Abstract
Rapid (“warm”) autopsies of patients with advanced metastatic cancer provide invaluable insight into the natural history, pathobiology, and morphology of advanced and treatment-resistant tumors. Here, we report a rapid autopsy case of a hereditary leiomyomatosis and renal cell carcinoma (HLRCC) patient with advanced metastatic renal cell carcinoma (RCC)—the first such case described for either a primary renal tumor or HLRCC-related cancer. Mutations in the fumarate hydratase (FH) gene underlie HLRCC, a rare syndrome involving cutaneous and uterine leiomyomata and aggressive kidney tumors. Loss of heterozygosity at the wild-type FH gene locus results in profound cellular metabolic derangement, “pseudohypoxic” upregulation of hypoxia-inducible factor 1[alpha] (HIF-1[alpha])-dependent transcription, and aberrant protein succination; these molecular changes drive oncogenesis of kidney tumors in HLRCC patients. The current index patient had a high-grade RCC with classic morphologic features of HLRCC, including large nuclei with prominent eosinophilic nucleoli and perinucleolar clearing. In addition, this patient’s RCC demonstrated extensive sarcomatoid and rhabdoid features—morphologies not previously well described in HLRCC-associated kidney tumors. Here, we report the extent of metastatic dissemination and supplement this unique tumor morphology with mitochondrial enzyme histochemistry and extended immunohistochemical analysis. Tumor cells strongly expressed PAX8, vimentin, CD10, and the HIF target GLUT1 and showed increased nuclear p53 accumulation; the expression of other RCC markers was negative. We also detail microscopic tubular epithelial changes in the grossly uninvolved ipsilateral renal parenchyma and demonstrate sporadic, aberrant upregulation of the HIF targets GLUT1 and CAIX in dysplastic peritumoral tubules.
Keywords: HLRCC, rapid autopsy, renal cell cancer
Introduction
Rapid autopsies—those performed within approximately 3 hours of death—of end-stage cancer patients help establish the nature and extent of metastatic disease, facilitate the procurement of invaluable tissue for research, and advance our understanding of the molecular mechanisms of treatment-resistant tumors.1 We have thus far performed 60 rapid autopsies on patients with metastatic hormone-refractory prostate cancer at the University of Michigan Health System, which have played a significant role in furthering our understanding of the morphologic spectrum and pathobiology of prostate cancer.2–7 Several other primary tumor types, including breast and pancreas, have been studied by rapid autopsy programs at various institutions 8,9; however, to the best of our knowledge, no rapid autopsy cases involving patients with metastatic renal cell carcinoma (RCC) have been described in the literature.
Hereditary leiomyomatosis and renal cell carcinoma (HLRCC), also known as Reed syndrome, is a rare familial syndrome characterized by cutaneous and uterine leiomyomata and kidney tumors.10 Reed et al 11 first described predisposition to this uncommon constellation of clinical findings in 2 families in 1973. Nearly 30 years later, Launonen et al 12 reported an additional 2 families with HLRCC and demonstrated that a subset of these patients had linkage disequilibrium at chromosome 1q42-44 and loss of heterozygosity (LOH) of the wild-type chromosome in tumor samples. Follow-up studies identified recurrent deleterious mutations in the fumarate hydratase (FH) gene in HLRCC families.13,14 FH encodes an essential enzyme in the Krebs (citric acid) cycle that catalyzes the production of malate from fumarate. LOH at the wild-type FH gene locus essentially eliminates FH protein function in tumor cells, which results in a metabolic shift from oxidative phosphorylation to aerobic glycolysis, stabilization of hypoxia-inducible factor 1[alpha] (HIF-1[alpha]), and upregulation of HIF target genes, such as GLUT1.15 These changes promote renal oncogenesis in HLRCC through a so-called “pseudohypoxic drive”, whereby tumor cells activate cellular hypoxia response pathways in the setting of normal oxygen availability. Reduced FH enzymatic activity also leads to a buildup of intracellular fumarate and widespread aberrant succination of protein cysteine residues (ie, S-[2-succinyl] cysteine or “2SC”), which can be assayed immunohistochemically and demonstrates high sensitivity and specificity for FH-mutant tumors.16
HLRCC kidney tumors were initially described as aggressive type II papillary RCCs; however, the histopathologic spectrum of these tumors has recently expanded.17 Although these tumors can have a papillary or solid architecture, the common feature and defining hallmark is the presence of tumor cells with large nuclei, prominent orangiophilic or eosinophilic nucleoli, and perinucleolar clearing. Immunohistochemistry for the low-molecular weight cytokeratins (CK) 7 and 20 and high-molecular weight cytokeratins (HMWCK; ie, CK1, 5, 10, and 14) are routinely negative, and molecular studies demonstrate frequent LOH for chromosome 1q. Uterine leiomyomata occur in >75% of women with HLRCC and frequently show variant histomorphology, including cellular and atypical patterns.18,19 Similar to kidney tumors in HLRCC, uterine leiomyomata show foci of cells with enlarged nuclei, prominent orangiophilic or eosinophilic nucleoli, and perinucleolar clearing, and molecular studies demonstrate frequent LOH for chromosome 1q. Interestingly, as opposed to uterine leiomyomata, cutaneous leiomyomata in HLRCC patients generally do not show these characteristic nuclear features.10 Here, we report a rapid autopsy case of advanced metastatic RCC in a patient with HLRCC, focusing on the scope and extent of metastatic dissemination and tumor morphology and immunophenotype.
