Abstract
Background
In light of the recent trend toward earlier dialysis initiation and its association with mortality among patients with end-stage renal disease, we hypothesized that frailty is associated with higher estimated glomerular filtration rate (eGFR) at dialysis start and may confound the relation between earlier dialysis initiation and mortality.
Methods
We examined frailty among participants of the Comprehensive Dialysis Study (CDS), a special study of the US Renal Data System, which enrolled incident patients from September 1, 2005, through June 1, 2007. Patients were followed for vital status through September 30, 2009, and for time to first hospitalization through December 31, 2008. We used multivariate logistic regression to model the association of frailty with eGFR at dialysis start and proportional hazards regression to assess the outcomes of death or hospitalization.
Results
Among 1576 CDS participants included, the prevalence of frailty was 73%. In multivariate analysis, higher eGFR at dialysis initiation was associated with higher odds of frailty (odds ratio [OR], 1.44 [95% CI, 1.23–1.68] per 5 mL/min/1.73 m2; P<.001). Frailty was independently associated with mortality (hazard ratio [HR], 1.57 [95% CI, 1.25–1.97]; P<.001) and time to first hospitalization (HR, 1.26 [95% CI, 1.09–1.45]; P<.001). While higher eGFR at dialysis initiation was associated with mortality (HR, 1.12 [95% CI, 1.02–1.23] per 5 mL/min/1.73 m2; P=.02), the association was no longer statistically significant after frailty was accounted for (HR, 1.08 [95% CI, 0.98–1.19] per 5 mL/min/1.73 m2; P=.11).
Conclusions
Frailty is extremely common among patients starting dialysis in the United States and is associated with higher eGFR at dialysis initiation. Recognition of signs and symptoms of frailty by clinicians may prompt earlier initiation of dialysis and may explain, at least in part, the well-described association between eGFR at dialysis initiation and mortality.
Over the past decade, the proportion of patients starting dialysis earlier in the course of chronic kidney disease (CKD) has risen sharply. In 1996, fewer than 1 in 5 patients started dialysis with an estimated glomerular filtration rate (eGFR) 10 mL/min/1.73 m2 or greater; one-third did so in 2000 and more than half (54%) started dialysis with higher eGFR in 2009.1 The proportion of patients started on dialysis with an eGFR of 10 mL/min/1.73 m2 or greater is particularly high among elderly individuals.2,3 In spite of this consistent trend, there is no evidence of survival benefit with earlier initiation of dialysis; rather, observational studies have suggested higher mortality with higher eGFR at dialysis initiation.4,5 Confounding by indication has been suggested by some to explain this association. However, Rosansky et al4 recently demonstrated an association of higher eGFR at dialysis initiation with higher mortality in even “healthy” patients by studying an incident cohort younger than 65 years and with no reported comorbidities other than hypertension.
To further elucidate the benefits and risks associated with earlier initiation of dialysis, it is important to explore which clinical factors influence health care providers’ decision to initiate dialysis. Frailty is a clinical syndrome highly prevalent in the population with end-stage renal disease (ESRD); we previously showed frailty to be strongly associated with mortality and the composite outcome of death and hospitalization.6
Using data from the Comprehensive Dialysis Study (CDS), a national cohort of patients new to dialysis, we aimed to describe the prevalence of frailty and to test the hypothesis that frailty is associated with earlier initiation of dialysis. We also aimed to determine whether the association between eGFR and mortality—described in large registry studies—might be explained, at least in part, by frailty.
METHODS
PATIENTS
The CDS is a special study of the US Renal Data System (US-RDS), and the details of its study design have been previously published.7 Briefly, the CDS was designed as a prospective study of incident patients receiving maintenance dialysis at 335 dialysis units throughout the United States. From September 1, 2005, through June 1, 2007, a total of 1678 eligible adult incident maintenance dialysis patients were enrolled (only 296 of the 335 dialysis units participated). Institutional review boards at the Nutrition Special Study Center (SSC) (University of California, San Francisco, and University of California, Davis), the Rehabilitation/Quality of Life SSC (Emory University), and the USRDS Coordinating Center (University of Minnesota) approved the study. All participants provided informed consent. For the current analysis, those with missing data to determine frailty status (n=49) or eGFR (n=14) were excluded, as well as those participants who were unable to ambulate or transfer (n=39), leaving 1576 participants from 295 dialysis units in the analytic cohort.
PATIENT DEMOGRAPHICS, CLINICAL MEASURES, AND OUTCOME VARIABLES
We obtained data on age; sex; race/ethnicity; smoking status; Medicaid coverage at the start of dialysis; comorbid conditions, including the inability to transfer or ambulate; use of erythropoietin; selected laboratory values (serum albumin, creatinine, and hemoglobin levels) within 45 days of dialysis initiation; and whether patients were under the care of a nephrologist before initiation of dialysis from the Medical Evidence Report (CMS form No. 2728). We calculated eGFR using the 4-variable Modification of Diet in Renal Disease (MDRD) study formula.8 We recorded treatment modality (peritoneal dialysis vs hemodialysis) at the time of CDS enrollment.
All CDS participants provided responses to a patient questionnaire via telephone interviews by trained interviewers at the DataBanque Research Services (Pittsburgh, Pennsylvania). The questionnaires included the RAND 12-item Short Form (SF-12) survey, version 0.2,9 the 36-item Kidney Disease Quality of Life (KDQOL-36) symptoms/problems scale,10 and the Human Activity Profile (HAP).11
We identified dates of death and hospitalizations from the USRDS Standard Analysis Files. We followed patients through September 30, 2009, for vital status and December 31, 2008, for hospitalization records. The difference in the follow-through dates was due to the lag in the availability of hospitalization data in the USRDS.
