2005). This leads to basement membrane assembly and inversion of polarity. Interestingly, Rac1 is not essential for laminin secretion but is required to correctly assemble laminin into a basement membrane (O'Brien et al., 2001). In order for basal laminin organisation to occur, Rac1 can initiate downstream signalling pathways by binding to the adaptor protein IRSp53 (encoded by BAIAP2) (Scita et al., 2008). MDCK cells lacking IRSp53 expression have disrupted basal stress fibres, reduced cell–matrix interactions and decreased laminin deposition (Cohen et al., 2011). One possible contribution of Rac1 to polarity is that it collaborates with IRSp53 to locally activate the actin polymerisation factors Mena (also known as ENAH) and WAVE proteins, resulting in the organisation of a basal actin network. This might provide the appropriate mechanical constraints at the basal surface in collaboration with Rho signalling to permit basement membrane assembly.
Rho has previously been identified as a key player in epithelial polarity; upon inhibition of β1 integrin or Rac1 in MDCK cells, polarity inversion is dependent on Rho–ROCK–myosin signalling (Yu et al., 2008). In 3D cultures of submandibular gland epithelia, the Rho substrate ROCK (Rho-associated kinase) restricts the expression of Par1b to basally located cells, which in turn is needed for deposition of the basement membrane at the basal surface of the developing tissue (Daley et al., 2012). Par1b is a serine/threonine kinase known for its role in phosphorylating Par3, which results in its sequestration by 14-3-3 proteins and inhibition of the formation of the Par complex (Benton and St Johnston, 2003). In MECs, Par1b-mediated phosphorylation of a separate substrate, the E3-ligase RNF41 (also known as NRDP1), is required for laminin deposition and apical–basal polarity (Lewandowski and Piwnica-Worms, 2014).
Studies in mammary epithelia in vivo and in 3D culture using Cre-lox technology have revealed that β1 integrins establish and maintain the orientation of polarity in the luminal epithelial cells (Akhtar and Streuli, 2013). This mouse model exhibits defective mammary acinar morphology in which the alveolar lumens are filled with cells, indicating that β1 integrin is essential for polarity and normal morphogenesis of breast epithelial acini and lobules. Unlike MDCK cells, MECs that genetically lack Rac1 retain the ability to establish polarity in vivo and in cells cultured in a 3D basement-membrane-rich matrix, demonstrating that Rac1 is not essential for polarity in all epithelial cells. Instead, the β1-integrin-interacting protein ILK is required. ILK has also been implicated in the maintenance of epithelial polarity in other cell types in vivo, including the epiblast, keratinocytes and hair follicles (Lorenz et al., 2007; Rudkouskaya et al., 2014; Sakai et al., 2003). Despite its original identification as a serine/threonine kinase, ILK appears to lack a functional kinase domain and instead is a platform for other effectors (Widmaier et al., 2012). Interestingly, different cell types, even within the mammary gland, might respond differently to integrin polarity cues. A mouse model that lacks β1 integrin expression specifically in basal MECs demonstrates that β1-integrin-dependent ECM interactions are essential to correctly orientate the basal cell division axis and segregate basal and luminal compartments (Taddei et al., 2008). Thus integrins are needed for correct orientation of polarity in luminal epithelial cells and for controlling the mitotic spindle axis in basal epithelial cells.
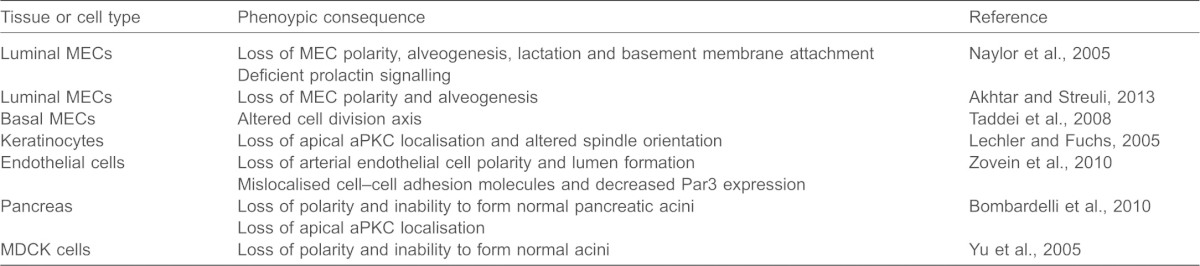
The conclusion of the above studies is that, in most cases, the orientation of epithelial polarity begins with outside-in signalling, when cell adhesion to an ECM protein activates β1 integrin. Epithelia normally contact basement membrane, so cells that inappropriately sit on stromal matrix activate Rac1 to cause an inside-out signal for accurate assembly of the basement membrane. Subsequently, β1-integrin-mediated adhesion to basement membrane establishes the correct intracellular orientation of polarity through a separate outside-in signalling pathway that involves ILK (Fig. 1).
Fig. 1.
A cascade of integrin signalling establishes polarity. (A) Integrin-binding to extracellular collagen initiates a Rac1-dependent pathway that stimulates the production of a basal actin cortex through IRSp53. (B) Laminin is assembled into a basement membrane in a Rac1-depdendent mechanism. In the Drosophila egg chamber, the ER exit site factor Tango1 is required for basal secretion, while Rab10 and the GEF Crag restrict basement membrane secretion and assembly basally. (C) Integrin binding to extracellular laminin that is organised into a basement membrane induces polarity signalling through the scaffolding factor ILK. The Par3 complex forms independently, but basal ECM–integrin positioning is needed to orientate the apical surface.
A remaining important question is whether this cascade of signalling is restricted to development and regeneration. Given that the epithelia of mature animals are normally attached to basement membrane, the signalling pathways for producing de novo basement membranes might only apply during tissue regeneration. For example, damaged keratinocytes migrate across collagen- or fibrin-containing ECM to repair wounds, but then they have to re-establish basement membranes and polarity in order to re-form an intact epithelium. In addition, mesenchymal to epithelial transformations occur in development and during the colonisation of certain cancer types at a secondary site. Again this process requires a transition from interaction of cells with the stromal ECM to those with basement membranes. In the future, it will be important to identify the requirement for β1 integrin and Rac1 signalling in such processes.
A further area to be clarified is whether the mechanisms linking integrins to polarity show differences across different epithelia. For example, although mammary and intestinal epithelia both form structures that contain hollow lumens, they differ fundamentally in that the former secrete proteins into the lumen whereas the latter absorb fluids from the lumen. Evidence for tissue-specific mechanisms of establishing polarity comes from C. elegans, where laminin is essential to orientate epithelial polarity in the pharynx but not the intestine (Rasmussen et al., 2012).
Sequential outside-in signals for basement membrane assembly and intracellular polarity might also be mediated by separate integrin heterodimers, although this has not been examined yet. For example, collagen might activate Rac1 through α1β1, α2β1 or α11β1 integrins, whereas the response to laminin for intracellular polarity is more likely to be mediated by α3β1 and α6β1. Other ECM receptors, such as the laminin-binding receptor dystroglycan, might contribute to basement membrane deposition and epithelial polarity in some but not all cell types (Esser et al., 2010; Masuda-Hirata et al., 2009; Weir et al., 2006). The collagen-binding discoidin domain receptor is involved in cell–cell adhesion and migration, but whether it has a role in polarity is not yet known (Wang et al., 2006; Yeh et al., 2011).