Materials and methods
Enrollment in the rapid autopsy program was secured, and consent for autopsy by the patient’s spouse was confirmed posthumously prior to performance of the autopsy at the University of Michigan Health System. The rapid autopsy protocol has been described previously,1,5 and the following adaptation was made for the specific circumstances of this case: no head or neck dissection was performed (per the family’s request). The entire gross dissection was performed simultaneously by the attending genitourinary pathologist (R.M.) and pathology resident (A.M.U.). Tissues procured at the time of autopsy were placed in O.C.T. medium (Sakura Finetek USA, Torrance, CA) or formalin for frozen or permanent histologic sections, respectively. Hematoxylin and eosin (H&E), Alcian blue, mucicarmine, and periodic acid-Schiff (PAS) staining (with and without diastase digestion) and enzyme histochemistry for succinate dehydrogenase (SDH), cytochrome oxidase, and NADH dehydrogenase (NADH-TR) were performed by the Department of Pathology at the University of Michigan Health System using routine laboratory methods. Immunohistochemistry by the Department of Pathology at the University of Michigan Health System was performed using a BenchMark ULTRA automated stainer and the ultraView Universal DAB Detection Kit (Ventana Medical Systems, Oro Valley, AZ). The following primary antibodies were used: PAX8 (predilute; Cell Marque, Rocklin, CA); vimentin (predilute; Ventana Medical Systems); CD10 (predilute; Ventana Medical Systems); pancytokeratin (AE1/AE3/Cam5.2; 1:200; Chemicon/Becton Dickinson, Franklin Lakes, NJ); CK7 (1:200; Cell Marque); CK20 (1:200; Cell Marque); [alpha]-methylacyl-CoA racemase (AMACR; 1:50; Dako, Denmark); RCC antigen (predilute; Ventana Medical Systems); CD117 (1:100; Cell Marque); HMWCK (CK34[beta]E12; 1:50; Dako); GLUT1 (predilute; Cell Marque); CAIX (1:1600; Novus Biologicals, Littleton, CO); and p53 (predilute; Ventana Medical Systems). Immunohistochemistry for 2SC was performed by the Department of Pathology at Memorial Sloan-Kettering Cancer Center using a rabbit polyclonal antibody 16,20 and the EnVision kit (Dako), according to the manufacturer’s protocol.
Results
Clinical history and sequence of events
The decedent was a 59-year-old Caucasian female with obesity, hyperlipidemia, insulin resistance, hypothyroidism, and eczema and a family history of breast cancer (in mother), prostate cancer (in father), and lung cancer (in a paternal uncle). Sixteen months before her death, she presented for dermatologic evaluation of numerous flesh-colored papules on her right flank and forearm; these lesions had been present for approximately 9 years without symptoms or recent change, and punch biopsy of one of the papules revealed a dermal population of smooth muscle–type spindle cells without prominent nucleoli, consistent with a cutaneous leiomyoma (Figs. 1A, B). She was subsequently referred by the consultant dermatologist/dermatopathologist to Medical Genetics for evaluation of possible HLRCC. A sample of whole blood was sent to a reference laboratory for direct exonic sequencing of the FH gene, and the patient was found to harbor a germline heterozygous A to C missense mutation at nucleotide position 320; this mutation results in substitution of threonine for asparagine at amino acid position 107 and has been previously reported in HLRCC patients.13 Follow-up ultrasonographic studies demonstrated uterine leiomyomata and left kidney cysts.
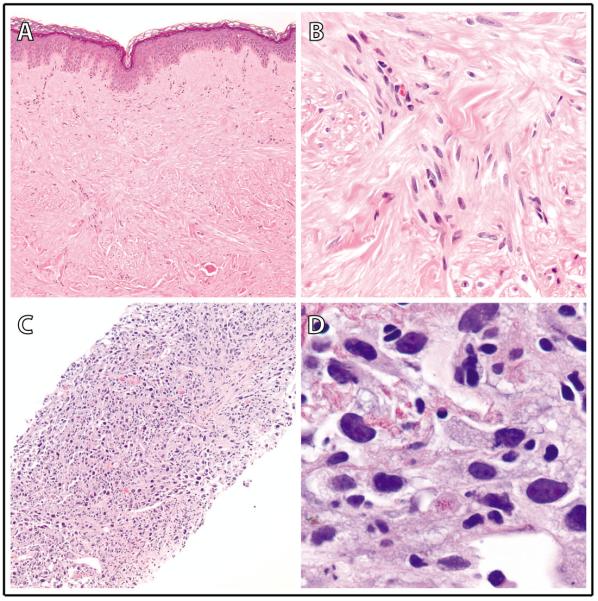
Figure 1.
Biopsy findings in a patient with hereditary leiomyomatosis and renal cell carcinoma (HLRCC) and metastatic renal cell carcinoma (RCC). (A,B) A skin punch biopsy of a right flank cutaneous leiomyoma (100X and 1000X in A and B, respectively) showed a dermal population of smooth muscle-type spindle cells, arranged in intersecting fascicles, with abundant eosinophilic cytoplasm and elongated, hyperchromatic nuclei without prominent nucleoli. (C, D) A core needle biopsy of a solitary large liver RCC metastasis (100X and 1000X in C and D, respectively) demonstrated high-grade malignant tumor cells with extensive nuclear pleomorphism and sarcomatoid features; peri-nucleolar clearing was not identified.
Four months prior to her death, the patient presented for evaluation of a 2-month history of worsening left lower back and hip pain. A computed tomography scan of the abdomen revealed an 11×8 cm exophytic, heterogenous mass arising within the left kidney, with probable tumor thrombus in the left renal vein and possible involvement of the pancreatic tail and splenic hilum. There was also scattered left retroperitoneal lymphadenopathy and a 5.7×5.0 cm lesion in the liver dome, concerning for metastatic disease. A core needle biopsy of the liver lesion revealed malignant tumor cells with extensive nuclear pleomorphism and sarcomatoid features (Fig. 1C); although high-grade nuclei were identified in this sample, the perinucleolar clearing characteristic of HLRCC-associated RCC was not present (Fig. 1D). The tumor cells demonstrated strong and diffuse expression of PAX8, with very focal AMACR expression and nonspecific cytoplasmic CAIX staining, but were negative for CK7 and p63 expression. Taken together, the clinicopathologic findings were consistent with a high-grade metastatic RCC.