FRAILTY DEFINITION
The phenotype of frailty was established using criteria similar to the earlier modification of Fried’s criteria (Fried et al12) by Johansen et al6 (eTable; http://www.archinternmed.com). However, since the CDS did not have data on weight loss, this variable was not included. We used a score of less than 75 on the Physical Function (PF) scale of the SF-12 as a marker for slowness and weakness. We classified patients as meeting the exhaustion criterion if they answered “a little of the time” or “none of the time” when asked about how much of the time during the past 4 weeks they felt they had a lot of energy, or if they reported that they felt “very much” or “extremely bothered” by feeling washed out or drained during the past 4 weeks. We defined low physical activity as the lowest quintile of Adjusted Activity Score of the HAP (stratified by age and sex based on normative data). The HAP has been previously validated in the ESRD population.13 In the initial modification by Woods et al,14 RAND-36 PF score lower than 75 was used to define both slowness and weakness and was therefore assigned 2 points in keeping with Fried’s definition. However, Johansen et al6 later found that the association of poor physical functioning with outcomes was similar to that of exhaustion and low physical activity among patients undergoing hemodialysis, suggesting that 1 point for this component of frailty was more appropriate. In our current analysis, we assigned 1 point for SF-12 PF scores lower than 75. Therefore, a total of 3 points were possible in the final frailty score, 1 each for slowness/weakness, exhaustion, and low physical activity; we defined patients with 2 or more points as frail.
STATISTICAL ANALYSIS
We a priori selected a set of baseline characteristics, including eGFR at the time of dialysis initiation, based on our clinical suspicion that they might be associated with frailty (Table 1). These factors were compared among frail and nonfrail patients using t test for continuous variables and the χ2 test for categorical variables. We converted serum albumin and hemoglobin concentrations to categorical variables based on clinically meaningful cut points; we also created a category of missing values. We assessed model discrimination using the concordance (“C”) statistic, equivalent to the area under the receiver operating characteristic curve. We used the Hosmer-Lemeshow test to assess model goodness of fit. We also assessed the relations among each of the frailty components and the same predictors using multivariate logistic regression.
Table 1.
Baseline Characteristics of Included Participants of the Comprehensive Dialysis Study (CDS)a
| Characteristic | No. (%)
|
P Value | ||
|---|---|---|---|---|
| Cohort (n = 1576) | Frail (n = 1155) | Not Frail (n = 421) | ||
| Age, mean (SD), y | 59.6 (14.2) | 60.0 (14.0) | 58.3 (14.6) | .03 |
| Male sex | 874 (55.5) | 594 (51.4) | 280 (66.5) | <.001 |
| White race | 1084 (68.8) | 809 (70.0) | 275 (65.3) | .07 |
| Current smoker | 111 (7.0) | 89 (7.7) | 22 (5.2) | .09 |
| Medicaid vs other payer | 373 (23.7) | 307 (26.6) | 66 (15.7) | <.001 |
| eGFR, mean (SD), mL/min/1.73 m2 | 10.0 (4.45) | 10.4 (4.52) | 8.8 (4.04) | <.001 |
| Albumin, mean (SD), g/dLb | 3.18 (0.71) | 3.15 (0.70) | 3.26 (0.71) | .02 |
| Hemoglobin, mean (SD), g/dLc | 10.08 (1.77) | 10.14 (1.76) | 9.92 (1.77) | .04 |
| Hemodialysis | 1411 (89.3) | 1038 (89.9) | 373 (88.6) | .53 |
| Comorbidity | ||||
| Diabetes mellitus | 820 (52.0) | 650 (56.3) | 170 (40.4) | <.001 |
| Congestive heart failure | 474 (30.1) | 381 (33.0) | 93 (22.1) | <.001 |
| Atherosclerotic heart disease | 367 (23.3) | 288 (24.9) | 79 (18.8) | .01 |
| CVA/TIA | 112 (7.1) | 96 (8.3) | 16 (3.8) | .002 |
| Peripheral vascular disease | 226 (14.3) | 189 (16.4) | 37 (8.8) | <.001 |
| COPD | 120 (7.6) | 104 (9.0) | 16 (3.8) | <.001 |
| Cancer | 95 (6.0) | 71 (6.2) | 24 (5.7) | .74 |
Abbreviations: COPD, chronic obstructive pulmonary disease; CVA/TIA, cerebrovascular accident/transient ischemic attack; eGFR, estimated glomerular filtration rate.
SI conversion factors: To convert albumin to grams per liter, multiply by 10; to convert hemoglobin to grams per liter, multiply by 10.
Data are presented as number (percentage) except where noted.
n=1199.
n=1412.
We used proportional hazards Cox regression to evaluate the outcomes of mortality or time to first hospitalization. We built 3 multivariate models. In addition to the variables selected a priori to study frailty, we included erythropoietin use prior to dialysis initiation and early nephrology referral in all 3 models. We included either frailty or eGFR at the time of dialysis initiation in each of the first 2 models. In the final model, we included both frailty and eGFR at the time of dialysis initiation simultaneously. We examined log (−log) plots and Schoenfeld residuals to test the proportionality assumption. To test whether frailty or eGFR at the time of dialysis initiation predicts mortality and hospitalization independent of each other, we tested the interaction between frailty and eGFR in our models.
