Abstract
BACKGROUND
Frailty is common in the elderly and in persons with chronic diseases. Few studies have examined the association of frailty with chronic kidney disease.
METHODS
We used data from the Third National Health and Nutrition Examination Survey to estimate the prevalence of frailty among persons with chronic kidney disease. We created a definition of frailty based on established validated criteria, modified to accommodate available data. We used logistic regression to determine whether and to what degree stages of chronic kidney disease were associated with frailty. We also examined factors that might mediate the association between frailty and chronic kidney disease.
RESULTS
The overall prevalence of frailty was 2.8%. However, among persons with moderate to severe chronic kidney disease (estimated glomerular filtration rate <45 mL/min/1.73 m2), 20.9% were frail. The odds of frailty were significantly increased among all stages of chronic kidney disease, even after adjustment for the residual effects of age, sex, race, and prevalent chronic diseases. The odds of frailty associated with chronic kidney disease were only marginally attenuated with additional adjustment for sarcopenia, anemia, acidosis, inflammation, vitamin D deficiency, hypertension, and cardiovascular disease. Frailty and chronic kidney disease were independently associated with mortality.
CONCLUSION
Frailty is significantly associated with all stages of chronic kidney disease and particularly with moderate to severe chronic kidney disease. Potential mechanisms underlying the chronic kidney disease and frailty connection remain elusive.
Keywords: Body composition, Chronic kidney disease, Frailty
Frailty is described in the geriatric literature as a multidimensional phenotype that reflects declining physical function and a global vulnerability to adverse outcomes in the setting of stress, such as illness or hospitalization.1–5 Multiple instruments to operationalize a definition of frailty have been created and validated.1 One well-validated index, proposed by Fried and colleagues,4 defines frailty as the presence of 3 or more of 5 criteria: unintentional weight loss, exhaustion, weakness, slow walking speed, and low physical activity.4 By using the criteria of Fried and colleagues, estimates of the prevalence of frailty vary from 7% of persons aged more than 65 years to 40% of persons aged more than 80 years.2–4 Frailty is even more prevalent among persons with diabetes and other chronic debilitating diseases.6 Frailty as defined by the Fried et al criteria is associated with increased risk of falls, hospitalization, disability, and death.4
Although chronic kidney disease in general and end-stage renal disease in particular are known to be associated with impaired health status and physical function, few studies have examined the association between chronic kidney disease and frailty.7–11 Shlipak et al12 found a strong association between chronic kidney disease and frailty in elderly participants in the Cardiovascular Health Study. Johansen et al13 found a strong association between end-stage renal disease and frailty, even among younger persons. However, the degree to which chronic kidney disease and frailty are linked across the population and the potential mediators leading to frailty in patients with chronic kidney disease are unknown. To explore these questions, we used data from the third National Health and Nutrition Evaluation Survey (NHANES III). We hypothesized that mild-to-moderate chronic kidney disease would be associated with frailty and that a loss of muscle mass (sarcopenia) associated with chronic kidney disease would explain a large fraction of the increased risk of frailty in persons with chronic kidney disease.
MATERIALS AND METHODS
Data Source
We obtained individual-level data from NHANES III, a nationally representative survey of the health status of persons residing in the United States collected between 1988 and 1994. NHANES III is a cross-sectional, multistage, stratified, clustered probability sample of the US civilian noninstitutionalized population conducted by the National Center for Health Statistics, a branch of the Centers for Disease Control and Prevention.14 The first of multiple planned mortality linkages was conducted in 2004, using a probabilistic matching algorithm linking NHANES III participants to the National Death Index. A public use version of the mortality linkage data is available and contains mortality status and months of follow-up, as well as cause of death data on all NHANES III participants older than 17 years for whom sufficient data were available. The institutional review board for the Centers for Disease Control and Prevention approved NHANES III, and all participants provided written consent. The present study was granted exempt status by the institutional review board of Stanford University School of Medicine.
Study Sample
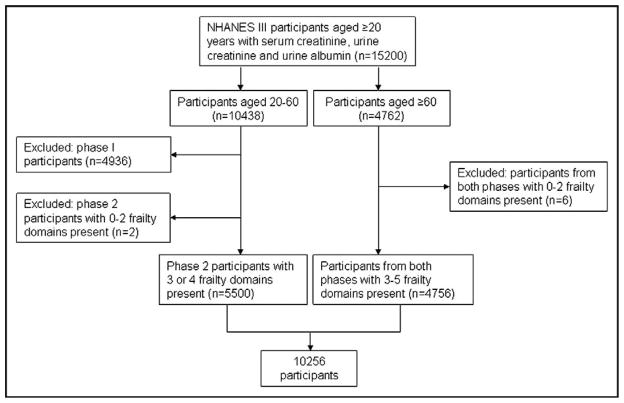
We identified all participants who completed an NHANES interview between 1988 and 1994 (n = 33,198). We limited the study population to persons aged 20 years or more with available serum creatinine, urine creatinine, and urine albumin measurements (n = 15,200). We excluded persons for whom sufficient data to assess frailty were unavailable, as described below (n = 4944). The analytic sample consisted of the remaining 10,256 persons (Figure 1).
