Abstract
The U.S. Preventive Services Task Force (USPSTF) recommends osteoporosis screening for women younger than 65 years whose 10-year predicted risk of major osteoporotic fracture is ≥ 9.3%. For identifying screening candidates among women aged 50-64 years, it is uncertain how the USPSTF strategy compares with the Osteoporosis Self-Assessment Tool (OST) and the Simple Calculated Osteoporosis Risk Estimate (SCORE). We examined data (1994-2012) from 5165 Women's Health Initiative participants aged 50-64. For the USPSTF (FRAX major fracture risk ≥ 9.3% calculated without BMD), OST (score <2), and SCORE (score >7) strategies, we assessed sensitivity, specificity, and area under the receiver operating characteristic curve (AUC) to discriminate between those with and without femoral neck (FN) T-score ≤ −2.5. Sensitivity, specificity, and AUC for identifying FN T-score ≤ −2.5 were 34.1%, 85.8%, and 0.60 for USPSTF (FRAX), 74.0%, 70.8%, and 0.72 for SCORE, and 79.8%, 66.3%, and 0.73 for OST. The USPSTF strategy identified about 1/3rd of women aged 50-64 with FN T-scores ≤ −2.5. Among women aged 50-64 years, the USPSTF strategy was modestly better than chance alone and inferior to conventional SCORE and OST strategies in discriminating between women with and without FN T-score ≤ −2.5.
Keywords: osteoporosis, fracture, bone mineral density, Fracture Risk Assessment Tool, USPSTF, OST, SCORE, FRAX
Introduction
One half of all postmenopausal women will have an osteoporosis-related fracture during their lifetime (1). Testing for and treating women with low bone mineral density (BMD) (BMD T-score -2.5 or less) can decrease the risk for subsequent fractures and fracture-related morbidity and mortality (1). In 2011, the United States Preventive Services Task Force (USPSTF) recommended routine screening for osteoporosis for all women aged 65 years and older and endorsed use of the Fracture Risk Assessment Tool (FRAX) to identify screening candidates among younger postmenopausal women aged 50-64 years (2). FRAX® is a Web-based tool that uses clinical risk factors with and without femoral neck BMD to estimate 10-year probability of hip and major osteoporotic (hip, clinical vertebral, humerus, or wrist) fractures. Specifically, the USPSTF recommends BMD testing for women aged 50-64 years whose 10-year predicted risk of major osteoporotic fracture (calculated using the FRAX model without BMD) is ≥ 9.3 % (equivalent to that of a 65-year-old white woman with no other FRAX clinical risk factors) (1).
Prior to the advent of FRAX, several tools were available for the prediction of osteoporosis risk, including the Osteoporosis Self-Assessment Tool (OST, based on weight and age) and the Simple Calculated Osteoporosis Risk Estimation Tool (SCORE, based on race, rheumatoid arthritis, history of non-traumatic fracture, age, prior estrogen therapy, and weight) (3-6). For identifying osteoporosis by BMD (T-score ≤ −2.5) among postmenopausal women, an OST score cutoff of < 2 has a sensitivity of 88%-95% and a specificity of 37%-52% (3-6). At a cutoff score of ≥ 7 (4,5) or ≥6 (6), the SCORE tool has a sensitivity of 88%-89% and a specificity of 40%-58%.
Among postmenopausal U.S. women aged 50-64 years, the ability of the USPSTF (FRAX-based) strategy, compared with OST and SCORE, to discriminate between women with and without osteoporosis is unknown. Using data from the Women's Health Initiative, we compared the proportions of postmenopausal women aged 50-64 years who would be identified for BMD testing by the USPSTF (FRAX ≥ 9.3%), OST (OST cutoff < 2), and SCORE (SCORE cutoff > 7) strategies. We then compared the sensitivity, specificity, and area under the receiver operating characteristic curves (AUC) of the 3 tools to discriminate women with and without osteoporosis (femoral neck T-score ≤ −2.5). Finally, for each of the 3 screening strategies, we calculated the thresholds that would correspond to a sensitivity range of 80%-99% for the detection of T-score ≤-2.5, and the associated specificity, and AUC.