Integrin-dependent microtubule dynamics control apical–basal polarity in epithelial cells
Microtubules are key to establishing and maintaining polarity. Disrupting microtubule assembly and turnover with either destabilising or stabilising drugs prevents epithelial cells from polarising properly. The mechanisms linking microtubules to epithelial polarity are not well understood, but clues can be gleaned from what is known about microtubules in migratory cells (Stehbens and Wittmann, 2012).
During migration, cells become polarised to generate a leading edge that protrudes forward and makes integrin-mediated contacts with the ECM, whereas the rear of the cell disassembles its focal adhesions and retracts (Huttenlocher and Horwitz, 2011). In migrating cells, the minus-ends of microtubules originate at the centrosome, which is close to the nucleus, and the plus-ends are orientated towards the leading edge. Plus-ends grow persistently until they near the base of the lamellipodia, where their instability increases and they undergo oscillations of lengthening and shortening (Waterman-Storer and Salmon, 1997). This microtubular dynamic instability contributes to actin remodelling within migratory protrusions, and promotes the rapid activation of migration should the need arise in development or regeneration. Microtubules also deliver specific cargos to the leading edge of the cell in order to maintain lateral polarity and thereby movement of the cell in a polarised direction (Gennerich and Vale, 2009).
In contrast to migrating cells, the centrosome of polarised epithelia is located apically and provides an organising centre for apical microtubules whose plus-ends are associated with primary cilia and adherens junctions (Sugioka and Sawa, 2012). These microtubules allow the transport machinery to deliver cilial regulatory proteins, and they promote cadherin clustering to maintain cell–cell junctions through the plus-end-binding protein CLIP170, and recruit myosin II for mechanical integrity at tight junctions (Bellett et al., 2009; Stehbens et al., 2006; Sumigray et al., 2012). However, the majority of the microtubules in epithelial cells are non-centrosomal and are aligned along the apical–basal axis, with their plus-ends orientated towards the basolateral membrane (Lüders and Stearns, 2007). It is not fully understood where the non-centrosomal microtubule minus-ends are anchored, but they can be stabilised at adherens junctions by adaptors such a calmodulin regulated spectrin-associated protein (CAMSAP) 2 or 3 (Meng et al., 2008). In polarised epithelial cells, non-centrosomal microtubules are crosslinked by microtubule crosslinking factor 1 (MTCL1). MTCL1 is recruited to microtubule bundles by the Par protein Par1b, and together these proteins determine the correct balance between dynamic and stable microtubules (Sato et al., 2013). Microtubules can also form self-associating networks at the basal surface of polarised epithelia (Reilein et al., 2005).
Integrins have a central role in controlling non-centrosomal microtubules in polarised epithelia. In mammary epithelia, deleting β1 intergins prevents microtubules from aligning along the apical–basal axis and reduces microtubule stability (Akhtar and Streuli, 2013). The mechanism for this is through indirect links that form between integrin adhesion complexes and the plus-end-binding protein EB1. Integrins and microtubules therefore have a close relationship in establishing the orientation of apical–basal polarity. This association with integrins is also extended to centrosomal microtubules because integrin deletion disrupts the distribution of the Golgi, which is located apical to the nucleus and in the vicinity of centrosomes. In mammary epithelia, the consequence of disrupted polarity is severe because glandular lumens cannot form, preventing the polarised secretion of differentiation products for normal tissue function.
Together, this indicates that in normal epithelial cell sheets, non-centrosomal microtubules are regulated by integrins and are essential for organising apical-basal polarity. It is most likely that integrin adhesions tether microtubule plus-ends, while microtubule motors transport adhesion complex proteins to assemble integrin junctions properly, and together these two mechanisms are intertwined in a positive feedback loop to enforce basal polarity.
It has not yet been established whether the plus-ends of microtubules in stationary polarised epithelia show similar dynamic instability to those in migrating cells. Although integrin trafficking by vesicles and motor proteins is a key feature of the polarity of migrating cells, their role in apical–basal epithelial polarity has not been established (Jacquemet et al., 2013). The plus-end-binding proteins that tether microtubules to integrins might also vary between tissues or species, because although EB1 is required for polarity in MECs, it is not essential in MDCK cells (Gierke and Wittmann, 2012). Live imaging of microtubules, plus-end-binding proteins and microtubule-organizing centre (MTOC) components in 3D-organotypic cultures will likely resolve these issues, and will also confirm whether microtubules are needed both to set up and to maintain apical–basal polarity.
ILK recruits microtubules to active β1 integrin in polarised epithelia
The mechanisms linking integrins to the plus ends of microtubules in polarised epithelia are beginning to be clarified. In mammary epithelial cells, ILK is required for the association between β1 integrin and EB1, and for the apical–basal orientation of non-centrosomal microtubules (Akhtar and Streuli, 2013). However, the exact adaptor proteins that form this linkage are unknown. The plus-ends of microtubules attach directly to EB1 through its N-terminus, whereas the C-terminus of EB1 binds a variety of stabilising plus-end-tracking (+TIP) proteins. These include cytoplasmic linker associated proteins (CLASPs) that can also bind to sides of microtubules, CLIP170 (also known as CLIP1), adenomatous polyposis coli (APC) and the spectraplakins (Akhmanova and Steinmetz, 2008). EB1, APC and the dynactin subunit p150glued (also known as DCTN1) all associate with and organise microtubules that are localised to the basal cortex of polarised epithelia (Reilein and Nelson, 2005). +TIP proteins and their interactors are therefore likely to link microtubules with integrin-containing adhesions in epithelia.
One candidate linker protein is the pleckstrin-homology-like domain family B members LL5α and LL5β (also known as PHLDB1 and PHLDB2, respectively). LL5 was identified in mass-spectrometry-based assays as a CLASP-binding partner, which recruits microtubules to the cell cortex (Lansbergen et al., 2006). In polarised MECs, LL5 localises to the basal surface at the sites where integrins bind to laminin-5 within the basement membrane (Hotta et al., 2010). However, no direct interaction occurs between LL5 and integrins, indicating that other adaptor proteins are also involved. In the pre-gastrulation epithelium of the epiblast, LL5 cooperates with CLASPs to maintain basement membrane integrity and anchors microtubules to the basal cell cortex (Nakaya et al., 2013). In these cells, CLASP1 and CLASP2 associate with α-dystroglycan, which is also a laminin receptor, raising the possibility that the ECM coordinates microtubular orientation by both dystroglycan and the laminin-binding integrins α3β1 and α6β1.
A few other studies have been performed in polarised epithelia to identify how microtubules link with the basement membrane. However, the adaptors that are known to link microtubules with focal adhesions at the cortex of spreading and migrating cells might also be relevant in this context. For example, CLASP1 and CLASP2 are also involved at the leading edge of polarised migrating cells, where glycogen synthase kinase-3β (GSK3β) controls their phosphorylation and thereby the extent of their association with the plus ends compared to the sides of microtubules (Kumar et al., 2009; Wittmann and Waterman-Storer, 2005). Similarly, Par proteins might also have a role. The Par–TIAM polarity complex, which consists of Par3–aPKC–TIAM1, stabilises microtubules at the leading edge of migrating keratinocytes to control persistent polarised migration (Pegtel et al., 2007). However, in both of these cases it has not been established how integrins or adhesion complexes link to microtubule-associated proteins.
