Abstract
We designed a randomized controlled trial of a home based intervention to improve antiretroviral adherence and to assess the impact of depressive symptoms among people living with HIV in Hunan, China. At baseline, 110 subjects reported taking 90% or less of prescribed medication. At 6 months, when the intervention ended, 56% of subjects in the control and 87% of subjects in the experimental group were adherent. This difference was maintained at 12 months. In multivariate analyses, controlling for baseline visual analogue adherence scale, stigma, social support, and Center for Epidemiological Studies Depression scale, the experimental group had a significantly higher proportion of people who were adherent (p=0.009). The high prevalence of significant depressive symptoms (67%) at baseline is of concern. It is of particular importance that future studies look at the types of depression likely to be seen in these patients and differentiate early between those likely to benefit from HIV-related support and those who will require additional depression-targeted interventions.
Introduction
By the end of 2012, China's national treatment program was delivering antiretroviral medications (ARV) to over 126,400 people in all provinces through the “Four Frees and One Care” (China CARES) national policy.1 The “Four Frees” include free ARV, free HIV counseling and screening, free intervention for prevention of mother to child transmission, and free education for AIDS orphans. “One Care” covers economic assistance to families living with HIV.
However, challenges remain. Although relatively few data are available describing prevalence and correlates of ARV adherence in China, it appears that, as elsewhere in the world, many Chinese patients fail to adhere successfully to a daily medication regimen. Prevalence data suggest that the proportion of Chinese patients reporting suboptimal adherence ranges from 20% to 40%.2–6 This is of concern in China because virological monitoring of treatment response and second-line drug regimens are limited. A national survey of 3667 unique patients identified HIV drug resistance in 19%; among the factors independently associated with drug resistance was self-reported poor medication adherence.7
Although data are limited, one qualitative study of 36 HIV-positive individuals in Yunnan province identified self-perceived facilitators and barriers to adherence. Facilitators included perceptions of the importance and benefits of therapy, while barriers were side effects, pill burden, stigma, and limited access to care.8 Another qualitative study, this one of patients, caregivers, and family members in Beijing, also described the profound impact of stigma on adherence behavior.9
There are gaps in the services offered by the China CARES program, some of which may be linked to medication adherence. It is of concern that treatment for conditions associated with HIV, including mental health problems, are not provided as part of China CARES. Depressive symptoms, such as anhedonia, feelings of worthlessness and hopelessness, and recurrent thoughts of suicide, disrupt the daily life and medication self-management for many people living with HIV (PLWH).10,11 Depression has been associated consistently with treatment non adherence.12 Unfortunately, data regarding depression among PLWH in China are limited, drawn from small sample sizes, and show rates of depressive symptoms that vary widely, from 20% to 79%.13
In response to these issues, we designed a randomized controlled trial to evaluate an intervention to improve ARV adherence and to assess the impact of depressive symptoms among PLWH in Hunan, China. The intervention, conducted between July 2010 and August 2012, was compared to usual care as implemented by the China CARES national program in Hunan Province.
Methods
Study sites and clinical management protocols
Hunan is the 11th largest province in China, located in the southeast region of the country. The Hunan HIV epidemic ranks 8th largest among Chinese provinces, with an estimated 20,000–30,000 HIV-infected individuals. There are five China CARES clinics in Hunan; the study was conducted at the two largest, Hengyang City and Changsha City. At these clinics, HIV-infected individuals receive clinical evaluation and free ARV if they meet clinical eligibility criteria, which at the time of the study were WHO Stage III and IV and/or CD4<200 cells/mm3. (The Chinese CD4 threshold for ARV has since been raised to 350 cell/mm3.)
The clinical sites follow the Chinese national protocol for ARV management, which includes pre-treatment adherence counseling, an initial 21-day course of trimethoprim/sulfamethoxazole (TMP-SMX) to prevent opportunistic infections and prepare for medication taking, a 2-week lead-in ARV regimen (most patients receive nevirapine), a follow up evaluation, a second 2-week supply of ARV, and finally a monthly schedule for medication distribution and evaluation.