Methods
Participants
The Women's Health Initiative was conducted at 40 clinical centers nationwide (7). Eligibility criteria for the clinical trials (WHI-CT) and the observational study (WHI-OS) included being aged 50-79 years at baseline, postmenopausal, and free from serious medical conditions (8,9). The WHI-CT consisted of randomized controlled trial evaluation of three interventions: a low-fat eating pattern, menopausal hormone therapy (HT), and calcium and vitamin D supplementation (9). Details are available at https://cleo.whi.org/about/SitePages/About%20WHI.aspx. All WHI participants were postmenopausal, defined as at least 6 months of amenorrhea for women aged ≥55 years, and at least 12 months of amenorrhea for women aged 50-54 years (10).
At enrollment, WHI-OS and WHI-CT participants at 3 of the 40 clinical centers (Tucson and Phoenix, Arizona; Pittsburgh, Pennsylvania; and Birmingham, Alabama) underwent hip and anteroposterior lumbar spine BMD testing by dual-energy x-ray absorptiometry (DXA, Hologic QDR2000 or QDR4500, Bedford, MA, USA) (11,12). Technologists used standard protocols for positioning and analysis of DXA measurements. The quality assurance program is available at https://biolincc.nhlbi.nih.gov/static/studies/whios/doc/whi/procedur/bone/1.pdf. Quality assurance included review of lumbar spine and hip phantom scans at each center, use of calibration phantoms across clinical sites, flagging of scans with specific problems, and review of a random sample of all scans (13). Femoral neck T-score classification was based on the National Health and Nutrition Examination III normative reference database (14).
Of the 11,488 participants at the 3 clinical centers that measured BMD, 6294 were aged 50-64 years at baseline. The current analysis is based on the 5165 participants aged 50-64 years at baseline who were not taking medications known to influence BMD (calcitonin, parathyroid hormone, bisphosphonates, selective estrogen receptor modulators, luteinizing hormone releasing-hormone agents, fertility medications, somatostatin agents) and for whom information regarding femoral neck T-score and osteoporosis risk factors was complete (Supplemental Figure 1).
Each institution obtained human subjects committee approval. Each participant provided written informed consent.
Outcomes
The primary outcomes were 1) the proportion of women for whom BMD testing would have been recommended according to each of the 3 risk assessment strategies (USPSTF, OST, SCORE) overall, and classified by femoral neck T-score category (T-score > −1, −1 ≥ T-score > −2.5, T-score ≤ −2.5); 2) the proportion of women with femoral neck T-score ≤ −2.5 who would be identified for screening under each strategy; and 3) the sensitivity, specificity, and area under the receiver operating characteristic curve (AUC) for identifying low BMD (T-score between −1 and −2.5) and osteoporosis (T-score ≤ −2.5) under each strategy.
In secondary analyses, we calculated the AUC of the 3 tools for identifying of T-score ≤ −2.5 at one or more of the following sites: lumbar spine, total hip, or femoral neck. We also estimated the cutoff score that would identify 90% of women with femoral neck T-scores ≤ −2.5.
Risk Assessment Strategies
Information regarding osteoporosis risk factors (age, race, rheumatoid arthritis, history of prior fracture, medication use, smoking, alcohol intake, and parental history of hip fracture) was obtained from baseline self-assessment questionnaires and weight and height measurements. Ten-year risk of major osteoporotic fracture was calculated for each participant by the WHO Collaborating Centre for Metabolic Bone Disease, using the FRAX tool without BMD (version 3.0) (2,15). Per the USPSTF screening guidelines, we defined participants with FRAX-predicted 10-year risk of major osteoporotic fracture ≥ 9.3% to be recommended for BMD testing. The calculation of SCORE and OST scores was based on prior publications (Supplemental Table 1) (3-6). SCORE values vary according to use of HT, but the USPSTF strategy does not account for HT. Thus, we present overall results and results stratified according to baseline current use or randomization to active treatment vs. non-use of HT (oral or transdermal patch).