We accounted for clustering by dialysis centers as previously described.15 In sensitivity analyses, we examined whether the results were altered by excluding patients with missing values for serum albumin and hemoglobin. We considered 2-tailed P < .05 as statistically significant. We conducted all analyses using STATA statistical software (version 11.1; StataCorp).
RESULTS
FRAILTY AND ITS ASSOCIATION WITH TIMING OF DIALYSIS INITIATION
Of 1576 patients included in the final analytic cohort, 73% were frail; even among patients younger than 40 years, the prevalence of frailty was 63%. Table 1 shows the baseline characteristics of included CDS participants and the unadjusted associations of patient characteristics and frailty. Frail patients had significantly higher mean eGFR at the initiation of dialysis (10.4 vs 8.8 mL/min/ 1.73 m2; P < .001). In multivariate analysis (Table 2), higher eGFR at the time of dialysis initiation was associated with higher odds of frailty independent of other predictors (odds ratio [OR], 1.44 [95% CI, 1.23–1.68] per 5 mL/min/1.73 m2; P < .001). Excluding those with missing values for either serum hemoglobin or albumin did not substantially change the results of the logistic regression. The model showed good discrimination (C statistic = 0.69) and was well calibrated (Hosmer-Lemeshow χ2 = 13.1; P = .11). Each of the frailty components was also significantly associated with higher eGFR at the time of dialysis initiation (OR, 1.47 [95% CI, 1.26–1.70] per 5 mL/min/1.73 m2 for slow and weak; OR, 1.17 [95% CI, 1.04–1.31] per 5 mL/min/1.73 m2 for exhaustion; OR, 1.62 [95% CI, 1.27–2.05] per 5 mL/min/1.73 m2 for physical inactivity).
Table 2.
Multivariate Model Examining Predictors of Frailtya
| Variable | Frail
|
|
|---|---|---|
| OR (95% CI) | P Value | |
| Age, per 10 y | 1.00 (0.90–1.11) | .98 |
| Male sex | 0.49 (0.39–0.62) | <.001 |
| White race | 1.25 (0.96–1.62) | .10 |
| Medicaid, vs other payers | 1.70 (1.22–2.36) | .002 |
| Current smoker | 1.27 (0.76–2.13) | .36 |
| eGFR, per 5 mL/min/1.73 m2 increase | 1.44 (1.23–1.68) | <.001 |
| Albumin quartiles/missing | .37 for trend | |
| Group 1: ≤2.5 g/dL | 0.98 (0.66–1.46) | .92 |
| Group 2: >2.5–3.0 g/dL | 1.12 (0.78–1.61) | .55 |
| Group 3: missing | 1.11 (0.80–1.54) | .53 |
| Group 4: >3.0–3.5 g/dL | 1 [Reference] | |
| Group 5: >3.5 g/dL | 0.85 (0.61–1.20) | .35 |
| Hemoglobin quartiles/missing | .07 for trend | |
| Group 1: ≤9 g/dL | 1 [Reference] | |
| Group 2: >9–10 g/dL | 1.07 (0.76–1.51) | .69 |
| Group 3: missing | 0.99 (0.63–1.57) | .97 |
| Group 4: >10–12 g/dL | 1.04 (0.77–1.42) | .80 |
| Group 5: >12 g/dL | 1.61 (1.02–2.53) | .04 |
| Hemodialysis | 1.04 (0.71–1.53) | .84 |
| Comorbidity | ||
| Diabetes mellitus | 1.52 (1.18–1.96) | .001 |
| Congestive heart failure | 1.27 (0.94–1.70) | .11 |
| Atherosclerotic heart disease | 0.96 (0.68–1.34) | .80 |
| CVA/TIA | 1.85 (1.04–3.28) | .04 |
| Peripheral vascular disease | 1.67 (1.16–2.41) | .006 |
| COPD | 1.77 (0.96–3.25) | .07 |
| Cancer | 1.19 (0.70–2.03) | .52 |
Abbreviations: COPD, chronic obstructive pulmonary disease; CVA/TIA, cerebrovascular accident/transient ischemic attack; eGFR, estimated glomerular filtration rate; OR, odds ratio.
SI conversion factors: To convert albumin to grams per liter, multiply by 10; to convert hemoglobin to grams per liter, multiply by 10.
Concordance statistic: C=0.69.