The patient initially received monochemotherapy with pazopanib—one of the standard, first-line therapeutic options for metastatic RCC at our institution. Follow-up imaging, however, indicated rapid disease progression; pazopanib was discontinued, and she subsequently received palliative radiation therapy. Due to persistent disease progression and poorly controlled, cancer-associated pain, she decided to enter a residential hospice program and, within a month, expired secondary to respiratory arrest. After death, the patient was transported to the University of Michigan Health System for participation in the rapid autopsy program.
Pathologic findings
A 12.5×8.5×6.0 cm tan-white, multinodular, partially necrotic, infiltrative tumor involved the majority of the left kidney and invaded the renal capsule, perinephric adipose tissue, renal sinus fat, and left adrenal gland (Fig. 2A). At least 3 fluid-filled, unilocular cysts (4.0 cm in greatest dimension) were present and surrounded by tumor in the left kidney. There was gross tumor extension into the inferior vena cava (below the diaphragm) through the renal vein. The retroperitoneum was directly involved by tumor, with extensive spread into the retroperitoneal soft tissue and partial destruction of the pancreatic parenchyma. The peritoneal and pelvic cavities were diffusely studded and/or caked by tumor, including the omentum, spleen, stomach, intestines, mesentery, right and left ovaries, diaphragm, and liver (Fig. 2B). Tumor invaded the ovarian parenchyma (bilaterally), splenic capsule and subjacent parenchyma, and stomach muscularis propria and submucosa. Tumor metastases included a single, well-circumscribed, tan-white, hemorrhagic, fleshy mass (greatest dimension 11.5 cm) in the liver (Fig. 2C) and 3 separate, well-circumscribed, white, intraparenchymal nodules (greatest dimension 0.8 cm) in the right lower lung lobe. The left supraclavicular and left iliac lymph nodes also demonstrated the presence of metastatic tumor (greatest dimension 4.5 cm). The right kidney, right adrenal gland, urinary bladder, mediastinal lymph nodes, and vertebral bone were not involved by tumor. The uterus was present and uninvolved by RCC; however, there was 1 grossly apparent subserosal uterine leiomyoma (0.9 cm in greatest dimension). At the time of autopsy, external examination did not reveal any grossly obvious cutaneous leiomyomata.
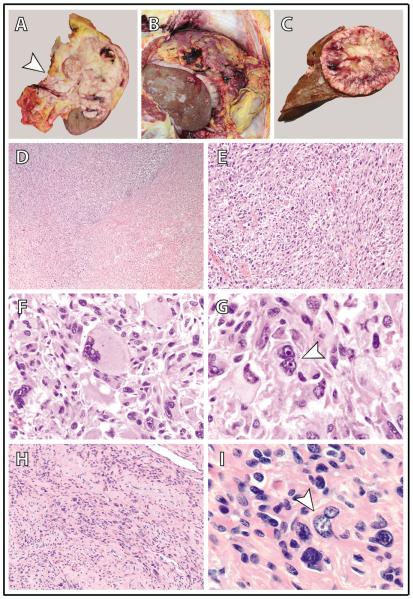
Figure 2.
Gross autopsy and histomorphologic findings in a patient with hereditary leiomyomatosis and renal cell carcinoma (HLRCC) and a high-grade, metastatic renal cell carcinoma (RCC). (A) The upper pole and middle of the left kidney were diffusely involved by a tan-white, infiltrative tumor that extended into the renal sinus and through the renal capsule into the perinephric adipose (arrowhead = renal sinus). (B) Diffuse intraperitoneal tumor metastasis (peritoneal carcinomatosis) with omental tumor caking. (C) A solitary large tumor metastasis in the liver. (D-G) The kidney tumor is comprised of sheets of highly malignant cells with extensive sarcomatoid (D and E; 40X and 200X magnification, respectively) and rhabdoid (F; 400X magnification) morphology; frequent admixed multinucleated tumor giants cells (arrowhead in G; 1000X magnification) are also identified. Tumor cells have enlarged nuclei with prominent eosinophilic nucleoli and peri-nucleolar clearing – the classic nuclear features of HLRCC-associated RCC. These features are readily seen in all histomorphologic patterns, including rhabdoid (F) and multinucleated tumor giant cells (G), and similar findings are present in all metastatic sites (not shown). (H,I) A grossly inapparent intramural uterine leiomyoma (100X and 1000X magnification in H and I, respectively); occasional cells have enlarged nuclei with eosinophilic nucleoli and peri-nucleolar clearing (arrowhead in I).
Microscopically, the primary renal carcinoma and all metastatic sites demonstrated sheets of high-grade malignant cells with extensive sarcomatoid and rhabdoid features, numerous multinucleated tumor giant cells, and focal necrosis (Figs. 2D–G). Many of the tumor cells exhibited large nuclei with prominent eosinophilic nucleoli and perinucleolar clearing; remarkably, such nuclear features were easily appreciable in the sarcomatoid and rhabdoid component of this tumor, as well as in tumor giant cells. The left renal cysts demonstrated entirely denuded epithelium. Adjacent to the tumor in the uninvolved left renal parenchyma, there were numerous irregular tubules lined by epithelial cells with a hobnail appearance (Fig. 4A). The nuclei of these hobnail tubular epithelial cells were large with irregular membranes, variably prominent nucleoli, and occasional nuclear grooves. There was frequent nuclear crowding and overlapping, and rare mitotic figures were identified. These hobnail tubular epithelial cells were only found in close proximity to the left kidney tumor and not in more distant areas of the left kidney or the uninvolved right kidney. Also found diffusely throughout the left renal parenchyma were irregularly shaped, distended tubules lined by epithelial cells with clear, vacuolated cytoplasm (Fig. 4F). The nuclei of these clear tubular epithelial cells were medium sized and round with small nucleoli. These irregular clear tubules were only rarely observed in the uninvolved right kidney. The grossly identified subserosal uterine leiomyoma showed degenerative and ancient change, without characteristic HLRCC nuclear features. An additional grossly inapparent mural uterine leiomyoma (incidentally identified at the time of microscopy) demonstrated a minority of cells with large nuclei, prominent eosinophilic nucleoli, and perinucleolar clearing (Figs. 2H, I).