Statistical Analysis
Using Chi-square tests, we compared the proportion of participants who would be identified for BMD testing using the three strategies (USPSTF FRAX ≥9.3%, OST score < 2, and SCORE score > 7). Next, within each T-score category (T-score ≥ −1, −1 > T-score > −2.5, T-score ≤-2.5), we used Chi-square tests to compare the proportion of participants who would be recommended for BMD testing by each of the strategies.
We determined the sensitivity, specificity, positive predictive value ([number of participants with T-score in the interval of interest who would be recommended for BMD testing/total number recommended for BMD testing]*100) and AUC curves of the strategies in discriminating participants with femoral neck T-score ≥ −1 (i.e. a normal T-score) from those with T-score ≤ −2.5 and −1 > T-score > −2.5. Because the scores of the 3 risk strategies were correlated with each other within the same women, we calculated differences in AUCs of the 3 tools for 10,000 bootstrap samples. We stratified the AUC results according to age categories chosen a priori: 50-54 years, 55-59 years, and 60-64 years. For each of the 3 scores, we constructed Receiver Operating Characteristic (ROC) curves for the identification of femoral neck T-scores ≤ −2.5. Finally, for each of the three screening strategies, we calculated the thresholds that would correspond to sensitivities in the range of 80% to 99% for the detection of femoral neck T-scores ≤ −2.5, along with the associated specificities and AUC values.
Our primary analyses focused on participants who were non-users of menopausal hormone therapy (n = 2857). In supplemental analyses, we stratified our results according to use vs. non-use of menopausal hormone therapy.
Analyses were performed using SAS for Windows Version 9.2.
Results
Study participant characteristics
Seventy-two percent of participants were white, 17% were black and 8% were Hispanic (Table 1). At baseline, approximately 1/3rd had body mass index ≥ 30 kg/m2 and 9.5% were current smokers. Average age was 57.7 years (median 58, interquartile range 54-61). Five percent of the analytic sample had femoral neck T-scores ≤ −2.5; 46% had −1 > femoral neck T-score ≥ −2.5. Mean 10-year predicted major osteoporotic fracture risk was 6.6% (median 5.8%, range 0.74%-47.7%).
Table 1.
Selected Baseline Characteristics of the 5165 Study Participants
| No. (%) of Participants | |
|---|---|
| Age, years | |
| 50-54 | 1371 (26.5) |
| 55-59 | 1744 (33.8) |
| 60-64 | 2050 (39.7) |
| Body mass index, kg/m2 | |
| Missing | 21 (0.4) |
| < 25 | 1628 (31.5) |
| 25 - < 30 | 1697 (32.9) |
| ≥ 30 | 1819 (35.2) |
| Race/Ethnicity | |
| Black | 894 (17.3) |
| Hispanic | 420 (8.1) |
| White | 3730 (72.2) |
| Other / unknown | 121 (2.3) |
| History of corticosteroid use | |
| Yes | 47 (0.9) |
| No | 5118 (99.1) |
| Rheumatoid arthritis | |
| Yes | 260 (5.0) |
| No | 4905 (95.0) |
| Smoking | |
| Missing | 57 (1.10) |
| Never smoker | 2701 (52.3) |
| Past smoker | 1916 (37.1) |
| Current smoker | 491 (9.5) |
| Hip or lower arm/wrist fracture after age 55 | |
| Yes | 62 (1.2) |
| No | 5103 (98.8) |
| Current menopausal hormone therapy | |
| Yes | 2308 (44.7) |
| No | 2857 (55.3) |
| ≥ 3 alcoholic drinks per day | |
| Yes | 36 (0.7) |
| No | 5110 (99.3) |
| Parental hip fracture | |
| Yes | 1887 (38.5) |
| No | 3010 (61.5) |
Compared with analytic sample participants, excluded participants were less likely to be African American (9.4% vs. 17.3%); distributions of age, smoking, BMI, alcohol use, and diabetes were not significantly different between groups (data not shown).