A further linker candidate is IQ motif containing GTPase activating protein (IQGAP1), which has been implicated in linking actin and microtubule dynamics in order to establish polarisation in migrating cells (Watanabe et al., 2004). IQGAP1 is a Rac1 and Cdc42 effector that is located at the leading edge of migratory cells and interacts with CLIP170 (Fukata et al., 2002). It is also an ILK-interacting partner that can anchor microtubules to immature focal adhesions in spreading keratinocytes. ILK recruits IQGAP1, which together with the formin, mDia1, stabilises microtubules at the cell cortex (Wickström et al., 2010). In addition to its role at the leading edge of migrating keratinocytes, ILK is involved with cancer cell migratory protrusions (Shibue et al., 2013). Interestingly, the activity and basolateral localisation of IQGAP1 is essential for correct lumen formation in MDCK cells, though this might be through a separate mechanism involving the accurate orientation of the mitotic spindle (Bañón-Rodríguez et al., 2014).
Together we conclude that β1 integrins are essential to maintain the apical–basal orientation of non-centrosomal microtubules in polarised epithelia, and they do so through ILK, LL5 and +TIP proteins (Fig. 2). Integrin–microtubule interactions appear to reduce microtubule dynamic instability and it is likely that, in turn, this positively reinforces the maintenance of epithelial polarity. However, we do not know the full details of the molecular links between adhesion complexes and +TIPs, for example, whether the adaptor proteins that link integrins to microtubules are specific to a cell type and whether they differ between spreading and/or migrating and stably polarised cells. Interestingly, dynamic imaging reveals an increased microtubule activity and a differential requirement for EB1 and +TIP proteins in the protrusions of cells that emigrate from monolayers of epithelial cells organised as 3D cysts (Gierke and Wittmann, 2012). Thus, integrin–microtubule interactions might have the function of anchoring microtubules in order to orientate their polarity, and these interactions may also play a role in integrin signalling.
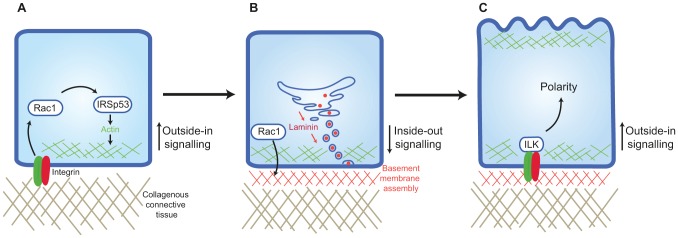
Fig. 2.
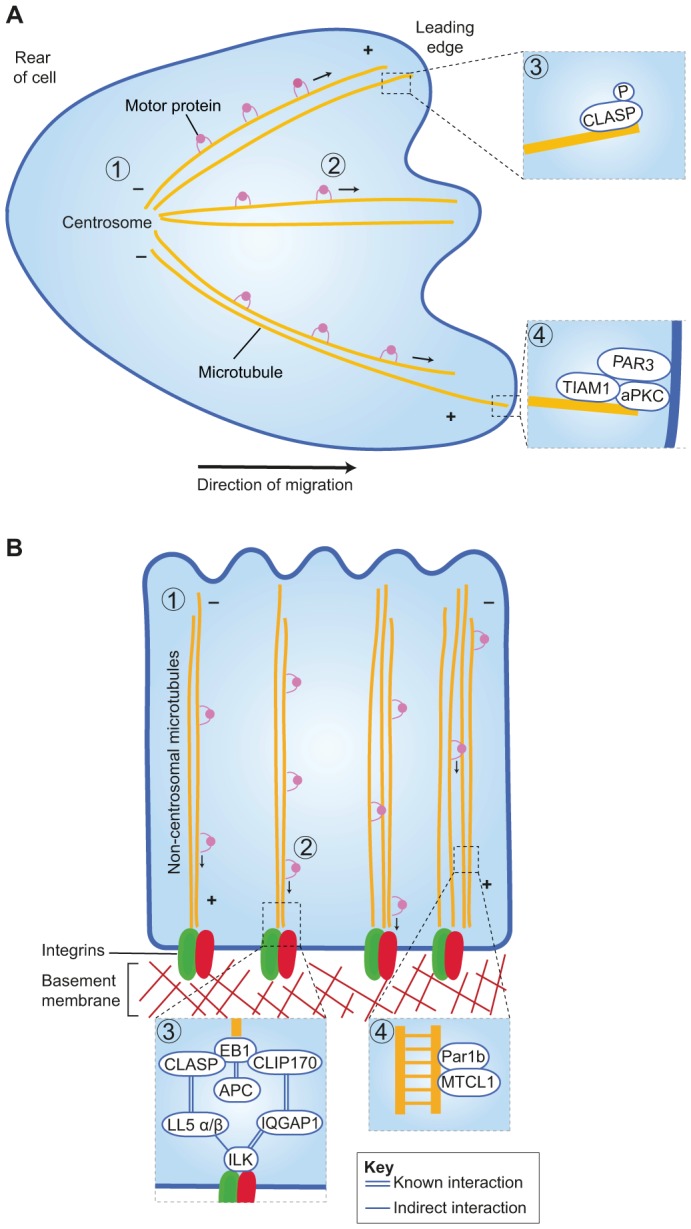
Microtubules polarise spreading cells for migration and epithelial cells in the apical–basal orientation. (A) In spreading and migrating cells, microtubules are orientated from the centrosome towards the periphery (1). Plus-end-directed microtubule motors transport cargoes to the leading edge (2). Phosphorylation of CLASP1 and/or CLASP2 controls the extent of their association with microtubule plus ends (3). The Par3–aPKC–TIAM1 complex stabilises microtubules at the leading edge to allow persistent migration (4). (B) In polarised epithelia, non-centrosomal microtubules are orientated along the apical–basal axis (1). Plus-end-directed motors transport adhesion proteins and other cargoes to the basal surface (2). Known and possible interactions linking integrins with microtubules (3). Par1b and the microtubule crosslinker MTCL1 determine the balance between stable and dynamic non-centrosomal apical–basal microtubules (4).
Microtubules are essential for transporting the proteins and lipids required for polarity
Microtubules also contribute to polarity through their involvement with protein transport. It is well established that the trafficking routes of intracellular proteins contribute to cell polarity (Shivas et al., 2010). There are three means by which proteins in epithelial cells are sorted to their correct destination: proteins can be transported directly to their destination through sorting at the trans-Golgi network, or to the basolateral membrane by default before being endocytosed and indirectly sorted to their correct destination. Alternatively, protein sorting might be random and influenced by other factors such as the rate of degradation at different areas of the plasma membrane (Carmosino et al., 2010). In addition, various endocytic pathways exist, including pathways that depend upon clathrin, caveolin-1 and flotillin. Each of these proteins binds to the plasma membrane to create an enriched domain, which becomes the site of the budding vesicle (Doherty and McMahon, 2009).