Subjects were receiving atazanavir or d4T, plus nevirapine or efavirenz, plus 3TC. These are the standard ARV medications provided by the Chinese government. Provincial China CDC personnel bring medications to the clinics for distribution by the clinic nurse. CD4 counts are obtained every 3 months; HIV-RNA is required before beginning treatment and recommended yearly.
Subjects and recruitment
Subjects were patients for whom ARV was prescribed at one of the two study sites. Potential subjects were told about the opportunity for study participation when they presented to the clinic for routine evaluation. Those beginning ARV treatment, as well as ARV experienced individuals, were eligible. Patients were eligible if they had detectable HIV-RNA, were prescribed ARV, and self-reported less than 90% adherence to either pre-ARV medications (TMP-SMX, multi-vitamins) or to ARV. ARV were prescribed for all participants at the time of the study.
Individuals with severe cognitive impairment and those who were not personally responsible for medication self-administration were not eligible. Because the intervention included home visits, willingness to receive those visits was an eligibility requirement. All subjects spoke either Mandarin Chinese or the local Hunan dialect.
Randomization and study protocol
After completing a baseline interview conducted by dedicated research staff, subjects were randomized to either the intervention or control condition. A stratified randomization procedure with a block size of 10 was used. A table was constructed using SAS programming to distribute intervention and control assignments in random order within three strata: CD4 of 50 or less; between 51–199; and 200 or greater cells/mm3, based on the most recent CD4 test in the clinical record. The China-based principal investigator (HHW) oversaw the randomization.
Subjects assigned to the intervention arm received the intervention for the subsequent 6 months. Subjects in both the control and intervention arms completed follow-up interviews 6 and 12 months after randomization. Subjects in both arms received 30 Yuan (approximately $5 US) at the completion of each study interview as partial compensation for time spent. The study protocol was reviewed and approved by the human subjects investigations oversight committees at Central South University (Xiangya School of Medicine), the University of California Los Angeles, and Yale University. All subjects provided written informed consent and agreed to the release of information regarding ARV regimen, treatment duration, date of diagnosis, CD4 cell count, and HIV-RNA.
Intervention
The intervention was guided by the pedagogical theory of Paolo Freire14 and led by nurses and peer educators who facilitated a self-directed discussion in which patients identified individual and social factors that influenced their adherence to medication regimens. The Freirian philosophy underlying the intervention is well known in China and was judged appropriate for the Chinese context.
In the intervention, known as Ai Sheng Nuo (Love, Life, Hope  ), the nurse and peer educator team made home visits twice a month for 3 months, followed by monthly visits for another 3 months. Between visits, the interventionists were available by phone. The intervention concluded at 6 months, after which time interventionists no longer interacted with subjects in person or via phone. Those subjects wishing additional support were referred to the clinical care site.
), the nurse and peer educator team made home visits twice a month for 3 months, followed by monthly visits for another 3 months. Between visits, the interventionists were available by phone. The intervention concluded at 6 months, after which time interventionists no longer interacted with subjects in person or via phone. Those subjects wishing additional support were referred to the clinical care site.
Usual care
Subjects assigned to both the intervention and to the control condition received standard adherence support services offered at all China CARES sites. These included pretreatment ARV education provided by a peer educator in the clinic, three scripted educational sessions provided by the prescribing physician, a 21-day lead-in course of TMP-SMX as pre ARV “medication taking practice”, and monthly adherence questioning and advice provided by the clinic nurse dispensing the ARV. The scripted educational sessions covered basic ARV information including the purpose of the medication, the need for adherence, and common side effects.
Intervention delivery and fidelity
A detailed intervention manual guided the delivery of the intervention. Members of the intervention team completed an initial training, which included both didactic information about HIV/AIDS, sexuality, substance abuse, and medication adherence as well as training in the use of Freirian educational techniques. To ensure fidelity and consistency in the implementation of the intervention, team members maintained detailed narrative logs of the content and process of each home visit. The logs were reviewed regularly by fellow team members and by the China-based principal investigator. Additional training for the interventionists was provided by US-based consultants.