Comparisons of the 3 risk assessment strategies
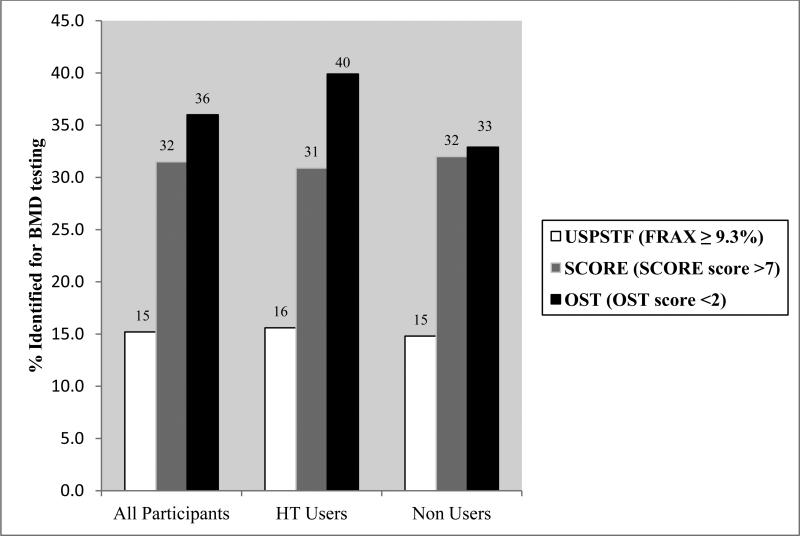
The USPSTF strategy identified 15.2% of all participants aged 50-64 for BMD screening, compared with 31.5% under the SCORE strategy, and 36.0% under the OST strategy (Chi-square p-value < 0.001, Figure 1).
Figure 1.
Proportion of women aged 50 to 64 years who would be identified for BMD testing according to each of the 3 strategies (n = 5165). Proportions are unadjusted. The USPSTF, SCORE, and OST strategies significantly differed from each other in dentifying participants for BMD testing (all pairwise Chi-Square p<0.001 for all participants group).
Among women with T-score ≤ −2.5 who were not using menopausal hormone therapy, the USPSTF strategy identified 33.3% for BMD testing, compared with 74.1% using SCORE and 79.3% using OST (Chi-square p-value < 0.001, Table 2, Supplemental Figure 2). The proportion of women with −1 > T-score > −2.5 who were identified for testing under the 3 strategies was: 17.5% for USPSTF, 42.2% for SCORE, and 48.9% for OST (Chi-square p-value < 0.001). Stratifying for baseline HT use did not notably alter these results. Results were similar among participants taking menopausal hormone therapy (Supplemental Table 2).
Table 2.
Proportion of women aged 50-64 years who would be identified for BMD testing by the three methods according to femoral neck T-score category1
| Non-users of menopausal hormone therapy (n = 2857) | USPSTF (FRAX ≥ 9.3%) | SCORE (SCORE score >7) | OST (OST score <2) |
|---|---|---|---|
| T-score ≤ −2.5 | 58 / 174 = 33.3% | 129 / 174 = 74.1% | 138 / 174 = 79.3% |
The proportion of women with T-scores ≤−2.5 who would have been identified for BMD testing differed significantly among the three screening strategies (Chi-square p-value < 0.001).