Figure 4.
Peri-tumoral tubular epithelial changes in grossly uninvolved ipsilateral renal parenchyma in a patient with a high-grade renal cell carcinoma (RCC) and hereditary leiomyomatosis and renal cell carcinoma (HLRCC). (A, F) H&E; (B, G) 2SC; (C, H) GLUT1; (D) CAIX; and, (E) p53. (A-E) Aberrant renal tubular epithelial cells with a hobnail appearance, irregular macronuclei, and occasional mitotic figures (A) are present adjacent to the left renal tumor. These dysplastic cells show focal, mild accumulation of S-(2-succinyl) cysteine (2SC) (B), patchy, strong membranous expression of GLUT1 (C) and CAIX (D), and sporadic nuclear accumulation of p53 (E). (F-H) Separate irregularly shaped tubules lined by epithelial cells with clear, vacuolated cytoplasm (F) are present throughout the left renal parenchyma. These clear cells demonstrate patchy, intermediate cytoplasmic and membranous expression of GLUT1 (H) but no expression of CAIX or p53 (not shown); there is no accumulation of 2SC (G). (All images at 400X magnification.)
Ancillary studies
Tumor cells demonstrated strong expression of PAX8, vimentin, and CD10, patchy expression of pancytokeratin, and very focal expression of CK20 (Figs. 3A, B; data not shown). The tumor cells were negative for AMACR, CK7, RCC, CD117, and HMWCK expression. GLUT1 was strongly expressed by tumor cells in a membranous pattern and showed moderate cytoplasmic staining, whereas CAIX staining was patchy, weak, and predominantly cytoplasmic (as opposed to membranous) (Figs. 3D, E). Tumor cells also demonstrated abundant accumulation of 2SC and diffuse stabilization of p53 (Figs. 3C, F). Relative to uninvolved right renal parenchyma, enzyme histochemistry of tumor cells revealed significantly decreased SDH activity, moderately decreased cytochrome oxidase activity, and comparable NADH dehydrogenase activity (Figs. 3G, H; data not shown). PAS staining with and without diastase showed mild intracellular glycogen accumulation and rare, diastase-resistant cytoplasmic globules in tumor cells.
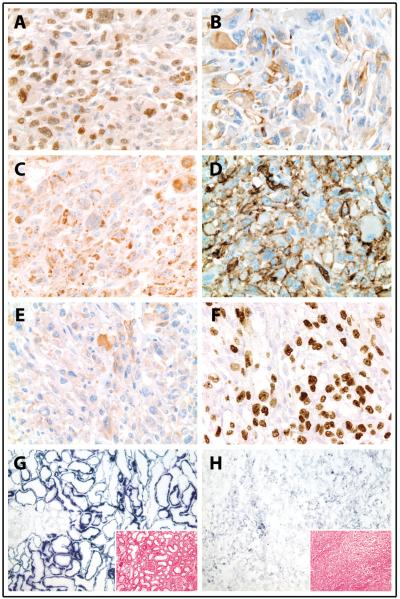
Figure 3.
Immunohistochemistry and ancillary studies of a high-grade renal cell carcinoma (RCC) in a patient with hereditary leiomyomatosis and renal cell carcinoma (HLRCC). (A-B) Tumor cells diffusely express PAX8 (A; 400X magnification) and show patchy expression of pan-cytokeratin (B; 400X magnification). (C) Diffuse S-(2-succinyl) cysteine (2SC) staining demonstrates significant accumulation of aberrantly succinated proteins in tumor cells (400X magnification). (D, E) The hypoxia inducible factor target GLUT1 (D; 400X magnification) is strongly expressed on tumor cell membranes and shows moderate cytoplasmic staining, while CAIX (E; 400X magnification) is only weakly and focally expressed in cytoplasm of tumor cells. (F) There is diffuse nuclear accumulation of p53 in tumor cells (400X magnification). (G,H) Enzyme histochemistry for succinate dehydrogenase (SDH; 100X magnification) demonstrates significantly decreased SDH activity in tumor cells (H) compared to uninvolved contralateral right kidney (G) (intensity of staining varies directly with enzymatic activity; inset, H&E, 100X magnification).
A subset of the aforementioned hobnail tubular epithelial cells strongly expressed GLUT1 and CAIX in a membranous pattern (Figs. 4C, D). Whereas scattered tubules in the uninvolved right renal parenchyma demonstrated intermediate, cytoplasmic GLUT1 expression, there was no strong, membranous GLUT1 expression, and, strikingly, no tubular epithelial cells in the uninvolved right renal parenchyma expressed CAIX. Unlike the adjacent tumor cells, the hobnail tubular epithelial cells showed only focal, mild accumulation of 2SC or p53 (Figs. 4B, E). These GLUT1-positive and CAIX-positive tubular epithelial cells also strongly expressed AMACR, CD10, and RCC; however, unlike the apical luminal distribution in normal tubules of the uninvolved right renal parenchyma, CD10 and RCC were more uniformly distributed on the cell membrane of hobnail tubular epithelial cells. These tubules also variably expressed CK7, CK20, and CD117, and PAS with and without diastase highlighted frequent diastase-resistant cytoplasmic globules in the apical portion of the hobnail tubular epithelial cells.
In contrast to the hobnail tubular epithelial cells, the clear cell tubules described above did not express CAIX, and, although GLUT1 demonstrated intermediate cytoplasmic and membranous GLUT1 staining, there was no accumulation of 2SC or p53 (Figs. 4G, H; data not shown). PAS with and without diastase demonstrated mild intracellular glycogen accumulation, and mucicarmine and Alcian blue were negative for intracellular mucin. These clear cell tubules also variably expressed CK7 and CD117 but were negative for AMACR, CD10, RCC, CK20, and HMWCK expression.