Measures
Adherence
A visual analogue scale (VAS) was used to assess adherence to ARV over the 30 days preceding each interview. The VAS consisted of a 10-cm horizontal line ranging from 0% to 100%. Each subject placed a cross on the line at the point showing his or her best estimate of how much medication he or she had taken in the preceding 30 days. 0% meant no medication was taken, and 100% meant all medication was taken. A number of studies have demonstrated a correlation between VAS scores and electronic monitoring data, pill counts, and HIV-RNA levels.15–17 Further, Amico and colleagues established construct validity for a VAS measure in relation to its association with specific barriers to ARV adherence.18
Depressive symptoms
The Center for Epidemiological Studies Depression Scale is a 20-item scale for epidemiological research that was developed by the US National Institute for Mental Health. The CES-D has a four-factor structure: depressive affect, somatic symptoms, positive affect, and interpersonal relations. Higher scores indicate increasing numbers of depressive symptoms. This instrument was chosen because it has good reliability and validity and has been translated into Chinese and widely used with Chinese populations.19,20
Social support and stigma
The Social Support Rating Scale (SSRS) is an established Chinese questionnaire. It is a 10-item scale comprising three subscales: objective social support, subjective social support, and utility of social support. The total maximum score is 50. Higher scores suggest higher levels of social support.
HIV/AIDS-related stigma is closely associated with local culture.21 In this instance, stigma was measured using a culturally sensitive Chinese scale. The scale comprises 34 items with five domains (disclosure concerns, public rejection, family stigma, internalized stigma, and health care providers' discrimination). Subjects are asked to rate the extent to which they agree that their personal experiences are mirrored in each statement, using a 5-point Likert scale. A higher score indicates a stronger sense of feeling stigmatized. The Cronbach's alpha of the scale is 0.90, content validity is 0.88, and the psychometric evaluation results are acceptable.22
Biological outcomes
Plasma samples were obtained at enrolment, 6 months, and 12 months for quantitative HIV-RNA and genotypic testing. The primary biological outcome was quantitative HIV-RNA, which was performed at the Hunan CDC laboratory. Standard HIV genotyping was performed at baseline and on all samples with detectable HIV-RNA at 6 or 12 months. The results of tests to innumerate CD4 lymphocytes were abstracted from the medical record.
Data collection and management
Trained interviewers collected data during structured face-to-face interviews conducted in a private area at the time of a regularly scheduled monthly visit to the China CARES site. Structured interviewer-administered questionnaires were used to assess demographic, clinical, and psychosocial variables. A second member of the study team reviewed each interview for completeness and accuracy. Information regarding ARV regimen, treatment duration, time of diagnosis, and CD4 cell count was abstracted from the medical record. HIV-RNA results were received directly from the laboratory. Data were collected at baseline, at the conclusion of the intervention phase (6-month data point), and 6 months after the conclusion of the intervention phase (12-month data point).
Analysis
Adherence was dichotomized as greater than 90% (adherent) and 90% or less (non-adherent). While it is known that different ARV regimens require different levels of adherence to achieve high levels of viral suppression,23 the specific requirements of individual regimens are not clear. In this case, 90% was chosen as a reasonable standard of measurement given the regimens being used.
Overall CES-D score was dichotomized as 16 or greater (suggestive of moderate to severe depressive symptoms) and less than 16 (suggestive of no or minimal depressive symptoms). Undetectable viral load was based on designation on lab report; undetectable in this case meant less than 400 copies per mm. CD4+ counts were categorized into 100 or less, 101–200, and greater than 200/mm3.
Data were double entered and stored in an Access® database. Descriptive and bivariate analyses were carried out using t-tests, Kruskal Wallis, and chi-square tests. Bivariate analyses on repeated measures included repeated measures ANOVA, and extended Cochran Maental Haenszel chi-square statistics. For dichotomous outcomes, generalized estimating equations (GEE) models were used to model changes over time and by group. Unstructured correlation was specified for the correlation structure. For outcomes with more than two levels, cumulative logit models with an independent correlation structure were used. Analyses were performed in SPSS® (17.0) and SAS® (9.3).