Among participants not using menopausal hormone therapy, of the 3 strategies, the USPSTF strategy had the lowest sensitivity (34.1%) for identifying femoral neck T-score ≤ −2.5, but the highest specificity (85.8%)(Table 3). Pairwise comparisons of the AUCs for the 3 strategies in identifying women with T-score ≤ −2.5 revealed significantly higher AUC for both SCORE and OST compared with the USPSTF strategy (p-values < 0.01, data not shown). The AUC for OST was not statistically significantly different from that of SCORE. Among women with femoral neck T-score ≤ −2.5, the positive predictive value under each of the 3 strategies was similar, approximately 11% in the overall sample. Results of a sensitivity analysis in which we included participants taking medications that influence BMD were similar (data not shown). Supplemental Figures 3a,b,c display the AUC curves under the 3 screening strategies. Results were similar among participants taking menopausal hormone therapy (Supplemental Table 3).
Table 3.
Sensitivity, specificity, positive predictive value (PPV), and area under the receiver operating characteristic curve (AUC) for identifying osteoporosis (T-score ≤ −2.5) at the femoral neck
| Non-users of menopausal hormone therapy (n=2163) | T-score ≤ −2.5 | |||
|---|---|---|---|---|
| Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | AUC (95% CI) | |
| USPSTF (FRAX ≥9.3) | 33.3 (26.3-40.4) | 86.4 (85.1-87.7) | 13.7 (10.4-17.0) | 0.60 (0.56-0.63) |
| SCORE (>7) | 74.1 (67.6-80.7) | 70.8 (69.1-72.5) | 14.1 (11.9-16.4) | 0.72 (0.69-0.76) |
| OST (<2) | 79.3 (73.2-85.4) | 70.1 (68.4-71.8) | 14.7 (12.4-16.9) | 0.75 (0.72-0.78) |
For the USPSTF strategy, a cutoff of 4.11 (i.e. ≥ 4.1% 10-year FRAX-predicted risk of major osteoporotic fracture) captured 90.7% of participants with femoral neck T-score ≤ −2.5. A SCORE score of > 5 captured 90.3% of participants with femoral neck T-score ≤ −2.5. An OST score of ≤ 2 captured 89.9%, of participants with femoral neck T-score ≤ −2.5.
The pattern of lower AUC for the USPSTF strategy compared with SCORE and OST was especially pronounced among women aged 50-54 years and 55-59 years (Supplemental Table 4). The AUC for identifying T-score ≤ −2.5 at any skeletal site (lumbar spine, femoral neck, total hip) was lower for the USPSTF strategy than for the OST or SCORE strategies (Supplemental Table 5). For all 3 tools, the AUC for identification of femoral neck T-score ≤ −2.5 was higher than the AUC for identification of T-score ≤ −2.5 at any site.
Table 4 displays the sensitivities, specificities, positive predictive values, and AUC values for alternative thresholds in identifying femoral neck T-score ≤ −2.5. The identification of 90% of participants with T-score ≥−2.5 corresponded to an OST score of <3, a SCORE score of >5, and a FRAX score of ≥4.11. For the range of thresholds that corresponded to sensitivities ≥90% in detection of femoral neck T-scores ≤ −2.5, we found AUC values greater than 0.70 for SCORE scores greater than >5 and for OST thresholds less than 3. A specificity of ≥70% was not found for any of the strategies at thresholds that had sensitivities of ≥80%.
Table 4.
