Abstract
In the present study, we have extensively continued our previous investigations of the nonsynonymous single-nucleotide polymorphisms (SNPs) in the human DNase I (DNASE1) gene potentially relevant to systemic lupus erythematosus (SLE); therefore, all of the 58 nonsynonymous SNPs registered in the NCBI dbSNP database could be evaluated and it could be checked as to whether these SNPs might serve as a functional SNP. From a compiled expression analysis of the amino-acid-substituted DNase I corresponding to each of the SNPs, it was possible to sort them into 23 SNPs while not affecting the activity: 12 abolishing it, 14 reducing it, and 9 increasing it. Among a total of 58 nonsynonymous SNPs, only 4 SNPs exhibited genetic polymorphisms in some of the populations examined; a minor allele producing a loss-of-function variant of each SNP was not distributed in 14 different populations derived from three ethnic groups. It could be assumed that a minor allele of these functional SNPs, despite their remarkably low genetic heterogeneity, could directly serve as a genetic risk factor for SLE. Furthermore, among the human DNase family genes, it seems that DNASE1 is able to tolerate the generation of nonsynonymous SNPs, and that the amino-acid substitutions resulting from the SNPs in DNASE1 easily alter the activity.
Introduction
Deoxyribonuclease I (DNase I, EC3.1.21.1) has been highlighted for its possible involvement in the pathogenesis of systemic lupus erythematosus (SLE) (Tsukumo and Yasutomo, 2004; Valle et al., 2008; Hedberg et al., 2011). It has been reported that DNase I-deficient mice develop an SLE-like syndrome (Napirei et al., 2000). In addition, a novel nonsense mutation (Yasutomo et al., 2001) and several missense (Dittmar et al., 2009) mutations resulting in the abolishment/reduction of the activity have been identified in patients with autoimmune diseases. These findings suggest that a single-nucleotide polymorphism (SNP) in DNASE1, producing forms with loss of function or markedly reduced activity, might be substantially responsible for the genetic background determining susceptibility to autoimmune diseases through failure in the breakdown of chromatin during apoptosis and/or necrosis (Counis and Trriglia, 2000; Mizuta et al., 2006; Napirei et al., 2006). Indeed, a previous finding (Shin et al., 2004) has indicated that the frequency of the homozygote for the G-allele in SNP p.Arg244Gln of DNASE1 in SLE patients is much higher in patients who have the corresponding autoantibodies than in patients who do not have them, for which production of the low activity-harboring DNase I isoform by the G-allele could partly account (Yasuda et al., 2010). Furthermore, the association of a nonsynonymous SNP in DNASE1 with SLE susceptibility has been demonstrated (Bodańo et al., 2006). Therefore, in order to clarify the genetic basis of the etiological role of DNase I in autoimmune diseases, we have continued our genetic and expression analysis of SNPs in the gene.
Many SNPs in DNASE1 have been screened, and they are now available on the NCBI dbSNP database (www.ncbi.nlm.nih.gov/projects/SNP, June 2013). We have been focusing on nonsynonymous SNPs potentially affecting the activity of DNase I in vivo through the corresponding amino-acid substitution as a functional SNP (Wu and Zeng, 2012), and, in fact, have previously performed genetic and expression analysis of such SNPs in DNASE1 (Yasuda et al., 2010; Fujihara et al., 2011; Ueki et al., 2014b). Since then, 14 nonsynonymous SNPs in DNASE1, which could potentially affect the activity, have been registered in the database. However, only limited population data are available for these SNPs. Especially, it is still unclear whether each SNP in DNASE1 may serve as a functional SNP affecting its in vivo activity. Therefore, comprehensive data on the biochemical-genetic aspects of these SNPs in DNASE1 potentially affecting the in vivo activity would likely be useful for clarifying their functionality in determining the genetic predisposition to SLE.
In the present study, we have extensively continued our previous studies (Yasuda et al., 2010; Ueki et al., 2014b) that are aimed at genetic and functional characterization of all the nonsynonymous SNPs in DNASE1. We investigated the genotype distribution of 14 nonsynonymous SNPs in DNASE1 in 14 different populations worldwide derived from three ethnic groups, using a novel genotyping method for each SNP, and the effects of all these SNPs on the enzymatic activity, in order to evaluate the functionality of each SNP. Furthermore, a comparison of DNASE1 with other members of the DNase family in terms of nonsynonymous SNPs was performed.