Results
Participant flow
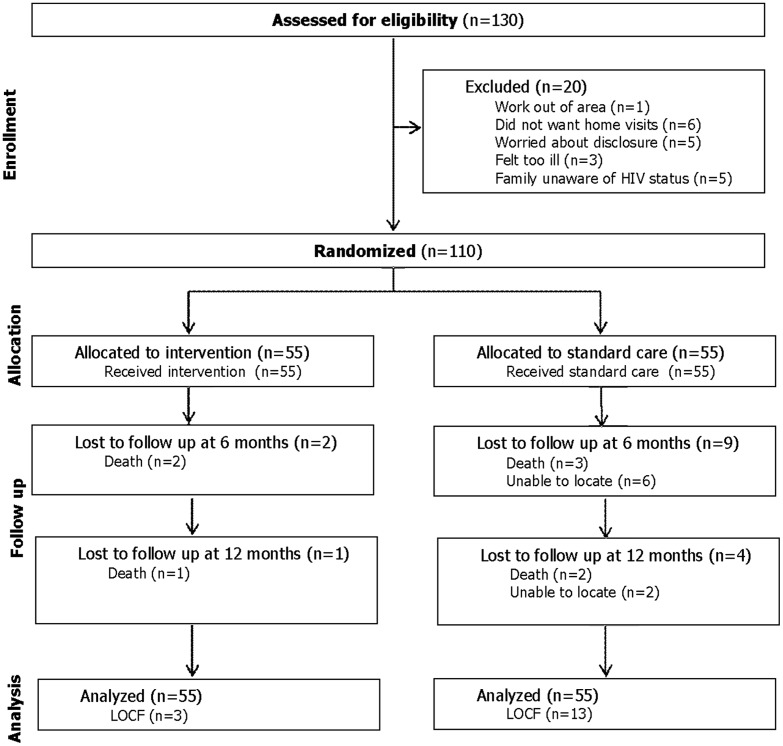
As seen in Fig. 1, 130 individuals were invited to participate, of whom 20 declined. Reasons for declining were plans to migrate out of the local area for work (N=1), did not want to receive home visitors (N=6), worried about possible disclosure of diagnosis to neighbors (N=5), feeling too ill (N=3), and family unaware of the diagnosis (N=5).
FIG. 1.
Flow diagram of study participants.
The final 110 subjects included 60 individuals beginning ARV who had been non-adherent to pre ARV medication regimens and 50 who were non-adherent to an existing ARV regimen. Fifty-five participants were randomized to the experimental condition and 55 to the control condition. At the 6-month follow-up, two participants were lost to death in the experimental group, while nine subjects were lost in the control condition (three deaths, six unable to locate). At the 12-month conclusion of the study, 52 subjects remained in the experimental group (one additional loss due to death), and 42 in the control group (two additional deaths and two additional unable to locate). The difference in attrition between the experimental (N=3) and control (N=13) conditions was statistically significant (p=0.05).
Participants
Table 1 summarizes key characteristics of the participants in the study by group assignment. The majority were males (N=78, 71%) in their mid- to late-thirties. About half were married or living with a partner (N=56, 51%); and while most had at least some high school education (N=95, 86%), less than a quarter (N=26, 24%) had stable employment. Almost one-third were currently using heroin (N=35, 32%) and many (N=27, 25%) had not disclosed their HIV status within the family. A majority (N=74, 67%) scored 16 or greater on the CES-D, indicating the presence of significant depressive symptoms. At baseline, mean social support scores on the SSRS were 34 (intervention group) and 29 (control group) on a scale of 0–50.
Table 1.