Discussion
Here, we describe a rapid autopsy case of advanced metastatic RCC in a patient with HLRCC. To our knowledge, this is the first rapid autopsy reported for a primary renal tumor and the first autopsy of any kind reported for an HLRCC-associated cancer. Our patient had the classic clinical features of HLRCC—cutaneous leiomyomata, uterine leiomyomata, and a high-grade renal tumor. Her treatment-resistant RCC demonstrated extensive local retroperitoneal invasion with subsequent peritoneal dissemination and distant metastasis to liver, lung, and left iliac and supraclavicular lymph nodes. While the tumor cells displayed the classic HLRCC nuclear features with prominent eosinophilic nucleoli and perinucleolar clearing, the extensive sarcomatoid and rhabdoid histomorphologies, as well as multinucleated tumor giant cells, had not previously been well described in HLRCC-associated renal tumors. Ancillary studies demonstrated expression of the HIF pathway target GLUT1, accumulation of aberrantly succinated proteins, and reduced SDH enzymatic activity in tumor cells, confirming the underlying defect in Krebs cycle metabolism of this HLRCC-associated cancer.
In addition to RCC, several other types of cancer have been reported in HLRCC patients, including uterine leiomyosarcoma and breast and bladder carcinomas, although the relative contribution of FH deficiency in these tumors is not completely understood.21 Despite a family history that included breast, prostate, and lung cancers, our patient had no personal history of other cancer diagnoses, and no evidence of other malignancies (aside from her RCC) was identified at autopsy. At last follow-up, none of the patient’s immediate family members had been tested for germline FH mutations, limiting further analysis of familial cancer predisposition.
Although the metastatic patterns of RCC have been extensively studied, HLRCC-associated RCC is relatively rare, and there are currently little data in the published literature to indicate whether these tumors metastasize in a similar manner. In their seminal work on the histomorphology of HLRCC-associated RCC, Merino et al 17 also documented the following tumor metastases: adrenal (4/14); lymph node (4/14), including cervical lymph node (1/14); liver (2/14); soft tissue (2/14); peritoneum (2/14); and sternum (1/14). Three additional detailed reports of HLRCC-associated metastatic renal tumors have been described, and no lung metastases were reported.18,22,23 Given that >70% of all metastatic RCCs involve the lungs,24 the lack of reported lung metastases in all previous HLRCC cases is notable. This is particularly intriguing given the relatively limited lung metastasis in our patient—despite extensive renal vein involvement, liver metastasis, and otherwise widely disseminated tumor. Interestingly, unlike some abdominal cancers, such as ovarian or gastric carcinomas, intraperitoneal dissemination (peritoneal carcinomatosis) is not a common feature of metastatic RCC. However, a recent case report of a 53-year-old man with a left-sided RCC documented extensive peritoneal carcinomatosis, and similar to our patient, the tumor showed sarcomatoid and rhabdoid histomorphologies.25
HLRCC-associated renal tumors often have a predominantly papillary architecture, although tubular and solid patterns have also been recognized, and some tumors may show focal areas of clear cell change.17 The defining cytologic characteristic of HLRCC-associated RCC is tumor cells with enlarged nuclei, prominent eosinophilic nucleoli, and perinucleolar clearing. Interestingly, there is at least 1 published report of an FH-deficient clear cell RCC in a patient with a heterozygous FH mutation; however, this tumor did not show the classic HLRCC nuclear features.26 In addition, multiple cases of collecting duct carcinomas have been reported in patients with FH mutations, although it is unclear whether these tumors have the classic HLRCC nuclear features.14 Here, we report a high-grade HLRCC-associated RCC with the classic HLRCC nuclear features, extensive sarcomatoid and rhabdoid differentiation, and the presence of multinucleated tumor giant cells. Remarkably, the sarcomatoid and rhabdoid areas in our patient’s tumor, as well as multinucleated tumor giant cells, demonstrated frequent HLRCC-related nuclear changes. Launonen et al 12 reported a single case of an RCC from a woman with presumed HLRCC that had “a sarcomatoid focus,” but the presence (or absence) of classic HLRCC nuclear features in this morphology was not explicitly described. In addition, a recently published cohort of 8 patients with HLRCC-associated RCC reported a single case with sarcomatoid differentiation 27; in all of these tumors, HLRCC-related nuclear changes were identified but not uniformly present. Our case similarly showed intratumoral heterogeneity of classic HLRCC nuclear features—both in the primary renal tumor and at metastatic sites. This suggests that some small core biopsies of HLRCC-associated RCC may fail to demonstrate the classic HLRCC-related nuclear changes, as was the case in our patient’s liver biopsy (Figs. 1C, D). Thus, depending on a given patient’s clinical features, practicing diagnostic pathologists must maintain a high level of suspicion for HLRCC and insist upon adequate tumor sampling in resection specimens.