Sensitivity, specificity, positive predictive value, and Area under the Receiver Operating Curve (AUC) for Identifying femoral neck T-score ≤ −2.5 using various construct cutpoints
| T-score ≤ −2.5 | ||||
|---|---|---|---|---|
| OST | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | AUC (95% CI) |
| < 2 | 79.8 (74.9-84.8) | 66.3 (64.9-67.6) | 11.1 (9.6-12.5) | 0.73 (0.71-0.76) |
| < 3 | 89.9 (86.2-93.6) | 53.3 (51.9-54.7) | 9.2 (8.1-10.3) | 0.72 (0.70-0.74) |
| < 4 | 94.6 (91.8-97.4) | 41.2 (39.8-42.6) | 7.8 (6.9-8.7) | 0.68 (0.66-0.69) |
| < 8 | 99.2 (98.1-100.0) | 12.4 (11.5-13.3) | 5.6 (5.0-6.3) | 0.56 (0.55-0.57) |
| SCORE | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | AUC (95% CI) |
|---|---|---|---|---|
| > 6 | 83.7 (79.2-88.3) | 61.3 (59.9-62.6) | 10.2 (8.9-11.5) | 0.73 (0.70-0.75) |
| > 5 | 90.3 (86.7-93.9) | 52.1 (50.7-53.5) | 9.0 (7.9-10.1) | 0.71 (0.69-0.73) |
| > 2 | 96.5 (94.3-98.8) | 29.9 (28.6-31.2) | 6.7 (5.9-7.6) | 0.63 (0.62-0.65) |
| > −6 | 99.2 (98.1-100.0) | 4.1 (3.5-4.6) | 5.2 (4.5-5.8) | 0.52 (0.51-0.52) |
| USPSTF (FRAX) | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | AUC (95% CI) |
|---|---|---|---|---|
| ≥ 5.04 | 80.2 (75.3-85.1) | 40.9 (39.5-42.3) | 6.7 (5.8-7.5) | 0.61 (0.58-0.63) |
| ≥ 4.59 | 85.3 (80.9-89.6) | 35.7 (34.3-37.0) | 6.5 (5.7-7.3) | 0.60 (0.58-0.63) |
| ≥ 4.11 | 90.7 (87.1-94.3) | 29.9 (28.6-31.2) | 6.4 (5.6-7.2) | 0.60 (0.58-0.62) |
| ≥ 3.51 | 95.3 (92.8-97.9) | 22.8 (21.6-24.0) | 6.1 (5.4-6.8) | 0.59 (0.58-0.60) |
| ≥ 2.24 | 99.2 (98.1-100.0) | 8.6 (7.8-9.3) | 5.4 (4.8-6.0) | 0.54 (0.53-0.55) |
Discussion
Current osteoporosis screening guidelines are based mostly on studies of women aged 65 years and over. In contrast, there are limited data regarding optimal osteoporosis screening strategies for younger postmenopausal women. In this study of women aged 50-64 years, under the USPSTF (FRAX-based) strategy, only 34.1% of women with T-score ≤ −2.5 would be recommended for BMD testing, compared with 74.0% with SCORE and 79.8% with OST. The positive predictive values of the 3 strategies for identifying women with femoral neck T-score ≤ −2.5 was similar, approximately 11%. The ability of the strategy to discriminate between women with and without densitometric osteoporosis was significantly lower for USPSTF (AUC 0.60) than for SCORE (AUC 0.72) or OST (AUC 0.73). In contrast, specificity of the USPSTF strategy was higher than SCORE and OST.
To our knowledge, prior studies have not compared the current USPSTF (FRAX) strategy to that of SCORE and OST among U.S. women aged 50-64 years. Although it did not examine the USPSTF strategy, one prior study of OST and SCORE among women aged 45-64 years found that the tools had similar AUC (0.77 for OST and 0.76 for SCORE) for identifying women with T-score ≤ −2.5 (5), as was the case in the current study.
The FRAX, OST, and SCORE thresholds that would be required to identify 90% of 50- to 64-year-olds with femoral neck T-score ≤ −2.5 are different from the cutoff scores traditionally recommended as screening thresholds. The alternative cutpoints for OST, SCORE, and FRAX that would have resulted in identification of ≥80% of women with femoral neck T-score ≥ −2.5 corresponded to specificities less than 70%.