Figure 1.
Study sample. NHANES = National Health and Nutrition Evaluation Survey.
Frailty
We defined frailty on the basis of a modification of previously validated frailty criteria originally reported by Fried et al.4 Our definition adheres to the 5 frailty domains established by the Fried et al criteria, but customizes the criteria for application to NHANES III data.
Low body weight for height, defined as Quetelet’s (body mass) index ≤ 18.5 kg/m2.
Slow walking, defined as the slowest quintile adjusted for gender, in a timed 8-foot walk.
Weakness, defined as present if participants answered “some difficulty,” “much difficulty,” or “unable to do” when asked how much difficulty they have “lifting or carrying something as heavy as 10 pounds (like a sack of potatoes or rice).”
Exhaustion, defined as present if participants answered “some difficulty,” “much difficulty,” or “unable to do” when asked how much difficulty they have “walking from one room to the other on the same level.”
Low physical activity, defined as present if participants answered “less active” when asked “Compared with most (men/women) your age, would you say that you are more active, less active, or about the same?”
Persons with available data for 3 or more frailty domains were included in our analysis. Timed walk was performed only on participants aged 60 years and older. In Phase 1 of the data collection (1988–1991), questions pertaining to weakness and exhaustion were asked only of participants aged 60 years and older. For this reason, no participants from Phase 1 younger than 60 years of age met inclusion criteria. Demographic and clinical characteristics of excluded Phase 1 participants were similar to those included from Phase 2 (see Appendix, available online).
Chronic Kidney Disease
Serum and urine creatinine were measured at a central laboratory (White Sands Research Center, Alamogordo, NM) by means of the modified kinetic Jaffe reaction using a Hitachi 737 analyzer (Boehringer Mannheim Corp, Indianapolis, Ind). Urine albumin was measured with a solid-phase fluorescent immunoassay (University of Minnesota School of Medicine, Minneapolis, Minn). Glomerular filtration rate (GFR) was estimated from serum creatinine using the Mayo quadratic equation.15 Microalbuminuria was defined as a urinary albumin-to-creatinine ratio ≥3.5 mmol/mg in women and ≥2.5 mmol/mg in men. Survey participants were stratified according to the following groups: normal kidney function (estimated [e]GFR >60 mL/min/1.73 m2), stage 1 and 2 chronic kidney disease (eGFR >60 mL/min/1.73 m2 with microalbuminuria), stage 3a chronic kidney disease (eGFR 45–59 mL/min/1.73 m2), and stage 3b, 4, and 5 chronic kidney disease (eGFR <45 mL/min/1.73 m2).
Bioelectrical Impedance Analysis
Body composition was estimated in participants aged 12 years and older with bioelectrical impedance analysis. The Valhalla Scientific Body Composition Analyzer 1990 B (San Diego, Calif) was used to introduce a low-amplitude (50 kHz) current across electrodes placed on the right hand and foot of participants. Two measurements were taken: resistance (R) and reactance (Xc). Resistance is the opposition to electrical current and is related to the length, diameter, and composition of the measured body segment. Resistance is low in lean tissue, which contains a high concentration of water and electrolytes, and high in adipose tissue and bone. Reactance reflects capacitance and is produced by cell membranes. Resistance and reactance can be considered separately or via one of several measures that incorporate the 2 into a single value, such as impedance (vector sum of resistance and reactance) and phase angle (arc tangent of Xc/R). Theoretically, phase angle can range from 0 to 90 degrees: 0 degrees if the circuit is only resistive (a system with no cell membranes) and 90 degrees if the circuit is only capacitive (a system of membranes with no fluid).
Other Explanatory Variables
Diabetes was defined as either self-reported physician-diagnosed diabetes or a hemoglobin A1c ≥6%. Liver disease was defined as aspartate aminotransferase >37 U/L or ala-nine aminotransferase >40 U/L for men and either aspartate aminotransferase or alanine aminotransferase >31 U/L for women. Cancer, arthritis, and chronic lung disease were each defined by self-reported physician diagnosis. Racial-ethnic classification was collected via self report; survey participants selected from the following categories: non-Hispanic white, non-Hispanic black, Mexican American, and other. Hemoglobin, bicarbonate, and C-reactive protein were measured according to protocols described elsewhere.17 Blood pressure was measured according to a protocol described elsewhere; hypertension was defined according to Joint National Committee 7 guidelines.18,19 We considered participants to have peripheral arterial disease if they reported calf pain with activity that was relieved with rest. Similarly, we considered participants to have coronary artery disease if they reported chest pain with activity that was relieved with rest or a prior myocardial infarction. We identified survey participants with congestive heart failure or history of stroke based on self-reported physician diagnosis. Questions used to identify these conditions are shown in the Appendix (available online). We considered the presence of one or more of the following: peripheral arterial disease, coronary artery disease, heart failure, or stroke as “overt cardiovascular disease.”