Little data are available regarding the immunophenotype of HLRCC-associated RCCs. In the largest set of reported cases, Merino et al 17 showed that the majority of tumors are negative for CK7, CK20, and HMWCK expression. To further supplement the histomorphology of our patient’s unique tumor, we performed immunohistocheminal analysis with an extended panel of markers commonly utilized to evaluate and differentiate RCC types. In agreement with previously published results, the tumor cells were negative for CK7 and HMWCK, although there was very focal expression of CK20; tumor cells also strongly expressed vimentin and CD10 but were negative for AMACR, RCC, and CD117 expression. This is not a classic RCC immunophenotype but probably most closely resembles that of clear cell RCC.28 However, unlike the vast majority of clear cell RCC, which express CAIX in a strong, membranous pattern, our patient’s tumor only showed patchy, weak cytoplasmic expression of CAIX (despite unambiguously strong expression of GLUT1, another HIF target) (see below). In addition, we were surprised to discover abundant nuclear accumulation of p53 in tumor cells, given recent data indicating decreased AMP-activated protein kinase–dependent expression of p53 in cultured cell lines from HLRCC patients in vitro.15,29 The expression of p53 has not been definitively studied in primary tissue samples of HLRCC-associated RCC. However, the intense nuclear accumulation of p53 in our patient’s tumor may be related to the accumulation of genetic abnormalities in the dedifferentiated sarcomatoid and rhabdoid histomorphologies, both of which are frequently associated with increased nuclear p53 expression.30,31
LOH of FH in HLRCC-associated RCC has multiple effects on tumor cell biology and metabolism.15 FH is a mitochondrial and cytosolic protein that catalyzes the conversion of fumarate to malate. Loss of FH enzymatic activity leads to increased intracellular accumulation of fumarate, an inhibitor of SDH (Fig. 3H) and HIF prolyl hydroxylase (PHD). HIF proteins are transcription factors whose stability is regulated by the von Hippel-Lindau (VHL) tumor suppressor by means of ubiquitin-mediated protein-degradation pathways. Hydroxylation of specific HIF proline residues by PHD is essential to promote VHL-dependent HIF protein degradation. Thus, in the setting of FH deficiency, increased intracellular fumarate inhibits PHD and reduces VHL-dependent HIF ubiquitination, leading to reduced turnover (ie, increased stabilization) of HIF transcription factors. In an analogous manner to FH in HLRCC, LOH of VHL underlies the development of clear cell RCC in patients with VHL syndrome.32 VHL is also frequently inactivated in sporadic clear cell RCC, and similar to intracellular fumarate accumulation, loss of VHL reduces HIF ubiquitination, leading to its overexpression. The overall effect of either of these molecular changes is activation of HIF target genes, including GLUT1 and vascular endothelial growth factor. Interestingly, GLUT1 and vascular endothelial growth factor expression have not been closely examined in HLRCC-associated RCC, nor has CAIX expression, another known HIF target that is strongly expressed by the vast majority of clear cell RCC.28 In our case, GLUT1 but not CAIX was strongly expressed by a high-grade HLRCC-associated RCC, in contrast to the peritumoral hobnail tubular epithelial cells, which expressed both GLUT1 and CAIX (see below). The reason for the discordance between GLUT1 and CAIX expression in our patient’s tumor is not immediately clear but may have to do with differential stabilization of HIF isoforms in HLRCC compared with sporadic or inherited-type clear cell RCC (ie, HIF-1[alpha] vs. HIF-2[alpha]).
In the grossly uninvolved renal parenchyma surrounding our patient’s tumor, we identified 2 types of tubular epithelial changes: “hobnail” and clear cell. Initially, given the predominant unilaterality of these changes, we wondered whether 1 or both of these changes could be preneoplastic. Several previous studies have highlighted the presence of dysplastic tubular epithelial cells in grossly uninvolved renal parenchyma surrounding RCC.33–35 These lesions are most commonly associated with collecting duct carcinomas or clear cell RCC but have been identified in association with all types of RCC, including those with sarcomatoid differentiation. Referred to either as intratubular epithelial dysplasia or as renal intratubular neoplasia, these lesions are thought by some to represent precursors to RCC; however, their natural history and malignant potential have not been clearly delineated.
Given that LOH of FH is thought to be a prerequisite in HLRCC-associated RCC and that 2SC is a sensitive and specific marker of increased intracellular fumarate in the absence of FH,15,16 we would expect preneoplastic HLRCC lesions to robustly express 2SC. However, whereas hobnail tubular epithelial cells showed sporadic, aberrant expression of the HIF targets GLUT1 and CAIX, there was only focal, weak accumulation of 2SC. In addition, there was only sporadic nuclear accumulation of p53. Thus, although these hobnail tubular epithelial cells may be dysplastic, we favor that they are not preneoplastic HLRCC lesions. Instead, we think that these tubular epithelial changes are a peritumoral effect—perhaps related to relatively decreased blood flow and oxygen delivery, leading to the observed expression of HIF targets GLUT1 and CAIX.
Similarly, because the clear cell tubular epithelial cells were essentially negative for 2SC, we believe that they are unlikely to be preneoplastic HLRCC lesions. Such clear cell change has been previously described in autopsy patients with poorly controlled diabetes mellitus; these collections of vacuolated tubular epithelial cells are referred to as Armanni-Ebstein lesions and result from intracellular glycogen accumulation in proximal convoluted tubules.36 In our patient, PAS staining with and without diastase demonstrated mild intracellular glycogen accumulation in these clear cell tubular epithelial cells, suggesting a low level of abnormal cellular metabolism. However, our patient did not have poorly controlled diabetes mellitus. This fact, coupled with the predominant unilaterality of these clear cell tubular lesions to the kidney involved by tumor, suggests that, similar to the hobnail tubular epithelial cells, these changes may be a peritumoral effect or a metabolic change related to the patient’s FH deficiency.
In summary, we present rapid autopsy findings from a unique patient with metastatic RCC with features consistent with HLRCC at the genotypic and phenotypic level. We plan to characterize further the novel molecular pathways that might be implicated in HLRCC, a subject which is beyond the scope of this current initial study. As we move forward with the next phase of our rapid autopsy program, expanding it to other RCC types and genitourinary malignancies, there are numerous opportunities to investigate new and different aspects of tumorigenesis and metastatic spread, including possible premalignant lesions, locally aggressive versus metastatic tumors, and variant histomorphologies with important clinical prognostic impact.
Acknowledgements
The authors thank the patient and her family for participation in the rapid autopsy program at the University of Michigan Health System; we are extremely indebted to the patient and her family, and this work is dedicated to her.
They are thankful to Drs Jeffrey M. Jentzen, Barbara J. McKenna, and Kenneth J. Pienta for help and guidance in maintenance of the rapid autopsy program. A special thanks to Rylen McDevitt, Nancy Stone, Breen Johnson, Diana French, and Irfan Asangani for assistance during the rapid autopsy. The authors are grateful to Dr Norma Frizzell (University of South Carolina School of Medicine) for providing the 2SC primary antibody. They also thank Drs Paul E. McKeever, Paul D. Killen, and Daniel R. Wahl for helpful suggestions during manuscript preparation and Tina Fields and the histology staff of the Departments of Pathology at the University of Michigan Health System and Memorial Sloan-Kettering Cancer Center for technical assistance. They are thankful to Karen Giles for assistance with manuscript submission.