Our results have potential clinical implications. The objective of BMD screening is to identify postmenopausal women with T-scores ≤ −2.5 because pharmacologic treatment to prevent fractures has been demonstrated to be effective in this group. (The efficacy of pharmacologic therapy in women at high fracture risk, but without T score - 2.5 or less or existing vertebral fractures, is uncertain.) Therefore, the ability of the USPSTF strategy to detect BMD T-score −2.5 is of great clinical importance. Our results suggest that the USPSTF FRAX threshold for screening women aged 50-64 would not identify the vast majority of women with T-score ≤ −2.5. This is concerning, since these women are considered treatment candidates. The high specificity of FRAX for identifying younger postmenopausal women with osteoporosis is consistent with its designated use as a treatment decision, not screening, tool (16). Our results demonstrate that for detecting women who have femoral neck T-score ≤ −2.5, alternative cutoff scores for the USPSTF, OST, and SCORE strategies may be required to improve the detection of U.S. women with T-score ≤ −2.5 in this age group. These findings highlight the pressing need for further prospective evaluation of all three tools in the identification of women with T-scores −2.5 or below with the goal of better targeting resources to at-risk young postmenopausal women.
Our study has limitations. In calculation of the SCORE risk, we lacked information regarding previous rib fractures and fractures between ages 45 and 54. However, the omission of rib fractures and fractures between ages 45 and 54 would likely have led to falsely low SCORE risk scores, resulting in an underestimate of the sensitivity of SCORE (the true difference in sensitivity between the SCORE and USPSTF approaches may be more marked than observed). Also, our FRAX fracture risk estimates did not account for secondary causes of osteoporosis, although we believe that secondary causes would be very uncommon in this study cohort of younger postmenopausal women. WHI participants are not a population sample and may be healthier than similarly-aged women in clinical practice. Strengths of our study include the large sample size and the systematic standardized data collection for fracture risk factors.
In conclusion, among women aged 50-64 years, the USPSTF strategy was modestly better than chance alone and inferior to SCORE and OST strategies in discriminating between women with and without femoral neck BMD T-score ≤ −2.5. These findings are important because efficacy of pharmacotherapy is proven for women with T-score ≤ −2.5, but not for women with T-score >−2.5 who have not experienced fractures. In women of this age group, a simple model (OST) based on weight and age discriminated between women with and without osteoporosis as well as the more complex USPSTF approach. Because the goal of osteoporosis screening is to identify postmenopausal women with BMD T-score ≤ −2.5 for pharmacologic therapy, these results could have substantial implications for osteoporosis screening of younger postmenopausal women in clinical practice.
Supplementary Material
Acknowledgements
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, Nancy Geller.
Clinical Coordinating Center: Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg
Investigators and Academic Centers: (Brigham and Women's Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker
Funding. The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C. Dr. Crandall received support from the Jonsson Comprehensive Cancer Center at the University of California, Los Angeles.
Footnotes
Authorship contributions:
Study concept and design: CC
Acquisition of data: AL, JC, JW-W, JR
Analysis and interpretation of data: CC, JL, MG, MD, AL, JC, JW-W, MG, JR, NW, KE
Drafting of the manuscript: CC
Critical revision of the manuscript for important intellectual content:
CC, JL, MG, MD, AL, JC, JW-W, MG, JR, NW, KE
Statistical expertise: JL
Obtained funding: AL, JC, JW-W, JR
- The following authors have no conflict of interest to report: CC, JL, JR, MD, JC, KE, JWW, M.L. Gourlay, M.L. Gass
- AL serves on an Amgen Scientific Methodology Advisory Committee for safety monitoring of Prolia.
- NW is stockholder and director, OsteoDynamics. He has received honoraria for lectures from the following companies in the past year: Amgen, Lilly, Novartis, Warner Chilcott. He has received consulting fees from the following companies in the past year: Abbott, Amgen, Baxter, Bristol-Myers Squibb, Imagepace, Johnson & Johnson, Lilly, Medpace, Merck, Novo Nordisk, Pfizer/Wyeth. Through his University, he has received research support from the following companies: Amgen, Merck, NPS.
JL had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The sponsor had no role in the design, analysis, writing, or review of this manuscript.