One role for integrin-mediated stabilisation of microtubules may be to control their ability to transport cargoes, i.e. proteins and specialised lipid domains that are required for the integrity of the basal cell surface, as well as basement membrane protein secretion. Kif3a is a component of the plus-end-directed motor kinesin-2, which is required for microtubular function at the leading edge of migrating kidney cells and for the formation of 3D epithelial acini, possibly through its vesicle transport function and/or by controlling microtubule dynamics (Boehlke et al., 2013). Another plus-end kinesin, Kif16b, mediates transcytosis to the apical membrane in polarised epithelial cells that lack expression of the clathrin-adaptor AP-1B1 (Perez Bay et al., 2013). Moreover, the kinesin Kif17 associates with +TIPs, stabilises microtubules and is required for apico-basal polarisation and lumen formation in 3D MDCK cell cultures (Jaulin and Kreitzer, 2010). Kinesins might also deliver proteins that control integrin activation at the basal cell surface of polarised epithelia. The small GTPase Rap1, which signals downstream of integrins, has been implicated in the establishment of polarity (Gérard et al., 2007; Itoh et al., 2007). Radil, an effector of Rap1, localises with microtubules by binding to kinesin Kif14, which may control the inside-out activation of integrins by limiting the availability of Radil (Ahmed et al., 2012). The ILK-mediated link between integrins and microtubules is also required for the fusion of caveolae to the plasma membrane of keratinocytes, thereby helping to establish caveolae-based signalling platforms on the basal epithelial surface (Wickström et al., 2010).
In addition to the possible role for integrins in transporting components towards the basal cell surface, integrins are also required to traffic some proteins away from this membrane. ECM overlay assays to re-orientate epithelial polarity revealed that β1 integrins have an indispensable role in the transport of the tight junction proteins zona occludens 1 (ZO-1) and claudins away from the apical pole during polarity inversion (Akhtar and Streuli, 2013). Internalisation of these proteins also relies on the GTPase dynamin, indicating a role for endocytic trafficking. During indirect protein transport, the early endosome acts a sorting centre where cargo can be re-routed to an alternative destination. Sorting is controlled by Rabs, for example Rab5 mediates vesicular fusion with the early endosome (Gorvel et al., 1991; Rubino et al., 2000). The expression of a dominant-negative Rab5 construct in mammary epithelia results in inhibition of lumen formation, indicating that early endosomal trafficking is essential to correctly establish polarity (Akhtar and Streuli, 2013).
Clathrin has also been implicated in trafficking of proteins from the Golgi to both the basolateral and apical membranes in order to maintain polarity (Gravotta et al., 2012). These processes may be linked to integrin-mediated control of polarity, because in epithelial cells that are cultured on patterned substrata, clathrin-mediated endocytosis of basally located receptors such as the transferrin receptor is enriched at cell-matrix adhesions (Grossier et al., 2014).
Taken together, these studies demonstrate that integrins contribute to the orientation of epithelial polarity by controlling vesicle trafficking, both towards and away from the basal cell membrane (Fig. 3). However the exact cargoes and vesicle trafficking systems that are used are not yet fully understood. It will be important to determine whether integrins can induce more than one endocytic pathway from the basal cell surface in order to establish polarity, and precisely which Rabs and their regulators are involved.
Fig. 3.
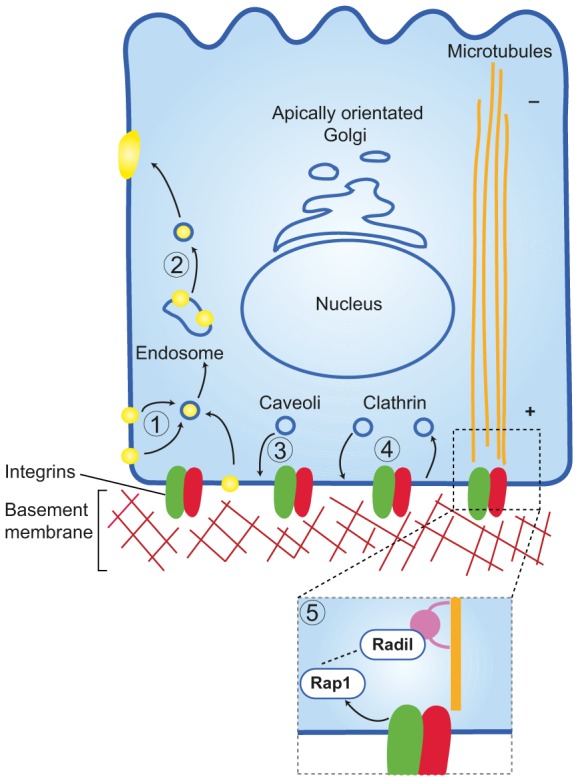
Trafficking events required to establish basal membrane identity. Several trafficking events are involved in establishing basolateral membrane polarity. During polarity inversion, apical components such as tight junction proteins are trafficked away from the apical surface in a β1-integrin-dependent manner to create a basally defined surface (1). Rab-dependent endosomal trafficking is also required to transcytose apical components away from the basal surface in order to establish epithelial polarity (2). Caveolae are delivered to the basolateral surface in an ILK-dependent mechanism to establish basal caveolae signalling platforms that provide a distinct lipid microenvironment at the basolateral membrane (3). Clathrin-dependent trafficking is implicated in delivering Golgi-derived basolateral membrane proteins, such as the transferrin and LDL receptors, to the basal membrane (4). Radil sequestration and transport by Klf14, a component of plus-end-directed microtubule motors, regulates Rap1-mediated integrin signalling, which might control polarity originating at the basolateral membrane (5).
Although the mechanisms of integrin internalisation and trafficking are under current investigation, little is known about the molecular links between integrins and the endocytosis of apical proteins. One possibility is that Par5 regulates the trafficking of Rab11 and other apical proteins away from the basal surface (Winter et al., 2012). It is not clear whether there is a trans-acting link between integrins and endocytosis, or whether integrins are constitutively recycled together with tight junction proteins in order to facilitate the redistribution of apical proteins. In addition, integrins are also responsible for endocytosis of various cell surface receptors, for example β1 integrin controls the internalisation of both platelet-derived growth factor (PDGF) and bone morphogenetic protein (BMP) receptors (Du et al., 2011; King et al., 2011). It is unknown whether this can contribute to the orientation of polarity.
Integrins and polarity in cancer
Loss of polarity and subsequent tissue disorganisation is a hallmark of cancer (Lee and Vasioukhin, 2008). Indeed, one of the defining changes in early cancer, for example the in situ lesions of breast cancer, is disrupted polarity. Oncogenes in disorganised tissues act differently to those with correct polar morphology. For example activated c-Myc causes hyperproliferation in MECs that have not established an ordered structure, but its transforming ability is suppressed by the polarity regulator liver kinase B1 (LKB1, also known as STK11) in organised 3D acini (Partanen et al., 2007). It is not clear whether the inability of c-Myc to transform epithelial cells organised in a 3D acini is caused by cell polarity suppressing c-Myc function or gene expression (Partanen and Klefstrom, 2012; Simpson et al., 2011). In any case the evidence suggests that polarity can act as a barrier to oncogenic transformation.
Studies such as these argue that polarity acts as a barrier against oncogenic signalling in epithelia. This concept is supported by studies of how integrins control the orientation of the spindle pole during division. For example, in the Drosophila midgut, integrin-dependent adhesion to the basement membrane induces cell polarity, which is accompanied by the asymmetric distribution of Par complex proteins, such as atypical protein kinase C (aPKC), and the production of two daughter cells with different cell fates. Perturbing integrin or aPKC expression results in the formation of intestinal tumours, indicating that integrin-mediated cell polarity suppresses oncogenesis in the fly midgut (Goulas et al., 2012).