FRAILTY, DIALYSIS INITIATION, MORTALITY, AND HOSPITALIZATION
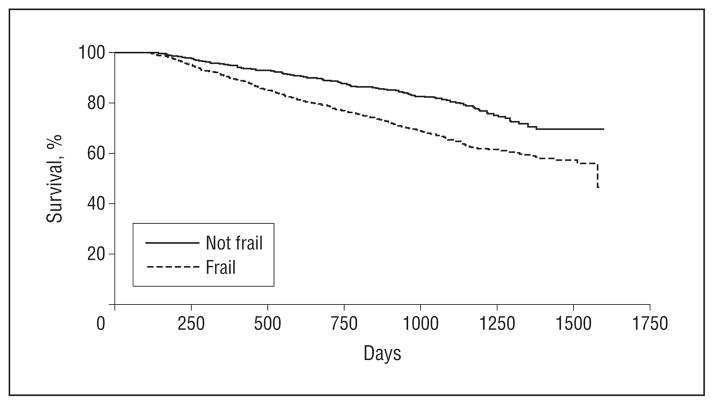
A total of 522 CDS participants (33%) died during a median follow-up of 2.9 years. In unadjusted analysis, those who were frail at baseline had a nearly 80% increase in the risk of death during follow-up (hazard ratio [HR], 1.79 [95% CI, 1.44–2.24]; P < .001) (Figure 1). Those who had higher eGFR at dialysis start had a 24% increase in the risk of death for every 5 mL/min/1.73 m2 increase in eGFR (HR, 1.24 [95% CI, 1.14–1.36]; P < .001). After adjustment for age, demographics, co-morbidities, tobacco use, Medicaid insurance status, serum albumin and hemoglobin values, erythropoietin use, nephrology referral, and modality, frailty (HR, 1.59 [95% CI, 1.27–1.99]; P < .001) and eGFR at dialysis initiation (HR, 1.12 [95% CI, 1.02–1.23] per 5 mL/min/1.73 m2; P = .02) were each individually associated with mortality (Table 3). When we included both frailty and eGFR in the same multivariate model, frailty remained significantly associated with higher mortality (HR, 1.57 [95% CI, 1.25–1.97]; P < .001), but eGFR was not (HR, 1.08 [95% CI, 0.98–1.19] per 5 mL/min/1.73 m2; P = .11), suggesting that the association between higher eGFR at dialysis initiation and mortality may have been confounded by frailty. There was no significant interaction between frailty and early start of dialysis (P = .73).
Figure 1.
Time to death. Kaplan-Meier plot of the unadjusted association between frailty and survival.
Table 3.
Correlates With Mortality in Multivariate Models
| Variable | HR (95% CI)a | P Value | HR (95% CI)b | P Value | HR (95% CI)c | P Value |
|---|---|---|---|---|---|---|
| Age, per 10 y | 1.39 (1.28–1.51) | <.001 | 1.38 (1.27–1.50) | <.001 | 1.38 (1.27–1.50) | <.001 |
| Male sex | 1.01 (0.86–1.20) | .87 | 0.93 (0.79–1.10) | .40 | 1.00 (0.85–1.18) | .99 |
| White race | 1.35 (1.10–1.65) | <.004 | 1.40 (1.15–1.71) | .001 | 1.35 (1.10–1.65) | .004 |
| Medicaid vs other payer | 1.16 (0.94–1.43) | .18 | 1.23 (1.00–1.51) | .05 | 1.15 (0.93–1.43) | .19 |
| Current smoker | 1.49 (1.06–2.10) | .02 | 1.47 (1.04–2.07) | .03 | 1.48 (1.05–2.08) | .03 |
| Frailty | 1.59 (1.27–1.99) | <.001 | 1.57 (1.25–1.97) | <.001 | ||
| eGFR per 5 mL/min/1.73m2 | 1.12 (1.02–1.23) | .02 | 1.08 (0.98–1.19) | .11 | ||
| Albumin quartiles/missing | <.001 for trend | .007 for trend | <.001 for trend | |||
| Group 1: ≤2.5 g/dL | 1.48 (1.14–1.92) | .003 | 1.45 (1.12–1.88) | .005 | 1.47 (1.13–1.90) | .004 |
| Group 2: >2.5–3.0 g/dL | 1.21 (0.90–1.62) | .21 | 1.18 (0.88–1.58) | .26 | 1.21 (0.90–1.63) | .21 |
| Group 3: missing | 1.16 (0.88–1.54) | .28 | 1.20 (0.91–1.59) | .20 | 1.16 (0.87–1.53) | .31 |
| Group 4: >3.0–3.5 g/dL | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| Group 5: >3.5 g/dL | 0.89 (0.68–1.17) | .40 | 0.90 (0.69–1.17) | .42 | 0.89 (0.67–1.17) | .40 |
| Hemoglobin quartiles/missing | .75 for trend | .75 for trend | .87 for trend | |||
| Group 1: ≤9 g/dL | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| Group 2: >9–10 g/dL | 1.06 (0.81–1.38) | .68 | 1.06 (0.82–1.37) | .68 | 1.06 (0.81–1.38) | .68 |
| Group 3: missing | 1.08 (0.77–1.52) | .65 | 1.06 (0.76–149) | .71 | 1.06 (0.76–1.49) | .73 |
| Group 4: >10–12 g/dL | 1.06 (0.86–1.31) | .60 | 1.02 (0.82–1.26) | .88 | 1.03 (0.83–1.28) | .78 |
| Group 5: >12 g/dL | 1.02 (0.75–1.40) | .88 | 0.96 (0.70–1.33) | .82 | 0.98 (0.71–1.36) | .92 |
| Hemodialysis vs PD | 0.74 (0.55–0.99) | .04 | 0.76 (0.57–1.00) | .06 | 0.74 (0.55–1.00) | .05 |
| Comorbidity | ||||||
| Diabetes mellitus | 1.02 (0.86–1.23) | .77 | 1.04 (0.87–1.25) | .65 | 1.00 (0.83–1.20) | >.99 |
| Congestive heart failure | 1.12 (0.92–1.36) | .27 | 1.12 (0.93–1.36) | .24 | 1.11 (0.91–1.35) | .30 |
| Atherosclerotic heart disease | 1.19 (0.99–1.46) | .10 | 1.17 (0.96–1.42) | .11 | 1.19 (0.97–1.46) | .09 |
| CVA/TIA | 0.88 (0.63–1.22) | .44 | 0.94 (0.70–1.28) | .70 | 0.87 (0.63–1.21) | .41 |
| Peripheral vascular disease | 1.23 (0.97–1.56) | .09 | 1.25 (0.99–1.58) | .06 | 1.27 (0.97–1.55) | .13 |
| COPD | 1.35 (0.99–1.84) | .06 | 1.32 (0.98–1.78) | .07 | 1.32 (0.97–1.81) | .08 |
| Cancer | 1.04 (0.74–1.46) | .83 | 1.12 (0.80–1.56) | .51 | 1.04 (0.74–1.46) | .83 |
| Erythropoietin use | .80 for homogeneity | .60 for homogeneity | .31 for homogeneity | |||
| Nonusers | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| Users | 1.01 (0.83–1.22) | .94 | 1.06 (0.86–1.29) | .59 | 1.02 (0.84–1.24) | .85 |
| Unknown or missing | 0.92 (0.69–1.21) | .55 | 0.92 (0.70–1.21) | .56 | 0.92 (0.70–1.22) | .58 |
| Early nephrology referral | .58 for homogeneity | .35 for homogeneity | .62 for homogeneity | |||
| No | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| Yes | 0.98 (0.79–1.23) | .89 | 0.96 (0.77–1.19) | .69 | 0.98 (0.79–1.23) | .87 |
| Unknown or missing | 1.17 (0.80–1.69) | .42 | 1.20 (0.83–1.74) | .32 | 1.15 (0.79–1.67) | .45 |
Abbreviations: COPD, chronic obstructive pulmonary disease; CVA/TIA, cerebrovascular accident/transient ischemic attack; eGFR, estimated glomerular filtration rate; HR, hazards ratio; PD, peritoneal dialysis.