Statistical Analysis
We conducted data analysis with SAS (version 9.1.3, SAS Institute, Inc, Cary, NC), accounting for oversampling, stratification, and clustering.20 We fitted a series of logistic regression models to determine the odds of frailty based on chronic kidney disease stage. Odds ratios and confidence intervals (CIs) were calculated from model parameter coefficients and standard errors, respectively. Because advanced age is known to be associated with chronic kidney disease and frailty, and estimates of GFR are dependent in part on age, sex, and race, our base model considered chronic kidney disease stage and the residual effects of age, sex, and race. We further adjusted for comorbid conditions that we had hypothesized to be related to frailty (diabetes, arthritis, cancer, chronic liver disease, and chronic lung disease) but not caused by chronic kidney disease. In subsequent analyses, we also adjusted for factors that often complicate chronic kidney disease to potentially explain any association identified between chronic kidney disease and frailty. These factors included sarcopenia (estimated using bioelectrical impedance analysis parameters), anemia (hemoglobin concentration), acidosis (serum bicarbonate concentration), inflammation (C-reactive protein concentration), vitamin D deficiency, and overt cardiovascular disease, as described above. We chose phase angle as the summary bioelectrical impedance analysis parameter in our primary analysis. Given a strong and nonlinear bivariate association between phase angle and frailty, phase angle was modeled with a linear spline with 1 knot at 6 degrees. Proxies of sarcopenia, anemia, acidosis, inflammation, vitamin D deficiency, and cardiovascular disease were added to the multivariable logistic regression model individually; we determined the change in odds ratio to reflect whether these factors helped to explain the chronic kidney disease and frailty association.
To determine whether frailty and chronic kidney disease were independently associated with survival, we performed a proportional hazards (Cox) regression analysis, accounting for the complex survey design and sample weights (SUDAAN version 10, Cary, NC). The base model adjusted for age, sex, race, chronic kidney disease category, and frailty. We fitted an additional model accounting for comorbid conditions associated with frailty. Multiplicative interaction terms (frailty × chronic kidney disease category) were used to evaluate effect modification.
RESULTS
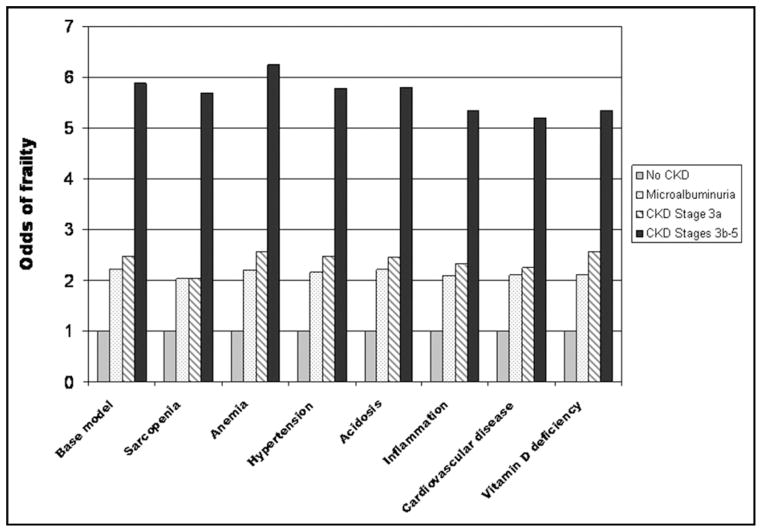
Of the 33,994 NHANES III participants, 10,256 participants, representative of approximately 100 million Americans, met inclusion criteria (Table 1). The overall rate of frailty was 2.8% (standard error, 0.34). The prevalence of frailty was significantly higher among participants with chronic kidney disease, particularly evident with eGFR less than 45 mL/min/1.73 m2 (Table 2). Rates of positive response in individual frailty domains ranged from 2.6% to 21.9%. The odds of frailty were substantially higher in participants with chronic kidney disease than in those without (Table 3). Further adjustment for potential mediators of a “chronic kidney disease effect” (sarcopenia, anemia, hypertension, acidosis, inflammation, vitamin D deficiency, and overt cardiovascular disease) failed to extinguish or significantly attenuate the chronic kidney disease and frailty association (Figure 2).