It is now established that polarity provides a barrier to oncogenesis in epithelia. This suggests that perturbations in the basement-membrane–integrin–ILK–microtubule axis might contribute to cancer progression. Supporting this model are the results of genomic sequencing approaches which reveal that mutations in a wide range of ECM components, integrins and cytoskeletal components occur in primary breast cancers (Ellis et al., 2012; Shah et al., 2012). Furthermore, activating mutations in β1 integrin increase the susceptibility for tumour formation derived from epithelial cells in the skin, whereas perturbed β1 integrin expression correlates with poor prognosis in breast cancer patients (dos Santos et al., 2012; Ferreira et al., 2009).
It will be important to test directly whether mutations in basement membrane proteins, β1 integrins, and the proximal intracellular components that link integrins with polarity result in disturbed epithelial polarity in vivo. We need to know which members of the basal polarity axis cooperate with oncogenes or the loss of tumour suppressors, whether this axis is a causal factor in the initiation or progression of cancers, and to identify how to rescue the polarity of primary cancers and thereby revert tumorigenesis.
Conclusions
Integrins provide the central mechanism for cells in multicellular organisms to interact with and sense their extracellular environment. They control every aspect of cell function and, here, we have presented a model for how integrins are involved in establishing the orientation of epithelial cell polarity. This model provides a starting point to identify components of the integrin polarity axis that are altered in disease, and to develop strategies to correct such defects for therapeutic gain.
Supplementary Material
Acknowledgments
The authors thank Pat Caswell, University of Manchester, for helpful appraisal of the manuscript.
Footnotes
Competing interests
The authors declare no competing interests.
Funding
The Wellcome Trust supports the Wellcome Trust Centre for Cell-Matrix Research, and J.L.L. on a 4-year PhD programme.
References
- Ahmed S. M., Thériault B. L., Uppalapati M., Chiu C. W., Gallie B. L., Sidhu S. S., Angers S. (2012). KIF14 negatively regulates Rap1a-Radil signaling during breast cancer progression. J. Cell Biol. 199, 951–967 10.1083/jcb.201206051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhmanova A., Steinmetz M. O. (2008). Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat. Rev. Mol. Cell Biol. 9, 309–322 10.1038/nrm2369 [DOI] [PubMed] [Google Scholar]
- Akhtar N., Streuli C. H. (2013). An integrin-ILK-microtubule network orients cell polarity and lumen formation in glandular epithelium. Nat. Cell Biol. 15, 17–27 10.1038/ncb2646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apodaca G., Gallo L. I., Bryant D. M. (2012). Role of membrane traffic in the generation of epithelial cell asymmetry. Nat. Cell Biol. 14, 1235–1243 10.1038/ncb2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bañón-Rodríguez I., Gálvez-Santisteban M., Vergarajauregui S., Bosch M., Borreguero-Pascual A., Martín-Belmonte F. (2014). EGFR controls IQGAP1 basolateral membrane localization and mitotic spindle orientation during epithelial morphogenesis. EMBO J. 33, 129–145 10.1002/embj.201385946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellett G., Carter J. M., Keynton J., Goldspink D., James C., Moss D. K., Mogensen M. M. (2009). Microtubule plus-end and minus-end capture at adherens junctions is involved in the assembly of apico-basal arrays in polarised epithelial cells. Cell Motil. Cytoskeleton 66, 893–908 10.1002/cm.20393 [DOI] [PubMed] [Google Scholar]
- Benton R., St Johnston D. (2003). Drosophila PAR-1 and 14-3-3 inhibit Bazooka/PAR-3 to establish complementary cortical domains in polarized cells. Cell 115, 691–704 10.1016/S0092-8674(03)00938-3 [DOI] [PubMed] [Google Scholar]
- Boehlke C., Kotsis F., Buchholz B., Powelske C., Eckardt K. U., Walz G., Nitschke R., Kuehn E. W. (2013). Kif3a guides microtubular dynamics, migration and lumen formation of MDCK cells. PLoS ONE 8, e62165 10.1371/journal.pone.0062165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombardelli L., Carpenter E. S., Wu A. P., Alston N., DelGiorno K. E., Crawford H. C. (2010). Pancreas-specific ablation of beta1 integrin induces tissue degeneration by disrupting acinar cell polarity. Gastroenterology 138, 2531–40, 2540.e1-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgewater R. E., Norman J. C., Caswell P. T. (2012). Integrin trafficking at a glance. J. Cell Sci. 125, 3695–3701 10.1242/jcs.095810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant D. M., Mostov K. E. (2008). From cells to organs: building polarized tissue. Nat. Rev. Mol. Cell Biol. 9, 887–901 10.1038/nrm2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant D. M., Datta A., Rodríguez-Fraticelli A. E., Peränen J., Martín-Belmonte F., Mostov K. E. (2010). A molecular network for de novo generation of the apical surface and lumen. Nat. Cell Biol. 12, 1035–1045 10.1038/ncb2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmosino M., Valenti G., Caplan M., Svelto M. (2010). Polarized traffic towards the cell surface: how to find the route. Biol. Cell 102, 75–91 10.1042/BC20090134 [DOI] [PubMed] [Google Scholar]
- Cohen D., Fernandez D., Lázaro-Diéguez F., Müsch A. (2011). The serine/threonine kinase Par1b regulates epithelial lumen polarity via IRSp53-mediated cell-ECM signaling. J. Cell Biol. 192, 525–540 10.1083/jcb.201007002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley W. P., Gervais E. M., Centanni S. W., Gulfo K. M., Nelson D. A., Larsen M. (2012). ROCK1-directed basement membrane positioning coordinates epithelial tissue polarity. Development 139, 411–422 10.1242/dev.075366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty G. J., McMahon H. T. (2009). Mechanisms of endocytosis. Annu. Rev. Biochem. 78, 857–902 10.1146/annurev.biochem.78.081307.110540 [DOI] [PubMed] [Google Scholar]
- dos Santos P. B., Zanetti J. S., Ribeiro-Silva A., Beltrão E. I. (2012). Beta 1 integrin predicts survival in breast cancer: a clinicopathological and immunohistochemical study. Diagn. Pathol. 7, 104 10.1186/1746-1596-7-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J., Chen X., Liang X., Zhang G., Xu J., He L., Zhan Q., Feng X. Q., Chien S., Yang C. (2011). Integrin activation and internalization on soft ECM as a mechanism of induction of stem cell differentiation by ECM elasticity. Proc. Natl. Acad. Sci. USA 108, 9466–9471 10.1073/pnas.1106467108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis M. J., Ding L., Shen D., Luo J., Suman V. J., Wallis J. W., Van Tine B. A., Hoog J., Goiffon R. J., Goldstein T. C. et al. (2012). Whole-genome analysis informs breast cancer response to aromatase inhibition. Nature 486, 353–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser A. K., Cohen M. B., Henry M. D. (2010). Dystroglycan is not required for maintenance of the luminal epithelial basement membrane or cell polarity in the mouse prostate. Prostate 70, 777–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S. (2013). Microtubules in cell migration. Annu. Rev. Cell Dev. Biol. 29, 471–499 10.1146/annurev-cellbio-101011-155711 [DOI] [PubMed] [Google Scholar]