SI conversion factors: To convert albumin to grams per liter, multiply by 10; to convert hemoglobin to grams per liter, multiply by 10.
Multivariate model in which frailty but not eGFR was included among predictor variables.
Multivariate model in which eGFR but not frailty was included among predictor variables.
Multivariate model in which both eGFR and frailty were included among predictor variables.
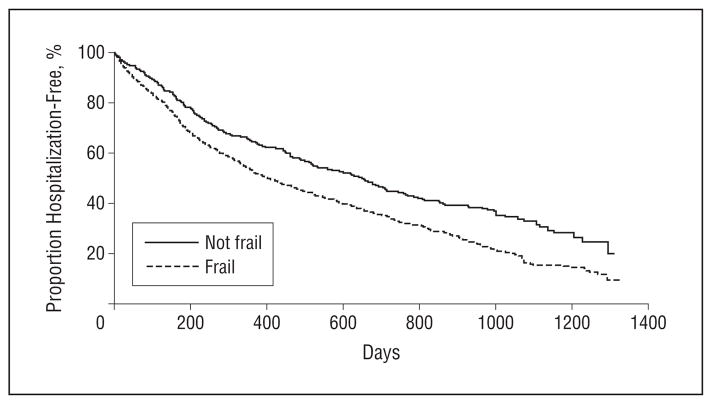
During a median follow-up of 1.2 years, 1148 of the participants (73%) were hospitalized at least once. In unadjusted analysis, frailty was associated with a higher risk of first hospitalization (HR, 1.44 [95% CI, 1.26–1.66]; P < .001) (Figure 2), as was higher eGFR at dialysis initiation (HR, 1.17 [95% CI, 1.10–1.24] per 5 mL/min/ 1.73 m2; P < .001). In a multivariate model (Table 4), when eGFR was not included, frailty was significantly associated with hospitalization (HR, 1.28 [95% CI, 1.11–1.48]; P = .001); when frailty was not included in the model, eGFR was significantly associated with hospitalization (HR, 1.09 [95% CI, 1.06–1.17] per 5 mL/min/ 1.73 m2; P = .006). When both frailty and eGFR were included in the multivariate model, both were significantly associated with time to first hospitalization (HR, 1.26 [95% CI, 1.09–1.45]; P = .001 for frailty; HR, 1.08 [95% CI, 1.01–1.15] per 5 mL/min/1.73 m2; P = .03 for eGFR). There was no significant interaction between frailty and eGFR at dialysis initiation (P = .90). Excluding patients with missing values for serum hemoglobin or albumin did not materially change the mortality or hospitalization model results.
Figure 2.
Time to first hospitalization. Kaplan-Meier plot of the unadjusted association between frailty and time to first hospitalization.
Table 4.