Materials and Methods
Samples from subjects
Genomic DNA was extracted using a QIAamp DNA Mini Kit (Qiagen, Chatsworth, CA) from blood or bloodstain samples randomly collected from healthy subjects (n=1752) derived from 14 different populations. The Asian populations included 110 Japanese (Shimane prefectures), 352 Koreans (Busan, South Korea), 193 South Chinese (Shenyang and Guangzhou of China), 112 Mongolians (Ulaanbaatar, Mongol), 153 Tibetans (Katmandu of Neparu), 35 Sri Lanka Tamils (Kandy of Sri Lanka), 48 Sri Lanka Sinhalese (Kandy of Sri Lanka), and 40 Tamangs (Kotyang of Nepal); the Caucasian population included 136 Turks (Adana area, Southern Turkey), 68 Germans (Munich), and 199 Mexicans (60 Mestizo, 88 Nahuas and 51 Huicholes); and the African populations included 126 Ovambos (Bantusin, Namibia), 105 Ghanaians (Ghana), and 75 Xhosas (Cape Town, South Africa). All of these populations were uniform in terms of ethnic variation. The samples were the same as those used for previous studies (Ueki et al., 2014b). Written informed consent was obtained from each participant. The study was approved by the Human Ethics Committees of the institute (the approval number 1024 for the Human Genome and Genetics Analysis Study).
Construction of expression vectors encoding human DNase I, and its amino-acid-substituted forms corresponding to each SNP
Expression pcDNA3.1 (+) vectors (Invitrogen, San Diego, CA) inserted with the entire coding sequence of human DNase I cDNA was separately prepared (Yasuda et al., 2010), and it was used as a wild-type construct; inserted cDNA was derived from the predominant haplotype in Japanese. The 58-amino-acid-substituted constructs, corresponding to each nonsynonymous SNP in DNASE1, including those previously prepared (Yasuda et al., 2010; Ueki et al., 2014b), were constructed using the KOD-Plus Mutagenesis kit (Toyobo Co., Ltd., Osaka, Japan) with the wild-type construct as a template. In these constructs, the amino-acid residue was replaced by the counterpart derived from a minor allele at each substitution site; for example, the A81T construct of DNase I, in which the Ala residue at position 81 in the protein is replaced by Thr derived from the minor allele, corresponds to SNP p.Ala81Thr. Furthermore, several substituted DNase I for SNP-related amino-acid residues were prepared in the same manner as described earlier. Nucleotide sequences of all the constructs were confirmed by DNA sequence analysis. Purification of two different clones derived from each construct used for transfection was performed using the Plasmid Midi kit (Qiagen).
Transient expression of the expression vectors and assay for DNase I activity
COS-7 cells were maintained in Dulbecco's modified Eagle medium containing 1 mM l-glutamine, 50 U/mL penicillin, 50 μg/mL, and 10% (v/v) fetal calf serum at 37°C under 5% CO2 in air. The cells were transiently transfected four times with 2 μg of each DNase I-related expression vector using Lipofectamine 2000 reagent (Invitrogen) according to the method previously described (Yasuda et al., 2010). At 48 h after transfection, the cells were harvested. Then, the cells were subjected to sonication using Bioruptor UCD-250 (Cosmo Bio Co., Ltd., Tokyo, Japan) to prepare lysates for the subsequent assay. Transfection efficiencies were estimated by co-transfecting with 600 ng of pSV-β-galactosidase vector (Promega, Madison, WI) and by subsequently assaying aliquots of cell lysates for β-d-galactosidase activity. The DNase I activity in both the lysates derived from the transfected cells and their conditioned medium was assayed by the single radial enzyme diffusion (SRED) method using a LAS-3000 imaging analyzer (Fuji Film, Tokyo, Japan) according to our previous report (Nadano et al., 1993; Yasuda et al., 2010). The activity of wild-type DNase I was defined as 1.0, and that of the amino-acid-substituted form was expressed relative to the wild type. The relative activity is expressed as mean±standard deviation (SD) derived from four transfections using two different clones derived from each construct. In order to investigate the thermal stability of each substituted DNase I, each cell medium transfected with the corresponding expression vector was incubated at 50°C for 20 min, and the remaining DNase I activity was determined by the SRED method. The remaining activity of wild-type DNase I was defined as 1.0.
Genotyping of nonsynonymous SNPs in DNASE1 by the polymerase chain reaction–restriction fragment length polymorphism method
Genotyping assays for each of 14 nonsynonymous SNPs in DNASE1 were separately performed using polymerase chain reaction (PCR) followed by restriction fragment length polymorphism (RFLP) analysis (Yasuda et al., 1995b), in the same manner as the other SNPs (Yasuda et al., 2010; Ueki et al., 2014b). Primers for the specific amplification of the DNA fragments encompassing a substitution site corresponding to each SNP were designed on the basis of the nucleotide sequences of the human DNASE1 (GenBank: accession no. D83195) (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/dna). Nucleotide and amino-acid residues of the enzyme were numbered from the 5′-terminus of the translation initiation codon and the N-terminal amino-acid residue of the precursor protein, respectively. SNP nomenclature is based on the recommendations for description of sequence variants (www.hgvs.org/mutnomen/examplesDNA.htlm); the sequence of DNase I (GenBank: accession no.AB188151) has been used as the coding DNA Reference Sequence.