- Ewald A. J., Brenot A., Duong M., Chan B. S., Werb Z. (2008). Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev. Cell 14, 570–581 10.1016/j.devcel.2008.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira M., Fujiwara H., Morita K., Watt F. M. (2009). An activating beta1 integrin mutation increases the conversion of benign to malignant skin tumors. Cancer Res. 69, 1334–1342 10.1158/0008-5472.CAN-08-3051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata M., Watanabe T., Noritake J., Nakagawa M., Yamaga M., Kuroda S., Matsuura Y., Iwamatsu A., Perez F., Kaibuchi K. (2002). Rac1 and Cdc42 capture microtubules through IQGAP1 and CLIP-170. Cell 109, 873–885 10.1016/S0092-8674(02)00800-0 [DOI] [PubMed] [Google Scholar]
- Gennerich A., Vale R. D. (2009). Walking the walk: how kinesin and dynein coordinate their steps. Curr. Opin. Cell Biol. 21, 59–67 10.1016/j.ceb.2008.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gérard A., Mertens A. E., van der Kammen R. A., Collard J. G. (2007). The Par polarity complex regulates Rap1- and chemokine-induced T cell polarization. J. Cell Biol. 176, 863–875 10.1083/jcb.200608161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierke S., Wittmann T. (2012). EB1-recruited microtubule +TIP complexes coordinate protrusion dynamics during 3D epithelial remodeling. Curr. Biol. 22, 753–762 10.1016/j.cub.2012.02.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorvel J. P., Chavrier P., Zerial M., Gruenberg J. (1991). rab5 controls early endosome fusion in vitro. Cell 64, 915–925 10.1016/0092-8674(91)90316-Q [DOI] [PubMed] [Google Scholar]
- Goulas S., Conder R., Knoblich J. A. (2012). The Par complex and integrins direct asymmetric cell division in adult intestinal stem cells. Cell Stem Cell 11, 529–540 10.1016/j.stem.2012.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravotta D., Carvajal-Gonzalez J. M., Mattera R., Deborde S., Banfelder J. R., Bonifacino J. S., Rodriguez-Boulan E. (2012). The clathrin adaptor AP-1A mediates basolateral polarity. Dev. Cell 22, 811–823 10.1016/j.devcel.2012.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossier J. P., Xouri G., Goud B., Schauer K. (2014). Cell adhesion defines the topology of endocytosis and signaling. EMBO J. 33, 35–45 10.1002/embj.201385284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupton S. L., Gertler F. B. (2010). Integrin signaling switches the cytoskeletal and exocytic machinery that drives neuritogenesis. Dev. Cell 18, 725–736 10.1016/j.devcel.2010.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigo S. L., Bilder D. (2011). Global tissue revolutions in a morphogenetic movement controlling elongation. Science 331, 1071–1074 10.1126/science.1199424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T. J., Tepass U. (2010). Adherens junctions: from molecules to morphogenesis. Nat. Rev. Mol. Cell Biol. 11, 502–514 10.1038/nrm2927 [DOI] [PubMed] [Google Scholar]
- Heasman S. J., Ridley A. J. (2008). Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat. Rev. Mol. Cell Biol. 9, 690–701 10.1038/nrm2476 [DOI] [PubMed] [Google Scholar]
- Hohenester E., Yurchenco P. D. (2013). Laminins in basement membrane assembly. Cell Adh. Migr. 7, 56–63 10.4161/cam.21831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta A., Kawakatsu T., Nakatani T., Sato T., Matsui C., Sukezane T., Akagi T., Hamaji T., Grigoriev I., Akhmanova A. et al. (2010). Laminin-based cell adhesion anchors microtubule plus ends to the epithelial cell basal cortex through LL5alpha/beta. J. Cell Biol. 189, 901–917 10.1083/jcb.200910095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher A., Horwitz A. R. (2011). Integrins in cell migration. Cold Spring Harb. Perspect. Biol. 3, a005074 10.1101/cshperspect.a005074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M., Nelson C. M., Myers C. A., Bissell M. J. (2007). Rap1 integrates tissue polarity, lumen formation, and tumorigenic potential in human breast epithelial cells. Cancer Res. 67, 4759–4766 10.1158/0008-5472.CAN-06-4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemet G., Humphries M. J., Caswell P. T. (2013). Role of adhesion receptor trafficking in 3D cell migration. Curr. Opin. Cell Biol. 25, 627–632 10.1016/j.ceb.2013.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaulin F., Kreitzer G. (2010). KIF17 stabilizes microtubules and contributes to epithelial morphogenesis by acting at MT plus ends with EB1 and APC. J. Cell Biol. 190, 443–460 10.1083/jcb.201006044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao R. M. (2013). The luminal connection: from animal development to lumopathies. Organogenesis 9, 111–117 10.4161/org.25225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King S. J., Worth D. C., Scales T. M., Monypenny J., Jones G. E., Parsons M. (2011). β1 integrins regulate fibroblast chemotaxis through control of N-WASP stability. EMBO J. 30, 1705–1718 10.1038/emboj.2011.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G., Langegger M., Timpl R., Ekblom P. (1988). Role of laminin A chain in the development of epithelial cell polarity. Cell 55, 331–341 10.1016/0092-8674(88)90056-6 [DOI] [PubMed] [Google Scholar]
- Kumar P., Lyle K. S., Gierke S., Matov A., Danuser G., Wittmann T. (2009). GSK3beta phosphorylation modulates CLASP-microtubule association and lamella microtubule attachment. J. Cell Biol. 184, 895–908 10.1083/jcb.200901042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansbergen G., Grigoriev I., Mimori-Kiyosue Y., Ohtsuka T., Higa S., Kitajima I., Demmers J., Galjart N., Houtsmuller A. B., Grosveld F. et al. (2006). CLASPs attach microtubule plus ends to the cell cortex through a complex with LL5beta. Dev. Cell 11, 21–32 10.1016/j.devcel.2006.05.012 [DOI] [PubMed] [Google Scholar]
- Lechler T., Fuchs E. (2005). Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 437, 275–280 10.1038/nature03922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M., Vasioukhin V. (2008). Cell polarity and cancer – cell and tissue polarity as a non-canonical tumor suppressor. J. Cell Sci. 121, 1141–1150 10.1242/jcs.016634 [DOI] [PubMed] [Google Scholar]
- Lerner D. W., McCoy D., Isabella A. J., Mahowald A. P., Gerlach G. F., Chaudhry T. A., Horne-Badovinac S. (2013). A Rab10-dependent mechanism for polarized basement membrane secretion during organ morphogenesis. Dev. Cell 24, 159–168 10.1016/j.devcel.2012.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski K. T., Piwnica-Worms H. (2014). Phosphorylation of the E3 ubiquitin ligase RNF41 by the kinase Par-1b is required for epithelial cell polarity. J. Cell Sci. 127, 315–327 10.1242/jcs.129148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz K., Grashoff C., Torka R., Sakai T., Langbein L., Bloch W., Aumailley M., Fässler R. (2007). Integrin-linked kinase is required for epidermal and hair follicle morphogenesis. J. Cell Biol. 177, 501–513 10.1083/jcb.200608125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüders J., Stearns T. (2007). Microtubule-organizing centres: a re-evaluation. Nat. Rev. Mol. Cell Biol. 8, 161–167 10.1038/nrm2100 [DOI] [PubMed] [Google Scholar]