Correlates With First Hospitalization in Multivariate Models
| Variable | HR (95% CI)a | P Value | HR (95% CI)b | P Value | HR (95% CI)c | P Value |
|---|---|---|---|---|---|---|
| Age, per 10 y | 1.12 (1.07–1.18) | <.001 | 1.13 (1.08–1.19) | <.001 | 1.12 (1.06–1.18) | <.001 |
| Male sex | 0.96 (0.84–1.08) | .49 | 0.91 (0.81–1.03) | .15 | 0.94 (0.83–1.07) | .36 |
| White race | 1.31 (1.14–1.51) | <.001 | 1.32 (1.15–1.52) | <.001 | 1.31 (1.14–1.51) | <.001 |
| Medicaid | 1.14 (0.98–1.32) | .09 | 1.15 (0.99–1.32) | .06 | 1.14 (0.98–1.32) | .09 |
| Current smoker | 1.24 (0.98–1.57) | .07 | 1.23 (1.05–2.09) | .03 | 1.23 (0.97–1.56) | .09 |
| Frailty | 1.28 (1.11–1.47) | .001 | 1.26 (1.09–1.45) | .001 | ||
| eGFR per 5 mL/min/1.73 m2 | 1.09 (1.03–1.17) | .006 | 1.08 (1.01–1.15) | .03 | ||
| Albumin quartiles/missing | <.001 for trend | <.001 for trend | <.001 for trend | |||
| Group 1: ≤2.5 g/dL | 0.26 (1.03–1.54) | .02 | 1.27 (1.05–1.54) | .01 | 1.26 (1.03–1.53) | .02 |
| Group 2: >2.5–3.0 g/dL | 1.19 (0.98–1.44) | .08 | 1.16 (0.96–1.41) | .12 | 1.19 (0.98–1.45) | .07 |
| Group 3: missing | 0.99 (0.80–1.22) | .92 | 0.99 (0.81–1.22) | .95 | 0.98 (0.80–1.22) | .89 |
| Group 4: >3.0–3.5 g/dL | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| Group 5: >3.5 g/dL | 0.85 (0.71–1.02) | .09 | 0.86 (0.71–1.02) | .09 | 0.85 (0.71–1.03) | .09 |
| Hemoglobin quartiles/missing | .10 for trend | .13 for trend | .18 for trend | |||
| Group 1: ≤9 g/dL | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| Group 2: >9–10 g/dL | 1.12 (0.93–1.36) | .23 | 1.10 (0.91–1.32) | .33 | 1.11 (0.92–1.34) | .27 |
| Group 3: missing | 1.47 (1.19–1.83) | <.001 | 1.45 (1.19–1.78) | <.001 | 1.45 (1.17–1.79) | .001 |
| Group 4: >10–12 g/dL | 1.13 (0.98–1.31) | .10 | 1.11 (0.96–1.29) | .15 | 1.10 (0.95–1.28) | .21 |
| Group 5: >12 g/dL | 1.20 (0.98–1.47) | .08 | 1.18 (0.96–1.45) | .12 | 1.16 (0.94–1.42) | .16 |
| Hemodialysis | 0.94 (0.78–1.13) | .51 | 0.93 (0.78–1.12) | .44 | 0.94 (0.78–1.13) | .51 |
| Comorbidity | ||||||
| Diabetes mellitus | 1.02 (0.91–1.15) | .73 | 1.13 (0.91–1.16) | .69 | 1.00 (0.89–1.13) | .96 |
| Congestive heart failure | 1.16 (1.02–1.32) | .03 | 1.12 (0.98–1.28) | .10 | 1.15 (1.00–1.31) | .04 |
| Atherosclerotic heart disease | 1.05 (0.90–1.23) | .55 | 1.03 (0.89–1.16) | .67 | 1.05 (0.90–1.23) | .55 |
| CVA/TIA | 1.22 (0.97–1.54) | .09 | 1.21 (0.96–1.52) | .10 | 1.22 (0.97–1.54) | .09 |
| Peripheral vascular disease | 1.13 (0.96–1.33) | .14 | 1.13 (0.96–1.32) | .14 | 1.12 (0.95–1.32) | .18 |
| COPD | 1.01 (0.79–1.30) | .91 | 1.01 (0.78–1.29) | .97 | 1.00 (0.78–1.29) | .99 |
| Cancer | 1.02 (0.77–1.35) | .90 | 1.04 (0.79–1.37) | .78 | 1.02 (0.77–1.35) | .89 |
| Erythropoietin use | .67 for homogeneity | .62 for homogeneity | .42 for homogeneity | |||
| Nonusers | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| Users | 0.99 (0.86–1.15) | .94 | 1.02 (0.89–1.18) | .75 | 1.01 (0.87–1.17) | .91 |
| Unknown or missing | 1.11 (0.87–1.40) | .41 | 1.12 (0.89–1.42) | .33 | 1.12 (0.88–1.42) | .36 |
| Early nephrology referral | .96 for homogeneity | .72 for homogeneity | .08 for homogeneity | |||
| No | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| Yes | 0.99 (0.84–1.17) | .93 | 0.95 (0.81–1.12) | .53 | 0.99 (0.84–1.17) | .89 |
| Unknown or missing | 1.03 (0.78–1.36) | .84 | 1.03 (0.78–1.36) | .83 | 1.02 (0.77–1.36) | .87 |
Abbreviations: COPD, chronic obstructive pulmonary disease; CVA/TIA, cerebrovascular accident/transient ischemic attack; eGFR, estimated glomerular filtration rate; HR, hazards ratio.
SI conversion factors: To convert albumin to grams per liter, multiply by 10; to convert hemoglobin to grams per liter, multiply by 10.
Multivariate model in which frailty but not eGFR was included among predictor variables.
Multivariate model in which eGFR but not frailty was included among predictor variables.
Multivariate model in which both eGFR and frailty were included among predictor variables.
COMMENT
Among participants of the CDS, we found a strikingly high prevalence of frailty (73%). Frailty was associated with higher eGFR at dialysis initiation. Frailty was also associated with higher mortality and attenuated the association between earlier dialysis initiation and death. Both frailty and eGFR at dialysis initiation were associated with shorter time to first hospitalization.