Table 1.
Sample Population Characteristics (n = 10256)
| Mean or Percent | Standard Error | 95% CI | |
|---|---|---|---|
| Age (y) | 49.59 | 1.30 | 46.97–52.20 |
| Gender (% male) | 47.07 | 0.71 | 45.64–48.51 |
| Race/ethnicity (%) | |||
| Non-Hispanic white | 77.27 | 2.28 | 72.69–81.85 |
| Non-Hispanic black | 10.05 | 1.13 | 7.79–12.32 |
| Mexican-American | 4.76 | 0.77 | 3.21–6.31 |
| Other | 7.92 | 1.45 | 4.99–10.84 |
| Poverty-income ratio | 3.17 | 0.10 | 2.97–3.37 |
| Highest grade completed | 12.23 | 0.14 | 11.93–12.52 |
| Self-reported health status (%) | |||
| Excellent | 18.64 | 0.96 | 16.70–20.58 |
| Very good | 30.48 | 1.12 | 28.23–32.73 |
| Good | 33.43 | 0.81 | 31.80–36.05 |
| Fair | 14.03 | 1.01 | 12.01–16.05 |
| Poor | 3.37 | 0.30 | 2.76–3.97 |
| eGFR (mL/min) | 106.21 | 1.19 | 103.82–108.60 |
| Chronic kidney disease (%) | |||
| Stage 1/2 | 9.66 | 0.65 | 8.36–10.97 |
| Stage 3A | 1.80 | 0.21 | 1.39–2.22 |
| Stage 3B-5 | 1.10 | 0.14 | 0.83–1.38 |
| Diabetes (%) | 13.01 | 0.85 | 11.30–14.71 |
| Cancer (%) | 4.75 | 0.45 | 3.85–5.65 |
| COPD (%) | 8.29 | 0.44 | 7.41–9.18 |
| Liver disease (%) | 8.96 | 0.64 | 7.67–10.25 |
| Arthritis (%) | 22.84 | 1.50 | 19.83–25.85 |
| 25 hydroxy vitamin D concentration, serum (ng/mL) | 29.38 | 0.55 | 28.27–30.49 |
| Hemoglobin (g/dL) | 14.20 | 0.04 | 14.11–14.28 |
| Bicarbonate, serum (mmol/L) | 27.62 | 0.24 | 27.15–28.10 |
| C-reactive protein, serum (units) | 0.44 | 0.01 | 0.41–0.46 |
| Hypertensiona (%) | 35.06 | 1.97 | 31.11–39.01 |
| Overt cardiovascular diseaseb (%) | 12.65 | 0.92 | 10.80–14.51 |
CI = confidence interval; eGFR = estimated glomerular filtration rate; COPD = chronic obstructive pulmonary disease.
Joint National Committee 7 guidelines.
Composite measure of peripheral arterial disease, coronary artery disease, congestive heart failure, and history of cerebrovascular accident.
Table 2.
Prevalence of Frailty and Domains
| Overall % (SE) | No CKD % (SE) | Stage 1/2 % (SE) | Stage 3a % (SE) | Stage 3b, 4, 5 % (SE) | |
|---|---|---|---|---|---|
| Shrinkage | 2.64 (0.20) | 2.32 (0.23) | 3.66 (0.62) | 2.07 (1.13) | 3.01 (2.13) |
| Weakness | 13.13 (0.83) | 10.45 (0.65) | 21.57 (1.87) | 36.20 (4.71) | 36.78 (4.82) |
| Exhaustion | 4.01 (0.38) | 2.55 (0.32) | 6.81 (1.18) | 11.31 (2.36) | 27.12 (4.24) |
| Low activity | 21.85 (0.70) | 20.82 (0.72) | 28.26 (1.96) | 25.77 (4.31) | 29.19 (4.81) |
| Slow walkinga | 13.35 (0.71) | 7.33 (0.51) | 15.17 (1.29) | 28.73 (4.11) | 31.54 (6.31) |
| Frailb | 2.77 (0.34) | 1.47 (0.21) | 5.94 (0.99) | 10.74 (2.36) | 20.90 (3.44) |
CKD = chronic kidney disease; SE = standard error.
The slowest-walking quintile adjusted for gender was defined before adjustment for complex survey design.
Frail persons exhibit ≥3 of 3 to 5 available frailty domains.
Table 3.
Odds Ratios for Frailty: Multivariable Model
| OR of Frailty | 95% CI | |
|---|---|---|
| No CKD | 1 (Reference) | — |
| CKD stage 1/2 | 2.21 | 1.49–3.28 |
| CKD stage 3a | 2.48 | 1.57–3.93 |
| CKD stage 3b-5 | 5.88 | 3.40–10.16 |
| Diabetes | 1.68 | 1.16–2.45 |
| COPD | 2.20 | 1.20–4.03 |
| Cancer | 1.89 | 1.19–2.99 |
| Arthritis | 3.34 | 2.08–5.38 |
OR = odds ratio; CI = confidence interval; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease.