References
- 1.Screening for osteoporosis: U.S. preventive services task force recommendation statement. Ann Intern Med. 2011;154(5):356–64. doi: 10.7326/0003-4819-154-5-201103010-00307. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization Collaborating Centre for Metabolic Bone Diseases UoS, UK . FRAX WHO Fracture Risk Assessment Tool, vol. 2012. World Health Organization Collaborating Centre for Metabolic Bone Diseases. University of Sheffield; UK: 2009. [Google Scholar]
- 3.Cadarette SM, McIsaac WJ, Hawker GA, Jaakkimainen L, Culbert A, Zarifa G, Ola E, Jaglal SB. The validity of decision rules for selecting women with primary osteoporosis for bone mineral density testing. Osteoporos Int. 2004;15(5):361–6. doi: 10.1007/s00198-003-1552-7. [DOI] [PubMed] [Google Scholar]
- 4.Geusens P, Hochberg MC, van der Voort DJ, Pols H, van der Klift M, Siris E, Melton ME, Turpin J, Byrnes C, Ross P. Performance of risk indices for identifying low bone density in postmenopausal women. Mayo Clin Proc. 2002;77(7):629–37. doi: 10.4065/77.7.629. [DOI] [PubMed] [Google Scholar]
- 5.Gourlay ML, Miller WC, Richy F, Garrett JM, Hanson LC, Reginster JY. Performance of osteoporosis risk assessment tools in postmenopausal women aged 45-64 years. Osteoporos Int. 2005;16(8):921–7. doi: 10.1007/s00198-004-1775-2. [DOI] [PubMed] [Google Scholar]
- 6.Lydick E, Cook K, Turpin J, Melton M, Stine R, Byrnes C. Development and validation of a simple questionnaire to facilitate identification of women likely to have low bone density. Am J Manag Care. 1998;4(1):37–48. [PubMed] [Google Scholar]
- 7.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women's Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13(9 Suppl):S107–21. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 8.Cauley JA, Wampler NS, Barnhart JM, Wu L, Allison M, Chen Z, Hendrix S, Robbins J, Jackson RD. Incidence of fractures compared to cardiovascular disease and breast cancer: the Women's Health Initiative Observational Study. Osteoporos Int. 2008;19(12):1717–23. doi: 10.1007/s00198-008-0634-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Control Clin Trials. 1998;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 10.Hays J, Hunt JR, Hubbell FA, Anderson GL, Limacher M, Allen C, Rossouw JE. The Women's Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13(9 Suppl):S18–77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 11.Beck TJ, Petit MA, Wu G, LeBoff MS, Cauley JA, Chen Z. Does obesity really make the femur stronger? BMD, geometry, and fracture incidence in the women's health initiative-observational study. J Bone Miner Res. 2009;24(8):1369–79. doi: 10.1359/JBMR.090307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LaCroix AZ, Beck TJ, Cauley JA, Lewis CE, Bassford T, Jackson R, Wu G, Chen Z. Hip structural geometry and incidence of hip fracture in postmenopausal women: what does it add to conventional bone mineral density? Osteoporos Int. 2010;21(6):919–29. doi: 10.1007/s00198-009-1056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Z, Arendell L, Aickin M, Cauley J, Lewis CE, Chlebowski R. Hip bone density predicts breast cancer risk independently of Gail score: results from the Women's Health Initiative. Cancer. 2008;113(5):907–15. doi: 10.1002/cncr.23674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, Johnston CC, Jr., Lindsay RL. Proximal femur bone mineral levels of US adults. Osteoporos Int. 1995;5(5):389–409. doi: 10.1007/BF01622262. [DOI] [PubMed] [Google Scholar]
- 15.Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19(4):385–97. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Osteoporosis Foundation . Clinician's Guide to Prevention and Treatment of Osteoporosis. National Osteoporosis Foundation; Washington, DC.: 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.