Baseline Characteristics of 110 Participants
| Characteristic | Intervention | Control | p Value |
|---|---|---|---|
| Male gender | 36 (65%) | 42 (76%) | 0.21 |
| Age (mean) | 38 | 37 | 0.88 |
| Married or living with partner | 30 (55%) | 26 (47%) | 0.45 |
| Some secondary school or above | 50 (95%) | 45 (81%) | 0.17 |
| Stably employed | 14 (25%) | 12 (22%) | 0.65 |
| Current heroin use | 14 (25%) | 21 (38%) | 0.15 |
| Has not disclosed HIV status at home | 17 (31%) | 10 (18%) | 0.15 |
| CES-D (Chinese) score ≥16 | 32 (58%) | 42 (76%) | 0.10 |
| HIV Stigma Scale (mean score) | 102 | 109 | 0.08 |
| Social Support Rating Scale (mean score) | 34 | 29 | 0.001 |
| Mean CD4 count (cells/mm3) | 149 | 137 | 0.552a |
| CD4 count by category | |||
| ≤50 | 12 (40%) | 19 (35%) | 0.279 |
| 51–199 | 22 (40%) | 16 (29%) | |
| ≥200 | 21 (38%) | 20 (36%) | |
Data are not normally distributed, nonparametric test was performed.
Intervention dose
Subjects in the experimental group received a mean of 8.5 in-person visits from a nurse and peer educator over the 6-month course of the intervention. Slight less than half (47%) of the visits were conducted in the subject's home, with the remainder occurring in cafes or other public venues. Family members participated in 46% of the in-person visits. Subjects also received a mean of 2.0 intervention visits by telephone.
Outcomes
Adherence
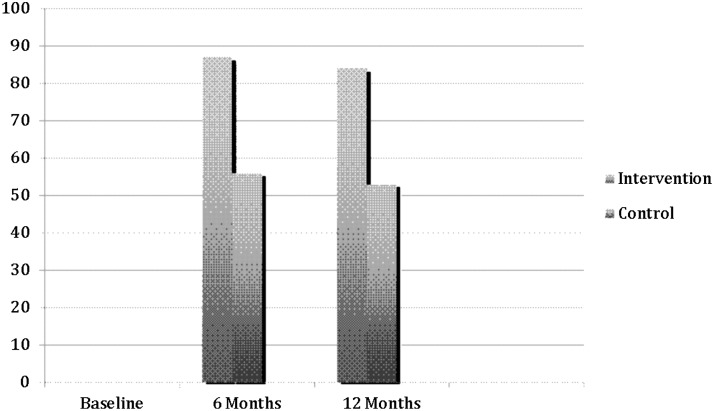
As seen in Fig. 2, at baseline all subjects reported taking 90% or less of prescribed medication, either pre-ARV (N=60) or ARV (N=50). Fifty-six percent of subjects in the control and 87% of subjects in the experimental group were adherent to ARV at 6 months. At 12 months, there was a slight reduction in the proportion of those who were adherent in both groups, with 53% of control subjects and 84% of experimental subjects adherent.
FIG. 2.
Proportion of subjects reporting >90% adherence by group.
In bivariate analyses, the difference in adherence between the two groups at 6 and 12 months was significant (p=0.003 and p=0.005). In multivariate analyses, controlling for baseline VAS adherence score, baseline stigma, baseline social support, and baseline CES-D (dichotomized), the experimental group had a significantly higher proportion of people who were adherent (p=0.009). The change in proportion of subjects who were adherent did not differ between the two groups over time (p=0.77). Baseline adherence level was not associated with greater than 90% adherence at 6 and 12 months (p=0.99).
Depressive symptoms
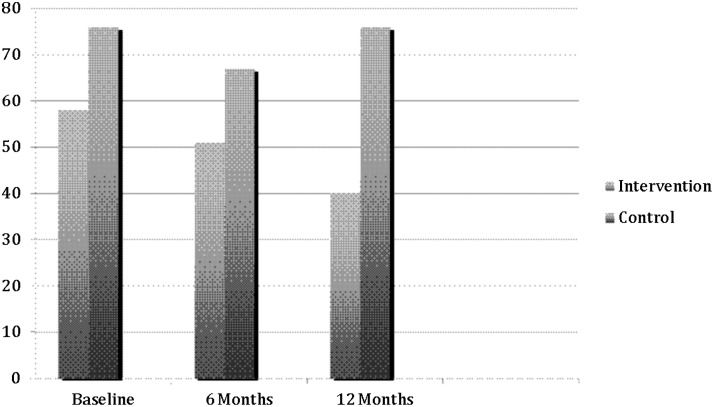
As seen in Fig. 3, at baseline 76% of control subjects and 58% of intervention subjects had a score on the CES-D of ≥16. Depressive symptoms decreased in both groups at 6 months, with 67% of control subjects and 51% of intervention subjects exhibiting a high number of depressive symptoms. However, at 12 months the proportion of control group subjects with a score ≥16 returned to 76%, while the proportion in the intervention group continued to decrease to a low of 40%.