Note: Chronic liver disease is excluded from this table because the association between chronic liver disease and frailty was not statistically significant.
Figure 2.
Potential mediators of frailty in CKD. CKD = chronic kidney disease.
We performed sensitivity analyses using 2 alternate equations to estimate GFR. By using the 4-variable Modification of Diet in Renal Disease (MDRD) equation, the odds of frailty were 2.3 (95% CI, 1.5–3.6) for persons with normal eGFR and microalbuminuria, 1.8 (95% CI, 1.1–3.0) for persons with stage 3a chronic kidney disease, and 5.5 (95% CI, 3.4–9.0) for persons with stage 3b to 5 chronic kidney disease. By using the Mayo equation with a correction factor for black race (1.21), the odds of frailty for persons with normal eGFR and microalbuminuria, stage 3a, and 3b to 5 chronic kidney disease were 2.2 (95% CI, 1.5–3.3), 2.6 (95% CI, 1.6–4.1), and 6.7 (95% CI, 3.9–11.4), respectively. Finally, we considered whether any medications prescribed to persons with chronic kidney disease might contribute to frailty. Additional adjustment for any antihypertensive agent did not materially alter the association of frailty and chronic kidney disease (data not shown).
To determine whether our findings were robust across other definitions of frailty, we considered a modification of the criteria proposed by the Study of Osteoporotic Fracture investigators.21 These results also were qualitatively similar to results obtained using our modified definition of the criteria by Fried et al4 (data not shown).
Frailty and chronic kidney disease stage were independently associated with the risk of death (Table 4, Figure 3); hazard ratios were only modestly attenuated after adjustment for comorbid conditions associated with frailty. Frailty × chronic kidney disease interaction terms were not statistically significant (all P values >.4).
Table 4.
Hazard Ratios for Mortality
| Model A Hazard Ratio (95% CI) |
Model Ba | |
|---|---|---|
| Frailty | 2.1 (1.6–2.8) | 2.0 (1.5–2.7) |
| Microalbuminuria | 2.1 (1.8–2.3) | 1.9 (1.7–2.1) |
| CKD stage 3a | 2.6 (2.1–3.3) | 2.4 (2.0–3.0) |
| CKD stage 3b-5 | 3.1 (2.3–4.3) | 3.0 (2.2–4.1) |
| Diabetes | — | 1.3 (1.2–1.6) |
| Chronic lung disease | — | 1.7 (1.4–2.0) |
| Cancer | — | 1.3 (1.1–1.6) |
| Arthritis | — | 0.9 (0.8–1.0) |
CI = confidence interval; CKD = chronic kidney disease.
Both models are adjusted for the complex survey design, age, sex, and race.
Also adjusted for diabetes, chronic lung disease, cancer, and arthritis.
Figure 3.
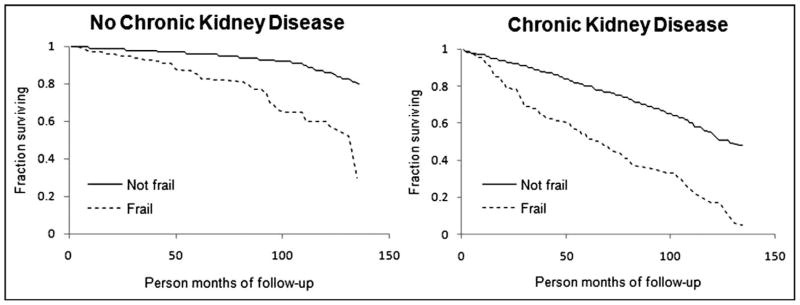
Frailty, chronic kidney disease, and survival.
DISCUSSION
We estimated that approximately 3% of US adults and approximately 5% of those aged more than 60 years were frail. Frailty was significantly more common in persons with chronic kidney disease. Even among persons with mild or early-stage chronic kidney disease, frailty was approximately twice as likely compared with those without chronic kidney disease. Persons with more severe chronic kidney disease were more likely to be frail. Frailty also was more common in persons with moderate to severe chronic kidney disease than in those with other chronic illnesses, such as vascular disease, cancer, and other degenerative diseases of aging. Finally, frailty and chronic kidney disease were independently associated with an increased risk of death.