Funding
This project is supported in part by the NCI Early Detection Research Network (U01 CA111275) and Prostate Cancer SPORE (P50 CA069568). A.A. is supported by the NIH National Center for Advancing Translational Sciences (KL2 TR000434). R.M. and A.M.C. are supported by the Prostate Cancer Foundation. A.M.C. is also supported by the Alfred A. Taubman Institute, the American Cancer Society, the Howard Hughes Medical Institute, and a Doris Duke Charitable Foundation Clinical Scientist Award.
Footnotes
Disclosures:
The authors have no conflicts of interest or funding to disclose.
References
- 1.Rubin MA, Putzi M, Mucci N, et al. Rapid ("warm") autopsy study for procurement of metastatic prostate cancer. Clin Cancer Res. 2000;6:1038–1045. [PubMed] [Google Scholar]
- 2.Shah RB, Mehra R, Chinnaiyan AM, et al. Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. Cancer Res. 2004;64:9209–9216. doi: 10.1158/0008-5472.CAN-04-2442. [DOI] [PubMed] [Google Scholar]
- 3.Varambally S, Yu J, Laxman B, et al. Integrative genomic and proteomic analysis of prostate cancer reveals signatures of metastatic progression. Cancer Cell. 2005;8:393–406. doi: 10.1016/j.ccr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Tomlins SA, Laxman B, Dhanasekaran SM, et al. Distinct classes of chromosomal rearrangements create oncogenic ETS gene fusions in prostate cancer. Nature. 2007;448:595–599. doi: 10.1038/nature06024. [DOI] [PubMed] [Google Scholar]
- 5.Mehra R, Tomlins SA, Yu J, et al. Characterization of TMPRSS2-ETS gene aberrations in androgen-independent metastatic prostate cancer. Cancer Res. 2008;68:3584–3590. doi: 10.1158/0008-5472.CAN-07-6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehra R, Kumar-Sinha C, Shankar S, et al. Characterization of bone metastases from rapid autopsies of prostate cancer patients. Clin Cancer Res. 2011;17:3924–3932. doi: 10.1158/1078-0432.CCR-10-3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grasso CS, Wu YM, Robinson DR, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487:239–243. doi: 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Embuscado EE, Laheru D, Ricci F, et al. Immortalizing the complexity of cancer metastasis: genetic features of lethal metastatic pancreatic cancer obtained from rapid autopsy. Cancer Biol Ther. 2005;4:548–554. doi: 10.4161/cbt.4.5.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singhi AD, Cimino-Mathews A, Jenkins RB, et al. MYC gene amplification is often acquired in lethal distant breast cancer metastases of unamplified primary tumors. Mod Pathol. 2012;25:378–387. doi: 10.1038/modpathol.2011.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lehtonen HJ. Hereditary leiomyomatosis and renal cell cancer: update on clinical and molecular characteristics. Fam Cancer. 2011;10:397–411. doi: 10.1007/s10689-011-9428-z. [DOI] [PubMed] [Google Scholar]
- 11.Reed WB, Walker R, Horowitz R. Cutaneous leiomyomata with uterine leiomyomata. Acta Derm Venereol. 1973;53:409–416. [PubMed] [Google Scholar]
- 12.Launonen V, Vierimaa O, Kiuru M, et al. Inherited susceptibility to uterine leiomyomas and renal cell cancer. Proc Natl Acad Sci U S A. 2001;98:3387–3392. doi: 10.1073/pnas.051633798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomlinson IP, Alam NA, Rowan AJ, et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet. 2002;30:406–410. doi: 10.1038/ng849. [DOI] [PubMed] [Google Scholar]
- 14.Toro JR, Nickerson ML, Wei MH, et al. Mutations in the fumarate hydratase gene cause hereditary leiomyomatosis and renal cell cancer in families in North America. Am J Hum Genet. 2003;73:95–106. doi: 10.1086/376435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linehan WM, Rouault TA. Molecular Pathways: Fumarate Hydratase-Deficient Kidney Cancer: Targeting the Warburg Effect in Cancer. Clin Cancer Res. 2013 doi: 10.1158/1078-0432.CCR-13-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bardella C, El-Bahrawy M, Frizzell N, et al. Aberrant succination of proteins in fumarate hydratase-deficient mice and HLRCC patients is a robust biomarker of mutation status. J Pathol. 2011;225:4–11. doi: 10.1002/path.2932. [DOI] [PubMed] [Google Scholar]
- 17.Merino MJ, Torres-Cabala C, Pinto P, et al. The morphologic spectrum of kidney tumors in hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome. Am J Surg Pathol. 2007;31:1578–1585. doi: 10.1097/PAS.0b013e31804375b8. [DOI] [PubMed] [Google Scholar]
- 18.Garg K, Tickoo SK, Soslow RA, et al. Morphologic features of uterine leiomyomas associated with hereditary leiomyomatosis and renal cell carcinoma syndrome: a case report. Am J Surg Pathol. 2011;35:1235–1237. doi: 10.1097/PAS.0b013e318223ca01. [DOI] [PubMed] [Google Scholar]
- 19.Sanz-Ortega J, Vocke C, Stratton P, et al. Morphologic and molecular characteristics of uterine leiomyomas in hereditary leiomyomatosis and renal cancer (HLRCC) syndrome. Am J Surg Pathol. 2013;37:74–80. doi: 10.1097/PAS.0b013e31825ec16f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagai R, Brock JW, Blatnik M, et al. Succination of protein thiols during adipocyte maturation: a biomarker of mitochondrial stress. J Biol Chem. 2007;282:34219–34228. doi: 10.1074/jbc.M703551200. [DOI] [PubMed] [Google Scholar]