FIG. 3.
Proportion of subjects reporting ≥16 CES-D by group.
In multivariate analyses, adjusting for baseline stigma, social support, and raw CES-D score, there was a significant difference in overall depression scores between the two groups (p=0.001), with the control group having a higher proportion of people with a CES-D score ≥16. Comparing baseline CES-D scores to 12-month CES-D scores, the intervention group showed a significant decrease in depressive symptoms compared to the control group (p=0.03). Comparing 6-month CES-D scores to 12-month CES-D scores, change in scores significantly differed between the two groups, with control subjects increasing in depressive symptoms and intervention subjects decreasing in depressive symptoms (p=0.05). Baseline raw CES-D and baseline stigma were significant predictors of CES-D scores (p<0.001 and p=0.003, respectively). Baseline social support did not predict CES-D scores.
Stratified analyses show that for those with a CES-D score ≥24 (severe depressive symptoms) at baseline, a higher proportion of intervention subjects were adherent compared to control subjects (90% vs. 33% at 6 months, 90% vs. 31% at 12 months). As well, among those with a CES-D <16 score (few depressive symptoms), a higher proportion of intervention subjects were adherent compared to control subjects (91% vs. 62% at 12 months).
Biological outcomes: HIV viral load
None of the subjects had an undetectable viral load at baseline. The proportion of those with an undetectable viral load increased in both groups at 6 months (24 or 44% in the control group and 31 or 57% in the intervention group), and at 12 months (31 or 59% in the control group and 39 or 72% in the intervention group). In multivariate analyses, controlling for baseline viral load copy number, baseline stigma, baseline social support, and baseline CES-D (dichotomized) there were no differences between groups (p=0.18).
We also examined whether viral load was associated with adherence. At baseline, none of the subjects had undetectable viral loads and all had adherence of 90% or less. At 6 months, 29% of those with adherence of 90% or less, and 59% of those with adherence greater than 90% had undetectable viral load. At 12 months, the proportions increased to 41% and 77%, respectively. In multivariate analyses controlling for baseline viral load, baseline stigma, baseline social support, baseline CES-D (dichotomized), and intervention group, there was a significant association between adherence and undetectable viral load (p=0.004). There was also a significant time effect, confirming that the proportion of subjects with an undetectable viral load increased over time (p=0.02). Baseline viral load copy number remained nonsignificant (p=0.28).
Biological outcomes: CD4 counts
CD4+ counts did not vary by group, but did increase over time in both groups. The largest increase was seen among those with baseline counts of 201 or greater cells/mm3. In multivariate analyses, controlling for baseline stigma, baseline social support, and baseline CES-D (dichotomized), CD4 did not differ by group (p=0.65). However, an overall significant increase in CD4+ count category was seen for all subjects between baseline and 12 months (p=0.003).
Discussion
Among this group of mostly men (71%) in their mid- to late-thirties, those who received 6 months of the Freirian intervention clearly improved their adherence during the course of the intervention and maintained that improvement over time. Adherence remained high in this group even 6 months after the intervention ceased. It seems likely that the opportunity to better understand the course of the disease and the treatment intervention contributed to the sustained improvement in treatment adherence.
However, it is not clear to what extent future access to adherence support will be important if treatment adherence is to be maintained throughout each person's lifetime. Given that this group was composed of both new antiretroviral patients as well as long-term patients, all of whom were having trouble adhering and all of whom responded equivalently, it is possible that intermittent access or access upon demand will be crucial to maintaining adherence at least among some patients.