Our study results confirm and extend the findings of Shlipak et al,12 who evaluated the prevalence of frailty in an elderly cohort (≥65 years). They found a 15% overall rate of frailty among persons with an elevated serum creatinine concentration. The odds of frailty were inversely related to the eGFR, and one fifth of persons with an eGFR of less than 40 mL/min/1.73 m2 were frail. We found a similar rate of frailty among persons with moderate to severe chronic kidney disease, the majority of whom were older than 60 years. Of note, we found similar rates of slow walking, weakness, exhaustion, and low activity when compared with the results of Shlipak et al. We found fewer individuals classified as low body weight for height, possibly because of the broader age range of the general population.
We were surprised that sarcopenia and other metabolic manifestations of chronic kidney disease failed to extinguish, or even significantly attenuate, the increased odds of frailty. One potential explanation for this finding is that subclinical vascular disease may contribute to the increased odds of frailty in chronic kidney disease. Measures of subclinical vascular disease are correlated with frailty, and persons with chronic kidney disease are likely to have a large burden of subclinical vascular disease that we were not able to account for in this study.22 The finding that patients with stage 1 and 2 chronic kidney disease also had a substantially increased odds of frailty may provide additional support for this hypothesis, because microalbuminuria is strongly associated with subclinical vascular disease but is unlikely to be associated with metabolic manifestations of chronic kidney disease when eGFR is normal.
For our analysis, we chose to use the Mayo quadratic equation rather than the MDRD equation to estimate kidney function.15 The Mayo equation was developed from a study population jointly enriched with healthy persons and persons with chronic kidney disease. In contrast, the MDRD equation was derived from a sample composed of persons with chronic kidney disease (iothalamate GFR 25–55 mL/ min/1.73 m2). Although none of the available equations are ideal in all settings, the MDRD equation tends to underestimate GFR in persons with normal or near normal kidney function; thus, it may overestimate the fraction of persons with clinically significant chronic kidney disease in a general population sample.23 Although the Mayo quadratic equation may be less apt to misclassify persons with near normal kidney function, it was derived in a less diverse (<1% black) population, and the relation between serum creatinine and GFR appears to differ significantly by race.16 Thus, we performed sensitivity analyses classifying chronic kidney disease stage by the 4-variable MDRD equation and a modification of the Mayo quadratic equation with a correction factor for black race. As expected, the MDRD equation yielded the highest proportion of persons classified as stage 3+ chronic kidney diseases. Odds ratios were somewhat attenuated when using the MDRD equation, suggesting a bias toward the null hypothesis owing to misclassification.
Our study’s strengths included analysis of a nationally representative sample of US adults with varying severity of chronic kidney disease. Our study has several important limitations. First, although our definition of frailty adheres to the 5 domains posited by Fried et al,4 it was necessary to modify the definition of individual domains to accommodate available data. For example, in the original study by Fried et al, the “weakness” domain was based on measured grip strength. However, grip strength measurements were not included in NHANES III. Other domains were based on interview data in both Fried et al’s study and our study, with slightly varied survey questions. Despite modification of the criteria by Fried et al, our NHANES III-compatible definition of frailty has strong face validity and remains faithful to the original domains by Fried et al. We also acknowledge that the dichotomous nature of this definition of frailty may obscure some potentially relevant distinctions. For example, persons who are weak and exhausted are likely to be different from persons with no Fried et al criteria present. Second, different data were collected on survey participants aged 20 to 60 years and those older than 60 years. The result is that more participants younger than 60 years have incomplete data from which to diagnose frailty than participants older than 60 years. For example, exhaustion and weakness questions were only asked of participants aged 60 years and older during the first phase of NHANES III data acquisition. In the second phase, these questions were asked of all adult participants. Because of these incompletely overlapping domains, only 4242 survey participants had data available for all 5 frailty domains; all of these survey participants were aged greater than 60 years. A total of 5944 survey participants had data available for 4 of the 5 domains, 90% of whom were aged 20 to 60 years. Rates of frailty were lower among participants with only 4 available domains, which we expected given their younger age (mean age of those with 4 vs 5 domains was 39.8 vs 70.9 years, respectively). Thus, it is likely that we have underestimated the population prevalence of frailty among persons younger than 60 years. However, because the majority of frail persons and the majority of persons with chronic kidney disease are aged more than 60 years, any limitations in available data for younger NHANES III participants would have been unlikely to qualitatively or quantitatively change our results or conclusions.
CONCLUSIONS
We confirmed the strong association between frailty and chronic kidney disease in the general US population. The association was especially strong among persons with an eGFR less than 45 mL/min/1.73 m2, but was substantial even among those with microalbuminuria and normal eGFR. Frailty and chronic kidney disease were independently associated with mortality. Given the prevalence of chronic kidney disease, especially among the elderly, and increasing evidence linking frailty to early mortality, morbidity, and disability, a better understanding of the mechanisms underlying the chronic kidney disease and frailty connection is clearly needed. Longitudinal studies of frailty and function in the population with chronic kidney disease, and interventions aimed at lessening the propensity toward frailty in this population, are clearly warranted.