- Macara I. G. (2004). Parsing the polarity code. Nat. Rev. Mol. Cell Biol. 5, 220–231 10.1038/nrm1332 [DOI] [PubMed] [Google Scholar]
- Mack N. A., Whalley H. J., Castillo-Lluva S., Malliri A. (2011). The diverse roles of Rac signaling in tumorigenesis. Cell Cycle 10, 1571–1581 10.4161/cc.10.10.15612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda-Hirata M., Suzuki A., Amano Y., Yamashita K., Ide M., Yamanaka T., Sakai M., Imamura M., Ohno S. (2009). Intracellular polarity protein PAR-1 regulates extracellular laminin assembly by regulating the dystroglycan complex. Genes Cells 14, 835–850 10.1111/j.1365-2443.2009.01315.x [DOI] [PubMed] [Google Scholar]
- McClatchey A. I. (2014). ERM proteins at a glance. J. Cell Sci. 127, 3199–3204 10.1242/jcs.098343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng W., Mushika Y., Ichii T., Takeichi M. (2008). Anchorage of microtubule minus ends to adherens junctions regulates epithelial cell-cell contacts. Cell 135, 948–959 10.1016/j.cell.2008.09.040 [DOI] [PubMed] [Google Scholar]
- Muthuswamy S. K., Xue B. (2012). Cell polarity as a regulator of cancer cell behavior plasticity. Annu. Rev. Cell Dev. Biol. 28, 599–625 10.1146/annurev-cellbio-092910-154244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya Y., Sukowati E. W., Sheng G. (2013). Epiblast integrity requires CLASP and Dystroglycan-mediated microtubule anchoring to the basal cortex. J. Cell Biol. 202, 637–651 10.1083/jcb.201302075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor M. J., Li N., Cheung J., Lowe E. T., Lambert E., Marlow R., Wang P., Schatzmann F., Wintermantel T., Schüetz G. et al. (2005). Ablation of beta1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. J. Cell Biol. 171, 717–728 10.1083/jcb.200503144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngok S. P., Lin W. H., Anastasiadis P. Z. (2014). Establishment of epithelial polarity - GEF who's minding the GAP? J. Cell Sci. 127, 3205–3215 10.1242/jcs.153197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien L. E., Jou T. S., Pollack A. L., Zhang Q., Hansen S. H., Yurchenco P., Mostov K. E. (2001). Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat. Cell Biol. 3, 831–838 10.1038/ncb0901-831 [DOI] [PubMed] [Google Scholar]
- Ojakian G. K., Schwimmer R. (1994). Regulation of epithelial cell surface polarity reversal by beta 1 integrins. J. Cell Sci. 107, 561–576 [PubMed] [Google Scholar]
- Partanen J. I., Klefstrom J. (2012). Impact of epithelial organization on Myc expression and activity—letter. Cancer Res. 72, 1035.author reply 1036 10.1158/0008-5472.CAN-11-2201 [DOI] [PubMed] [Google Scholar]
- Partanen J. I., Nieminen A. I., Mäkelä T. P., Klefstrom J. (2007). Suppression of oncogenic properties of c-Myc by LKB1-controlled epithelial organization. Proc. Natl. Acad. Sci. USA 104, 14694–14699 10.1073/pnas.0704677104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegtel D. M., Ellenbroek S. I., Mertens A. E., van der Kammen R. A., de Rooij J., Collard J. G. (2007). The Par-Tiam1 complex controls persistent migration by stabilizing microtubule-dependent front-rear polarity. Curr. Biol. 17, 1623–1634 10.1016/j.cub.2007.08.035 [DOI] [PubMed] [Google Scholar]
- Perez Bay A. E., Schreiner R., Mazzoni F., Carvajal-Gonzalez J. M., Gravotta D., Perret E., Lehmann Mantaras G., Zhu Y. S., Rodriguez-Boulan E. J. (2013). The kinesin KIF16B mediates apical transcytosis of transferrin receptor in AP-1B-deficient epithelia. EMBO J. 32, 2125–2139 10.1038/emboj.2013.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen J. P., Reddy S. S., Priess J. R. (2012). Laminin is required to orient epithelial polarity in the C. elegans pharynx. Development 139, 2050–2060 10.1242/dev.078360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilein A., Nelson W. J. (2005). APC is a component of an organizing template for cortical microtubule networks. Nat. Cell Biol. 7, 463–473 10.1038/ncb1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilein A., Yamada S., Nelson W. J. (2005). Self-organization of an acentrosomal microtubule network at the basal cortex of polarized epithelial cells. J. Cell Biol. 171, 845–855 10.1083/jcb.200505071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney N., Streuli C. H. (2011). How integrins control mammary epithelial differentiation: a possible role for the ILK-PINCH-Parvin complex. FEBS Lett. 585, 1663–1672 10.1016/j.febslet.2011.05.014 [DOI] [PubMed] [Google Scholar]
- Rubino M., Miaczynska M., Lippé R., Zerial M. (2000). Selective membrane recruitment of EEA1 suggests a role in directional transport of clathrin-coated vesicles to early endosomes. J. Biol. Chem. 275, 3745–3748 10.1074/jbc.275.6.3745 [DOI] [PubMed] [Google Scholar]
- Rudkouskaya A., Welch I., Dagnino L. (2014). ILK modulates epithelial polarity and matrix formation in hair follicles. Mol. Biol. Cell 25, 620–632 10.1091/mbc.E13-08-0499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T., Li S., Docheva D., Grashoff C., Sakai K., Kostka G., Braun A., Pfeifer A., Yurchenco P. D., Fässler R. (2003). Integrin-linked kinase (ILK) is required for polarizing the epiblast, cell adhesion, and controlling actin accumulation. Genes Dev. 17, 926–940 10.1101/gad.255603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Akitsu M., Amano Y., Yamashita K., Ide M., Shimada K., Yamashita A., Hirano H., Arakawa N., Maki T. et al. (2013). The novel PAR-1-binding protein MTCL1 has crucial roles in organizing microtubules in polarizing epithelial cells. J. Cell Sci. 126, 4671–4683 10.1242/jcs.127845 [DOI] [PubMed] [Google Scholar]
- Scita G., Confalonieri S., Lappalainen P., Suetsugu S. (2008). IRSp53: crossing the road of membrane and actin dynamics in the formation of membrane protrusions. Trends Cell Biol. 18, 52–60 10.1016/j.tcb.2007.12.002 [DOI] [PubMed] [Google Scholar]