- 21.Lehtonen HJ, Kiuru M, Ylisaukko-Oja SK, et al. Increased risk of cancer in patients with fumarate hydratase germline mutation. J Med Genet. 2006;43:523–526. doi: 10.1136/jmg.2005.036400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Refae MA, Wong N, Patenaude F, et al. Hereditary leiomyomatosis and renal cell cancer: an unusual and aggressive form of hereditary renal carcinoma. Nat Clin Pract Oncol. 2007;4:256–261. doi: 10.1038/ncponc0773. [DOI] [PubMed] [Google Scholar]
- 23.van Spaendonck-Zwarts KY, Badeloe S, Oosting SF, et al. Hereditary leiomyomatosis and renal cell cancer presenting as metastatic kidney cancer at 18 years of age: implications for surveillance. Fam Cancer. 2012;11:123–129. doi: 10.1007/s10689-011-9491-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss L, Harlos JP, Torhorst J, et al. Metastatic patterns of renal carcinoma: an analysis of 687 necropsies. J Cancer Res Clin Oncol. 1988;114:605–612. doi: 10.1007/BF00398185. [DOI] [PubMed] [Google Scholar]
- 25.Esnakula AK, Naab TJ, Green W, et al. Extensive peritoneal carcinomatosis secondary to renal cell carcinoma with sarcomatoid and rhabdoid differentiation. BMJ Case Rep. 2013;2013 doi: 10.1136/bcr-2013-008725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lehtonen HJ, Blanco I, Piulats JM, et al. Conventional renal cancer in a patient with fumarate hydratase mutation. Hum Pathol. 2007;38:793–796. doi: 10.1016/j.humpath.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 27.Toubaji A, Al-Ahmadie HA, Fine SW, et al. Clinicopathologic Features of Hereditary Leiomyomatosis and Renal Cell Carcinoma (HLRCC) Encountered as Sporadic Kidney Cancer. Mod Pathol. 2013;26:252A–252A. [Google Scholar]
- 28.de Peralta-Venturina M, Moch H, Amin M, et al. Sarcomatoid differentiation in renal cell carcinoma: a study of 101 cases. Am J Surg Pathol. 2001;25:275–284. doi: 10.1097/00000478-200103000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Shuch B, Bratslavsky G, Linehan WM, et al. Sarcomatoid renal cell carcinoma: a comprehensive review of the biology and current treatment strategies. Oncologist. 2012;17:46–54. doi: 10.1634/theoncologist.2011-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delahunt B. Sarcomatoid renal carcinoma: the final common dedifferentiation pathway of renal epithelial malignancies. Pathology. 1999;31:185–190. doi: 10.1080/003130299104945. [DOI] [PubMed] [Google Scholar]
- 31.Gokden N, Nappi O, Swanson PE, et al. Renal cell carcinoma with rhabdoid features. Am J Surg Pathol. 2000;24:1329–1338. doi: 10.1097/00000478-200010000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Kuroiwa K, Kinoshita Y, Shiratsuchi H, et al. Renal cell carcinoma with rhabdoid features: an aggressive neoplasm. Histopathology. 2002;41:538–548. doi: 10.1046/j.1365-2559.2002.01427.x. [DOI] [PubMed] [Google Scholar]
- 33.Leroy X, Zini L, Buob D, et al. Renal cell carcinoma with rhabdoid features: an aggressive neoplasm with overexpression of p53. Arch Pathol Lab Med. 2007;131:102–106. doi: 10.5858/2007-131-102-RCCWRF. [DOI] [PubMed] [Google Scholar]
- 34.Chapman-Fredricks JR, Herrera L, Bracho J, et al. Adult renal cell carcinoma with rhabdoid morphology represents a neoplastic dedifferentiation analogous to sarcomatoid carcinoma. Ann Diagn Pathol. 2011;15:333–337. doi: 10.1016/j.anndiagpath.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Humphrey PA. Renal cell carcinoma with rhabdoid features. J Urol. 2011;186:675–676. doi: 10.1016/j.juro.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 36.Truong LD, Shen SS. Immunohistochemical diagnosis of renal neoplasms. Arch Pathol Lab Med. 2011;135:92–109. doi: 10.5858/2010-0478-RAR.1. [DOI] [PubMed] [Google Scholar]
- 37.Tong WH, Sourbier C, Kovtunovych G, et al. The glycolytic shift in fumarate-hydratase-deficient kidney cancer lowers AMPK levels, increases anabolic propensities and lowers cellular iron levels. Cancer Cell. 2011;20:315–327. doi: 10.1016/j.ccr.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oda H, Nakatsuru Y, Ishikawa T. Mutations of the p53 gene and p53 protein overexpression are associated with sarcomatoid transformation in renal cell carcinomas. Cancer Res. 1995;55:658–662. [PubMed] [Google Scholar]
- 39.Shehata BM, Stockwell CA, Castellano-Sanchez AA, et al. Von Hippel-Lindau (VHL) disease: an update on the clinico-pathologic and genetic aspects. Adv Anat Pathol. 2008;15:165–171. doi: 10.1097/PAP.0b013e31816f852e. [DOI] [PubMed] [Google Scholar]
- 40.Mourad WA, Nestok BR, Saleh GY, et al. Dysplastic tubular epithelium in "normal" kidney associated with renal cell carcinoma. Am J Surg Pathol. 1994;18:1117–1124. doi: 10.1097/00000478-199411000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Yorukoglu K, Aktas S, Mungan MU, et al. Tubular dysplasia and carcinoma in situ: precursors of renal cell carcinoma. Urology. 1999;53:684–689. doi: 10.1016/s0090-4295(98)00580-9. [DOI] [PubMed] [Google Scholar]
- 42.Van Poppel H, Nilsson S, Algaba F, et al. Precancerous lesions in the kidney. Scand J Urol Nephrol Suppl. 2000:136–165. [PubMed] [Google Scholar]
- 43.Zhou C, Yool AJ, Nolan J, et al. Armanni-Ebstein lesions: a need for clarification. J Forensic Sci. 2013;58(Suppl 1):S94–98. doi: 10.1111/j.1556-4029.2012.02274.x. [DOI] [PubMed] [Google Scholar]