To our knowledge, the association of frailty with higher eGFR at dialysis initiation has not previously been reported. Although the difference of 1.6 mL/min/1.73 m2 in mean eGFR between the frail and nonfrail patients is not large, it should be noted that there was a wide range of eGFRs from 1.4 mL/min/1.73 m2 to 29.5 mL/min/ 1.73 m2 at which patients were started on dialysis. Furthermore, based on the data from the recent Initiating Dialysis Early and Late (IDEAL) trial, this difference could correspond to approximately 3 additional dialysis-free months among the nonfrail group. This is particularly interesting given the recent trend toward earlier initiation of dialysis. Because the signs and symptoms of ESRD can be nonspecific, it is possible that the very clinical presentation of frailty could be judged as signs or symptoms of uremia, and this judgment could lead to earlier dialysis initiation of the frail population. In addition, it has been argued that because eGFR may overestimate true GFR among patients with lower muscle mass, initiation of dialysis at a higher eGFR in such patients is appropriate.16 Finally, frail patients might be more amenable to starting dialysis than nonfrail patients, either in the hope that dialysis might address their frailty or because they have fewer competing activities to be disrupted by dialysis.
Several observational studies have shown a survival disadvantage with higher eGFR at the start of dialysis even after adjustment for age and comorbidities,4,17,18 but these studies have not been able to rule out confounding by frailty. Rosansky et al4 recently demonstrated an association of higher eGFR at dialysis initiation with higher mortality in even “healthy” patients by studying an incident cohort younger than 65 years and with no reported comorbidities other than hypertension. However, given the high prevalence of frailty even in those younger than 40 years among patients new to dialysis in the CDS, it is likely that the study by Rosansky et al4 included many patients who were frail, which could have confounded the observed results. The IDEAL trial, a randomized trial comparing earlier vs standard timing of initiation of dialysis, did not show harm associated with early dialysis initiation, although group separation was limited.19 While frailty was not captured in the trial, the randomized design would have been expected to yield balance in the proportion of frail patients in each group.
It remains to be seen whether frailty or other outcomes, such as functional status, improve or worsen with earlier dialysis initiation. Recently, Kurella Tamura et al20 showed that in a cohort of nursing home residents, initiation of dialysis was associated with decline in functional status. By the end of the 12 months after dialysis initiation, more than half had died, and only 1 in 8 was alive with stable or improved functional capacity. Given that frailty has been shown to be a precursor to functional dependence, it is likely that the cohort in the study by Kurella Tamura et al20 was largely frail at baseline.
In light of these prior findings, it is important to note that our data did not show a benefit with early start of dialysis regardless of baseline frailty status. If anything, those who were started at higher eGFRs had a higher risk for hospitalization even after adjustment for frailty, suggesting that early start of dialysis could be harmful. Likewise, although eGFR was no longer statistically significantly associated with mortality after inclusion of frailty in the model, the 95% CI of the point estimate encompassed mostly greater than 1. Therefore, our data were insufficient to rule out the association between early start of dialysis and mortality, even after frailty is accounted for. In addition, since quality of life could be adversely affected by dialysis start, the utility of early dialysis initiation should be carefully considered. The results of our study highlight the importance of considering factors other than eGFR to determine the timing of dialysis initiation and suggest that frailty should not be considered one of the clinical considerations or characteristic complications of kidney failure that National Kidney Foundation guidelines state may prompt initiation of therapy before stage 5 CKD.21
One of the major strengths of our study is that we used a relatively large cohort that was geographically and racially/ethnically diverse with a sizable number of patients undergoing peritoneal dialysis, a modality used by relatively few patients in the United States but by most patients in many other countries. Our study has several limitations. First, we calculated eGFR using the 4-variable MDRD study equation, which could have overestimated residual kidney function in patients with sarcopenia. Because sarcopenia is conceptually a salient feature of frailty, glomerular filtration rate estimated by the MDRD formula would have been expected to be higher in frail patients than in nonfrail patients. However, it is important to note that sarcopenia is but 1 component of frailty, a multisystem clinical syndrome with many other features. In fact, we did find that the exhaustion component of frailty, which would not be likely to be mediated by low muscle mass, was also associated with higher eGFR at the start of dialysis. In addition, we found that frail patients had higher hemoglobin values than nonfrail patients at dialysis start, suggesting that the level of kidney function may indeed have been higher. Second, we used a modified definition of frailty to accommodate available data collected from questionnaires, which assessed physical function rather than physical performance as assessed by grip strength or gait speed in the original Fried’s criteria (Fried et al12) for frailty. We did not have data on weight loss either, which was a criterion in Fried’s original definition. However, others have modified Fried’s frailty definition based on questionnaires of physical functioning and without weight loss information and have shown frailty to be an independent predictor of mortality in populations both with and without ESRD.6,14 Finally, to allow these data to be compared with those from other populations with chronic diseases, we used a fairly standard definition of frailty. Because such a large fraction of patients undergoing dialysis are frail, it may be valuable to consider another construct that has more predictive power (eg, “frail-plus”) to further differentiate the largely frail population in ESRD based on important clinical outcomes, such as trajectory of functional status, physical activity, and quality of life with renal replacement therapy.
In summary, frailty is exceptionally common in patients new to dialysis and is associated with higher eGFR at dialysis initiation. Frailty is also associated with mortality and attenuates the association between earlier dialysis initiation and mortality. In light of the recent trend toward earlier initiation of dialysis despite the absence of supportive evidence, it will be important to determine whether overall health and functional capacity improve with dialysis. Comprehensive efforts other than dialysis aimed to improve functional capacity in this population should also be considered.
Acknowledgments
Funding/Support: This work was supported through contracts N01-DK-7-0005 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Dr Bao’s effort was supported by grant No. 69734 from the Graduate Medical Education funding through the VA and by T32 2T32DK007418-31 from the National Institutes of Health. Dr Dalrymple’s effort was supported by grant No. UL1 RR024146 from the National Center for Research Resources. A portion of Dr Johansen’s effort was supported by 1K24DK085153 from the NIDDK.