- Shah S. P., Roth A., Goya R., Oloumi A., Ha G., Zhao Y., Turashvili G., Ding J., Tse K., Haffari G. et al. (2012). The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature 486, 395–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibue T., Brooks M. W., Weinberg R. A. (2013). An integrin-linked machinery of cytoskeletal regulation that enables experimental tumor initiation and metastatic colonization. Cancer Cell 24, 481–498 10.1016/j.ccr.2013.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivas J. M., Morrison H. A., Bilder D., Skop A. R. (2010). Polarity and endocytosis: reciprocal regulation. Trends Cell Biol. 20, 445–452 10.1016/j.tcb.2010.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson D. R., Yu M., Zheng S., Zhao Z., Muthuswamy S. K., Tansey W. P. (2011). Epithelial cell organization suppresses Myc function by attenuating Myc expression. Cancer Res. 71, 3822–3830 10.1158/0008-5472.CAN-10-3782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrosal G., Pérez L., Herranz H., Milán M. (2010). Scarface, a secreted serine protease-like protein, regulates polarized localization of laminin A at the basement membrane of the Drosophila embryo. EMBO Rep. 11, 373–379 10.1038/embor.2010.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Johnston D., Ahringer J. (2010). Cell polarity in eggs and epithelia: parallels and diversity. Cell 141, 757–774 10.1016/j.cell.2010.05.011 [DOI] [PubMed] [Google Scholar]
- Stehbens S., Wittmann T. (2012). Targeting and transport: how microtubules control focal adhesion dynamics. J. Cell Biol. 198, 481–489 10.1083/jcb.201206050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehbens S. J., Paterson A. D., Crampton M. S., Shewan A. M., Ferguson C., Akhmanova A., Parton R. G., Yap A. S. (2006). Dynamic microtubules regulate the local concentration of E-cadherin at cell-cell contacts. J. Cell Sci. 119, 1801–1811 10.1242/jcs.02903 [DOI] [PubMed] [Google Scholar]
- Streuli C. H., Akhtar N. (2009). Signal co-operation between integrins and other receptor systems. Biochem. J. 418, 491–506 10.1042/BJ20081948 [DOI] [PubMed] [Google Scholar]
- Sugioka K., Sawa H. (2012). Formation and functions of asymmetric microtubule organization in polarized cells. Curr. Opin. Cell Biol. 24, 517–525 10.1016/j.ceb.2012.05.007 [DOI] [PubMed] [Google Scholar]
- Sumigray K. D., Foote H. P., Lechler T. (2012). Noncentrosomal microtubules and type II myosins potentiate epidermal cell adhesion and barrier formation. J. Cell Biol. 199, 513–525 10.1083/jcb.201206143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei I., Deugnier M. A., Faraldo M. M., Petit V., Bouvard D., Medina D., Fässler R., Thiery J. P., Glukhova M. A. (2008). Beta1 integrin deletion from the basal compartment of the mammary epithelium affects stem cells. Nat. Cell Biol. 10, 716–722 10.1038/ncb1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner K., Mori H., Mroue R., Bruni-Cardoso A., Bissell M. J. (2012). Coherent angular motion in the establishment of multicellular architecture of glandular tissues. Proc. Natl. Acad. Sci. USA 109, 1973–1978 10.1073/pnas.1119578109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townley A. K., Schmidt K., Hodgson L., Stephens D. J. (2012). Epithelial organization and cyst lumen expansion require efficient Sec13-Sec31-driven secretion. J. Cell Sci. 125, 673–684 10.1242/jcs.091355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. Z., Ojakian G. K., Nelson W. J. (1990). Steps in the morphogenesis of a polarized epithelium. II. Disassembly and assembly of plasma membrane domains during reversal of epithelial cell polarity in multicellular epithelial (MDCK) cysts. J. Cell Sci. 95, 153–165 [DOI] [PubMed] [Google Scholar]
- Wang C. Z., Su H. W., Hsu Y. C., Shen M. R., Tang M. J. (2006). A discoidin domain receptor 1/SHP-2 signaling complex inhibits alpha2beta1-integrin-mediated signal transducers and activators of transcription 1/3 activation and cell migration. Mol. Biol. Cell 17, 2839–2852 10.1091/mbc.E05-11-1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Lacoche S., Huang L., Xue B., Muthuswamy S. K. (2013). Rotational motion during three-dimensional morphogenesis of mammary epithelial acini relates to laminin matrix assembly. Proc. Natl. Acad. Sci. USA 110, 163–168 10.1073/pnas.1201141110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Wang S., Noritake J., Sato K., Fukata M., Takefuji M., Nakagawa M., Izumi N., Akiyama T., Kaibuchi K. (2004). Interaction with IQGAP1 links APC to Rac1, Cdc42, and actin filaments during cell polarization and migration. Dev. Cell 7, 871–883 10.1016/j.devcel.2004.10.017 [DOI] [PubMed] [Google Scholar]
- Waterman-Storer C. M., Salmon E. D. (1997). Actomyosin-based retrograde flow of microtubules in the lamella of migrating epithelial cells influences microtubule dynamic instability and turnover and is associated with microtubule breakage and treadmilling. J. Cell Biol. 139, 417–434 10.1083/jcb.139.2.417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir M. L., Oppizzi M. L., Henry M. D., Onishi A., Campbell K. P., Bissell M. J., Muschler J. L. (2006). Dystroglycan loss disrupts polarity and beta-casein induction in mammary epithelial cells by perturbing laminin anchoring. J. Cell Sci. 119, 4047–4058 10.1242/jcs.03103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickström S. A., Lange A., Hess M. W., Polleux J., Spatz J. P., Krüger M., Pfaller K., Lambacher A., Bloch W., Mann M. et al. (2010). Integrin-linked kinase controls microtubule dynamics required for plasma membrane targeting of caveolae. Dev. Cell 19, 574–588 10.1016/j.devcel.2010.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmaier M., Rognoni E., Radovanac K., Azimifar S. B., Fässler R. (2012). Integrin-linked kinase at a glance. J. Cell Sci. 125, 1839–1843 10.1242/jcs.093864 [DOI] [PubMed] [Google Scholar]
- Winter J. F., Höpfner S., Korn K., Farnung B. O., Bradshaw C. R., Marsico G., Volkmer M., Habermann B., Zerial M. (2012). Caenorhabditis elegans screen reveals role of PAR-5 in RAB-11-recycling endosome positioning and apicobasal cell polarity. Nat. Cell Biol. 14, 666–676 10.1038/ncb2508 [DOI] [PubMed] [Google Scholar]
- Wittmann T., Waterman-Storer C. M. (2005). Spatial regulation of CLASP affinity for microtubules by Rac1 and GSK3beta in migrating epithelial cells. J. Cell Biol. 169, 929–939 10.1083/jcb.200412114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh Y. C., Wu C. C., Wang Y. K., Tang M. J. (2011). DDR1 triggers epithelial cell differentiation by promoting cell adhesion through stabilization of E-cadherin. Mol. Biol. Cell 22, 940–953 10.1091/mbc.E10-08-0678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Datta A., Leroy P., O'Brien L. E., Mak G., Jou T. S., Matlin K. S., Mostov K. E., Zegers M. M. (2005). Beta1-integrin orients epithelial polarity via Rac1 and laminin. Mol. Biol. Cell 16, 433–445 10.1091/mbc.E04-05-0435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Shewan A. M., Brakeman P., Eastburn D. J., Datta A., Bryant D. M., Fan Q. W., Weiss W. A., Zegers M. M., Mostov K. E. (2008). Involvement of RhoA, ROCK I and myosin II in inverted orientation of epithelial polarity. EMBO Rep. 9, 923–929 10.1038/embor.2008.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zovein A. C., Luque A., Turlo K. A., Hofmann J. J., Yee K. M., Becker M. S., Fassler R., Mellman I., Lane T. F., Iruela-Arispe M. L. (2010). Beta1 integrin establishes endothelial cell polarity and arteriolar lumen formation via a Par3-dependent mechanism. Dev. Cell 18, 39–51 10.1016/j.devcel.2009.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