The intervention was not associated with a significant difference in biological outcomes, although this is not surprising given the small sample size and relatively short follow up. Over time, improved adherence is likely to be associated with better biological outcomes. Of note, the difference in viral load outcomes, while not statistically significant, did trend in the correct direction. This finding is consistent with previous work suggesting that even brief self-report measures can be robust.24
There was also an increase in the relationship between adherence and CD4+ outcome over time. The lack of an association of improvement in CD4+ outcome with intervention group is most likely due to the large proportion of adherent subjects in both study groups and the increased time required to demonstrate improvement in this area. It is also likely that because treatment began relatively late in the course of illness, some subjects will not show immunological improvement regardless of improved virological status. This situation is likely to change as China moves toward earlier initiation of ARV therapy.
Also of great interest was the high prevalence (67%) of significant depressive symptoms at baseline. A complete understanding of the complexities of depression among this group of patients awaits more sophisticated tools, as the CES-D used in this study does not differentiate fully between depressive symptoms in response to changeable external symptoms, such as HIV infection, and a true depressive illness that requires specific intervention.
Patients living with HIV are likely more vulnerable to depressive symptoms associated with their disease status, as well as to depression itself in association with their underlying risk factor for disease. Differentiating between these two states could be an important treatment strategy, as those with depressive symptoms associated with HIV disease status may well respond to effective HIV disease management, as did the majority of those in the treatment group reported here, while those with underlying true depression may require depression therapy. As noted in an earlier study, HIV clinics can routinely assess clients for depressive symptoms, but should also consider more significant psychiatric diagnoses, particularly among those patients who do not improve in the face of improving HIV status.25
This study confirms that a simplified adherence intervention, using a Freirian philosophy and aimed at improving antiretroviral adherence, can effectively improve adherence among a range of Chinese patients living with HIV in remote as well as in highly sophisticated areas. Dialogic teaching that takes place in the home or nonclinical setting clearly facilitated communication and participant-centered teaching. This helped participants identify their own barriers and resources regarding HIV management. In the Chinese context, family participation was also extremely beneficial in creating an environment that would be critical for optimal adherence to treatment.
That said, home visits or off-site clinical visits were very time consuming and difficult to arrange. It will be valuable in the future to study the extent to which home visits of the research team can be reduced further, especially for those living significant distances from the clinic site. However, it does seem likely that at least a few initial visits by the team will be important to establishing long-term trust between the patients, their families, and the clinical team.
The study also confirms the importance of depression as an indicator of concomitant mental health. The results of this study suggest that future interventions address both adherence and depression. It is of particular importance that future studies look at the types of depression likely to be seen in these patients and differentiate early between those likely to benefit from HIV-related support and those that will require additional, depression targeted interventions.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ministry of Health People's Republic China. 2012China AIDS Response Progress Report 2012. Beijing, China [Google Scholar]
- 2.Wang H, Zhou J, He G, et al. . Consistent ART adherence is associated with improved quality of life, CD4 counts, and reduced hospital costs in central China. AIDS Res Hum Retroviruses 2009;25:757–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang H, He G, Li X, et al. . Self-reported adherence to antiretroviral treatment among HIV-infected people in central China. AIDS Patient Care STDs 2008;22:71–80 [DOI] [PubMed] [Google Scholar]