Footnotes
Financial Disclosure: Dr Chertow serves on the board of directors of Satellite Healthcare Inc and on the scientific advisory board of DaVita Clinical Research.
Disclaimer: The interpretation and reporting of the data presented herein are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government.
Online-Only Material: The eTable is available at http://www.archinternmed.com.
Author Contributions: Dr Bao had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Bao, Chertow, and Johansen. Acquisition of data: Johansen. Analysis and interpretation of data: Bao, Dalrymple, Chertow, Kaysen, and Johansen. Drafting of the manuscript: Bao. Critical revision of the manuscript for important intellectual content: Bao, Dalrymple, Chertow, Kaysen, and Johansen. Statistical analysis: Bao. Obtained funding: Chertow, Kaysen, and Johansen. Administrative, technical, and material support: Bao and Chertow. Study supervision: Chertow and Johansen.
References
- 1.Collins AJ, Foley RN, Chavers B, et al. United States Renal Data System 2011 Annual Data Report: atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis. 2012;59(1 suppl 1):A7, e1–e420. doi: 10.1053/j.ajkd.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 2.Rosansky SJ, Clark WF, Eggers P, Glassock RJ. Initiation of dialysis at higher GFRs: is the apparent rising tide of early dialysis harmful or helpful? Kidney Int. 2009;76(3):257–261. doi: 10.1038/ki.2009.161. [DOI] [PubMed] [Google Scholar]
- 3.Collins AJ, Foley RN, Herzog C, et al. US Renal Data System 2010 Annual Data Report. Am J Kidney Dis. 2011;57(1 suppl 1):A8, e1–e526. doi: 10.1053/j.ajkd.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Rosansky SJ, Eggers P, Jackson K, Glassock R, Clark WF. Early start of hemodialysis may be harmful. Arch Intern Med. 2011;171(5):396–403. doi: 10.1001/archinternmed.2010.415. [DOI] [PubMed] [Google Scholar]
- 5.Wright S, Klausner D, Baird B, et al. Timing of dialysis initiation and survival in ESRD. Clin J Am Soc Nephrol. 2010;5(10):1828–1835. doi: 10.2215/CJN.06230909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johansen KL, Chertow GM, Jin C, Kutner NG. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007;18(11):2960–2967. doi: 10.1681/ASN.2007020221. [DOI] [PubMed] [Google Scholar]
- 7.Kutner NG, Johansen KL, Kaysen GA, et al. The comprehensive dialysis study (CDS): a USRDS special study. Clin J Am Soc Nephrol. 2009;4(3):645–650. doi: 10.2215/CJN.05721108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D Modification of Diet in Renal Disease Study Group. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 9. [Accessed April 11, 2011];The SF-12: an even shorter health survey. http://www.sf-36.org/tools/sf12.shtml.
- 10.Hays RD, Kallich JD, Mapes DL, Coons SJ, Carter WB. Development of the kidney disease quality of life (KDQOL) instrument. Qual Life Res. 1994;3(5):329–338. doi: 10.1007/BF00451725. [DOI] [PubMed] [Google Scholar]
- 11.Fix A, Daughton D. Human Activity Profile (HAP) Manual. Odessa, FL: Psychological Assessment Resources Inc; 1986. [Google Scholar]
- 12.Fried LP, Tangen CM, Walston J, et al. Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 13.Johansen KL, Painter P, Kent-Braun JA, et al. Validation of questionnaires to estimate physical activity and functioning in end-stage renal disease. Kidney Int. 2001;59(3):1121–1127. doi: 10.1046/j.1523-1755.2001.0590031121.x. [DOI] [PubMed] [Google Scholar]
- 14.Woods NF, LaCroix AZ, Gray SL, et al. Women’s Health Initiative. Frailty: emergence and consequences in women aged 65 and older in the Women’s Health Initiative Observational Study. J Am Geriatr Soc. 2005;53(8):1321–1330. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- 15.Johansen KL, Chertow GM, Kutner NG, Dalrymple LS, Grimes BA, Kaysen GA. Low level of self-reported physical activity in ambulatory patients new to dialysis. Kidney Int. 2010;78(11):1164–1170. doi: 10.1038/ki.2010.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiner DE, Stevens LA. Timing hemodialysis initiation: a call for clinical judgment. Am J Kidney Dis. 2011;57(4):562–565. doi: 10.1053/j.ajkd.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Kazmi WH, Gilbertson DT, Obrador GT, et al. Effect of comorbidity on the increased mortality associated with early initiation of dialysis. Am J Kidney Dis. 2005;46(5):887–896. doi: 10.1053/j.ajkd.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Clark WF, Na Y, Rosansky SJ, et al. Association between estimated glomerular filtration rate at initiation of dialysis and mortality. CMAJ. 2011;183(1):47–53. doi: 10.1503/cmaj.100349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooper BA, Branley P, Bulfone L, et al. IDEAL Study. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363(7):609–619. doi: 10.1056/NEJMoa1000552. [DOI] [PubMed] [Google Scholar]
- 20.Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361(16):1539–1547. doi: 10.1056/NEJMoa0904655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemodialysis Adequacy 2006 Work Group. Clinical practice guidelines for hemodialysis adequacy, update 2006. Am J Kidney Dis. 2006;48(suppl 1):S2–S90. doi: 10.1053/j.ajkd.2006.03.051. [DOI] [PubMed] [Google Scholar]