CLINICAL SIGNIFICANCE.
Distinct from disability or comorbidity, frailty is a clinical syndrome that confers increased risk of hospitalization and death.
Risk of frailty is increased approximately 2-fold in mild chronic kidney disease and approximately 6-fold in persons with moderate to severe chronic kidney disease.
The relationship between frailty and chronic kidney disease cannot be fully explained by the increased prevalence of sarcopenia, anemia, acidosis, inflammation, and overt cardiovascular disease among persons with chronic kidney disease.
Acknowledgments
Funding: This research was funded by a grant from the Stanford University School of Medicine Medical Scholars Program.
APPENDIX
This table compares demographic, laboratory, and other data from excluded survey participants aged 20 to 60 years (Phase 1 of data collection, 1988–1991) and included participants aged 20 to 60 years (Phase 2, 1991–1994).
| Phase 1 Mean or Percent |
Phase 2 Mean or Percent |
|
|---|---|---|
| Age (y) | 37.7 | 38.0 |
| Gender (% male) | 49.3 | 49.2 |
| Race/ethnicity (%) | ||
| Non-Hispanic white | 75.9 | 73.2 |
| Non-Hispanic black | 11.0 | 11.4 |
| Mexican-American | 5.7 | 6.1 |
| Other | 7.5 | 9.4 |
| Poverty-income ratio | 3.0 | 3.3 |
| Highest grade completed | 12.7 | 12.8 |
| Self-reported health status (%) | ||
| Excellent | 23.2 | 21.4 |
| Very good | 32.6 | 33.5 |
| Good | 31.9 | 33.4 |
| Fair | 10.6 | 10.2 |
| Poor | 1.7 | 1.4 |
| eGFR (mL/min) | 117.8 | 117.0 |
| Chronic kidney disease (%) | ||
| Stage 1/2 | 6.6 | 6.1 |
| Stage 3A | 0.1 | 0.2 |
| Stage 3B-5 | 0.1 | 0.1 |
| Diabetes (%) | 6.0 | 7.2 |
| Cancer (%) | 2.0 | 2.5 |
| COPD (%) | 6.2 | 5.9 |
| Liver disease (%) | 6.5 | 11.0 |
| Arthritis (%) | 10.9 | 11.3 |
| 25 hydroxy vitamin D concentration, serum (ng/mL) | 29.8 | 30.3 |
| Hemoglobin (g/dL) | 14.0 | 14.2 |
| Bicarbonate, serum (mmol/L) | 28.9 | 27.0 |
| C reactive protein, serum (units) | 0.4 | 0.4 |
| Hypertensiona (%) | 18.0 | 17.8 |
| Overt cardiovascular diseaseb (%) | 6.8 | 6.7 |
Definition of “overt cardiovascular disease.” Overt cardiovascular disease was defined as having any peripheral arterial disease, coronary artery disease, congestive heart failure, or history of cerebrovascular accident. Interview questions defining these conditions are summarized below.
Peripheral arterial disease. A positive result requires all the responses listed in the right-hand column of the following table:
| Interview Question | Response Sequence Required for Definition |
|---|---|
| Do you get pain in either leg while you are walking? | Yes |
| Does this pain ever begin while you are standing still or sitting? | No |
| In what part of your legs do you feel it? | Calves |
| Do you get it if you walk uphill or hurry? | Yes |
| Does this pain ever disappear while you are walking? | No |
| What happens if you stand still? Is the pain relieved? | Yes, relieved |
Coronary artery disease. A positive result requires all of the first 3 responses listed in the right-hand column of the following table, or the final response:
| Interview Questions | Response Sequence Required for Definition |
|---|---|
| Have you ever had any pain or discomfort in your chest? | Yes |
| Do you get it when you walk uphill or hurry? | Yes |
| If you stand still, what happens to it? Is the pain or discomfort relieved or not relieved? | Yes, relieved |
| Has a doctor ever told you that you had a heart attack? | Yes |
Congestive heart failure. A positive result requires a “yes” answer to the question, “Has a doctor ever told you that you have congestive heart failure?”
Cerebrovascular accident. A positive result requires a “yes” answer to the question, “Has a doctor ever told you that you had a stroke?”
Footnotes
Conflict of Interest: None.
Authorship: All authors had access to the data and played a role in writing this manuscript.