- 4.Wang X, Wu Z. Factors associated with adherence to antiretroviral therapy among HIV/AIDS patients in rural China. AIDS 2007;21:S149–S155 [DOI] [PubMed] [Google Scholar]
- 5.Simoni J, Chen W, Huh D, et al. . A preliminary randomized controlled trial of a nurse-delivered medication adherence intervention among HIV positive outpatients initiating antiretroviral therapy in Beijing, China. AIDS Behav 2011;15:919–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang L, Li L, Zhang Y, et al. . Self-efficacy, medication adherence, and quality of life among people living with HIV in Hunan Province of China. JANAC 2013;24:145–153 [DOI] [PubMed] [Google Scholar]
- 7.Xing H, Ruan Y, Li J, et al. . HIV drug resistance and its impact on antiretroviral therapy in Chinese HIV-infect patients. PLoS One 2013;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabin L, Desilva M, Hamer D, et al. . Barriers to adherence to antiretroviral medications among patients living with HIV in southern China. AIDS Care 2008;20:1242–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fredriksen-Goldsen K, Shui C, Starks H, et al. . “You must take the medications for you and for me” Family caregivers promoting HIV medication adherence in China. AIDS Patient Care STDs 2011;25:737–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tedaldi E, Berg-Wolf M, Richardson J, et al. . Sadness in the SUN: Using computerized screening to analyze correlates of depression and adherence in HIV-infected adults in the United States. AIDS Patient Care STDs 2012;26:718. [DOI] [PubMed] [Google Scholar]
- 11.Jin H, Atkinson J, Yu X, et al. . Depression and suicidality in HIV/AIDS in China. J Affect Disord 2006;94:269–275 [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez J, Batchelder A, Psaros C, et al. . Depression and HIV/AIDS treatment nonadherence: A review and meta-analysis. J Acquir Immune Defic Syndr 2011;58:181–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren Y, Yang S. Epidemiology of depression in HIV/AIDS patients. J Guangdong Pharmaceutical College 2009;25:219–221 [Google Scholar]
- 14.Freire P. Pedagogy of the Oppressed. Continuum. New York City, 1986 [Google Scholar]
- 15.Walsh J, Mandalia S, Gazzard B. Responses to a 1 month self-report on adherence to antiretroviral therapy are consistent with electronic data and virological treatment outcomes. AIDS 2002;16:269–277 [DOI] [PubMed] [Google Scholar]
- 16.Oyugi J, Byakika-Tusiime J, Charlebois E, et al. . Multiple validated measures of adherence indicate high level of adherence to generic HIV antiretroviral therapy in a resource-limited setting. JAIDS 2004;36:1100:02 [DOI] [PubMed] [Google Scholar]
- 17.Giordano T, Guzman D, Clark R, et al. . Measuring adherence to antiretroviral therapy in a diverse population using a visual analogue scale. HIV Clin Trials 2004;5:74–79 [DOI] [PubMed] [Google Scholar]
- 18.Amico K, Fisher W, Corman D, et al. . Visual analogue scale of ART adherence: Association with 3-day self-report and adherence barriers. JAIDS 2006;42:455–459 [DOI] [PubMed] [Google Scholar]
- 19.Zhang J. Measuring Chinese psychological well-being with Western developed instruments. J Pers Assess 2002;79:492–511 [DOI] [PubMed] [Google Scholar]
- 20.Stall D, Sum C, Lum S, et al. . Screening for depressive symptoms: Validation of the CES-D scale in a multi-ethnic group of patients with diabetes. Diabetes Care 2008;31:1119. [DOI] [PubMed] [Google Scholar]
- 21.Okoror T, Falade C, Olorunlana A, et al. Exploring the cultural context of HIV stigma on antiretroviral therapy adherence among people living with HIV/AIDS in southwest Nigeria. AIDS Patient Care STDs 2013;27:55. [DOI] [PubMed] [Google Scholar]
- 22.Li X, He G, Wang H, et al. . Development and evaluation of HIV/AIDS related stigma and discrimination scale. Chin J Nursing 2010;45:496–499 [Google Scholar]
- 23.Bangsberg D. Less than 95% adherence to can lead to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. CID 2006;43:939. [DOI] [PubMed] [Google Scholar]
- 24.Simoni J, Kurth A, Pearson C, et al. . Self-report measures of antiretroviral therapy adherence: A review with recommendations for HIV research and clinical management. AIDS Behav 2006;10:227–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schumacher J, McCullumsmith C, Mugavero M, et al. Routine depression screening in an HIV clinic cohort. AIDS Behav 2013;17:2781–2791 [DOI] [PMC free article] [PubMed] [Google Scholar]