References
- 1.Jones DM, Song X, Rockwood K. Operationalizing a frailty index from a standardized comprehensive geriatric assessment. J Am Geriatr Soc. 2004;52:1929–1933. doi: 10.1111/j.1532-5415.2004.52521.x. [DOI] [PubMed] [Google Scholar]
- 2.Morley JE, Haren MT, Rolland Y, Kim MJ. Frailty. Med Clin North Am. 2006;90:837–847. doi: 10.1016/j.mcna.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 3.Slaets JP. Vulnerability in the elderly: frailty. Med Clin North Am. 2006;90:593–601. doi: 10.1016/j.mcna.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 5.Hoogerduijn JG, Schuurmans MJ, Duijnstee MS, et al. A systematic review of predictors and screening instruments to identify older hospitalized patients at risk for functional decline. J Clin Nurs. 2007;16:46–57. doi: 10.1111/j.1365-2702.2006.01579.x. [DOI] [PubMed] [Google Scholar]
- 6.Morley JE. Diabetes, sarcopenia, and frailty. Clin Geriatr Med. 2008;24:455–469. doi: 10.1016/j.cger.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Brogan DJ, Haber M, Kutner NG. Functional decline among older adults: comparing a chronic disease cohort and controls when mortality rates are markedly different. J Clin Epidemiol. 2000;53:847–851. doi: 10.1016/s0895-4356(00)00207-9. [DOI] [PubMed] [Google Scholar]
- 8.Hailpern SM, Melamed ML, Cohen HW, Hostetter TH. Moderate chronic kidney disease and cognitive function in adults 20 to 59 years of age: Third National Health and Nutrition Examination Survey (NHANES III) J Am Soc Nephrol. 2007;18:2205–2213. doi: 10.1681/ASN.2006101165. [DOI] [PubMed] [Google Scholar]
- 9.Kurella M, Ireland C, Hlatky MA, et al. Physical and sexual function in women with chronic kidney disease. Am J Kidney Dis. 2004;43:868–876. doi: 10.1053/j.ajkd.2003.12.050. [DOI] [PubMed] [Google Scholar]
- 10.Kurella M, Mapes DL, Port FK, Chertow GM. Correlates and outcomes of dementia among dialysis patients: the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant. 2006;21:2543–2548. doi: 10.1093/ndt/gfl275. [DOI] [PubMed] [Google Scholar]
- 11.Kurella M, Yaffe K, Shlipak MG, et al. Chronic kidney disease and cognitive impairment in menopausal women. Am J Kidney Dis. 2005;45:66–76. doi: 10.1053/j.ajkd.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 12.Shlipak MG, Stehman-Breen C, Fried LF, et al. The presence of frailty in elderly persons with chronic renal insufficiency. Am J Kidney Dis. 2004;43:861–867. doi: 10.1053/j.ajkd.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 13.Johansen KL, Chertow GM, Jin C, Kutner NG. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007;18:2960–2967. doi: 10.1681/ASN.2007020221. [DOI] [PubMed] [Google Scholar]
- 14.Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–1994. Vital Health Stat. 1994:32. [PubMed] [Google Scholar]
- 15.Rule AD, Larson TS, Bergstralh EJ, et al. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141:929–937. doi: 10.7326/0003-4819-141-12-200412210-00009. [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 17.Gunter EW, Lewis BG, Koncikowski SM. Laboratory Procedures Used for the Third National Health and Nutrition Examination Survey (NHANES III), 1988–1994. Hyattsville, MD: US Department of Health and Human Services, Public Health Service, Centers for Disease Control; National Center for Health Statistics; 1996. [Google Scholar]
- 18.Reference Manual: Physician Examiner’s Training Manual. Rockville, MD: National Center for Health Statistics; 1991. [Google Scholar]
- 19.Lenfant C, Chobanian AV, Jones DW, Roccella EJ. Seventh Report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7): resetting the hypertension sails. Hypertension. 2003;41:1178–1179. doi: 10.1161/01.HYP.0000075790.33892.AE. [DOI] [PubMed] [Google Scholar]
- 20.SAS OnlineDoc 9.1.3. Cary, NC: SAS Institute; 2002–2008. Introduction to survey sampling and analysis procedures. [Google Scholar]
- 21.Ensrud KE, Ewing SK, Taylor BC, et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008;168:382–389. doi: 10.1001/archinternmed.2007.113. [DOI] [PubMed] [Google Scholar]
- 22.Lin J, Knight EL, Hogan ML, Singh AK. A comparison of prediction equations for estimating glomerular filtration rate in adults without kidney disease. J Am Soc Nephrol. 2003;14:2573–2580. doi: 10.1097/01.asn.0000088721.98173.4b. [DOI] [PubMed] [Google Scholar]
- 23.Rule AD, Gussak HM, Pond GR, et al. Measured and estimated GFR in healthy potential kidney donors. Am J Kidney Dis. 2004;43:112–119. doi: 10.1053/j.ajkd.2003.09.026. [DOI] [PubMed] [Google Scholar]