PCR amplification was performed in a 25 μL reaction mixture using ∼5 ng of DNA. The reaction mixture contained a 1× buffer (15 mM Tris-HCl, pH 8.0, 50 mM KCl), 1.5 mM MgCl2, 0.5 μM of each primer, 200 μM dNTPs, and 1.25 U of Taq polymerase (AmpliTaq Gold; Applied Biosystems, Foster City, CA). PCR was performed with a protocol consisting of initial denaturation at 94°C for 7 min, followed by 30 cycles with denaturation at 94°C for 30 s, annealing at 55–65°C for 30 s, and extension at 72°C for 1 min, followed by a final extension at 72°C for 7 min. Two microliters of the PCR product obtained using each pair of primers was digested with 5 U of each enzyme listed in Supplementary Table S1 (New England Biolabs, Ipswich, MA) at 37°C for 3 h in a final reaction mixture volume of 15 μL to determine the genotype of each SNP. The digested products (5 μL) were separated in an 8% polyacrylamide gel, and the patterns on the gels were visualized by silver or ethidium bromide staining, as previously described (Yasuda et al., 2010).
Analytical methods
Multiple alignment analyses of the amino-acid sequences of animal DNase I were performed using DNASIS Pro V3.0 (Hitachi Solutions, Ltd., Tokyo, Japan); animal DNase I was surveyed using the Kyoto Encyclopedia of Genes and Genomes database (www.genome.jp/kegg), in which the amino-acid sequences of human DNase I were used as query sequences for Basic Local Alignment Search Tool (BLAST) searches of each genome database.
DNase I activity was compared between wild-type and substituted enzymes by means of the unpaired, Student's t-test. Differences at p<0.05 were considered statistically significant.
Results
Effect of amino-acid substitution resulting from nonsynonymous SNPs in DNase1 on expression of the enzyme activity
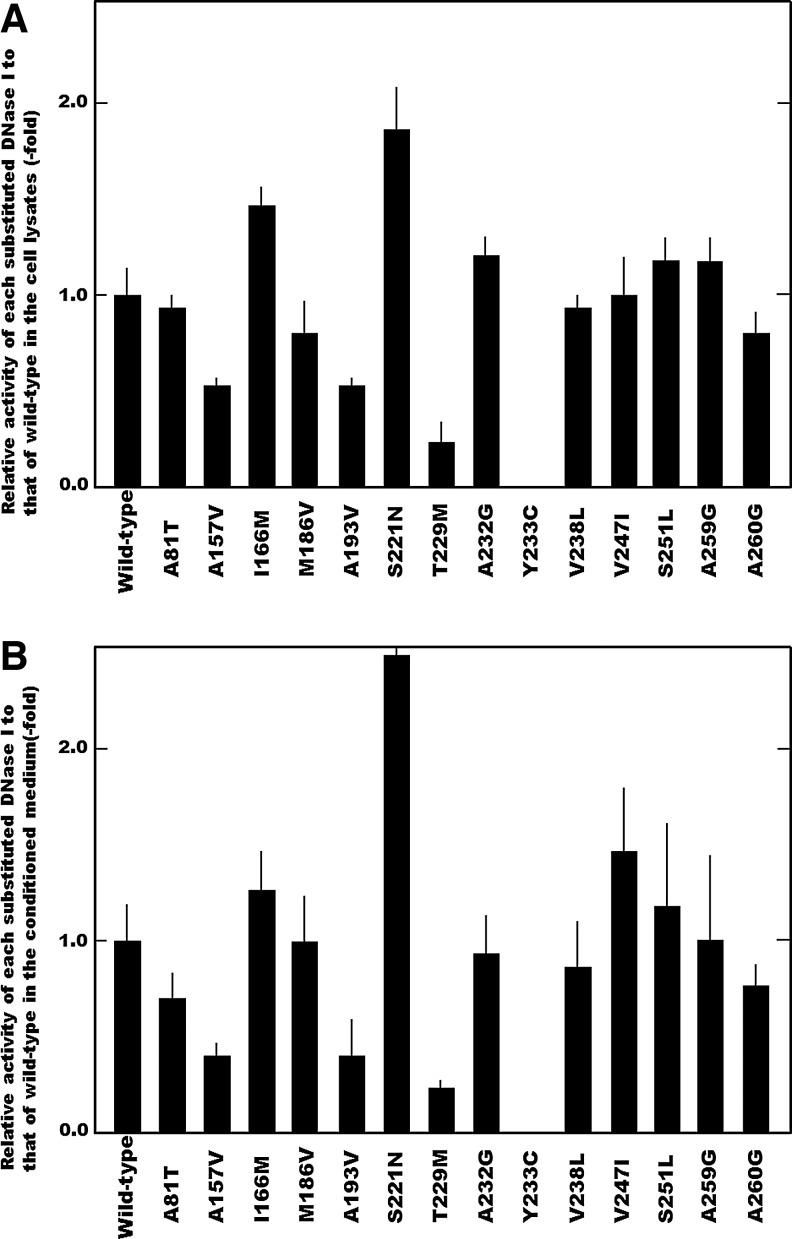
Fifty-eight amino-acid-substituted constructs in the DNase I protein derived from the minor allele in each SNP, along with those characterized in previous studies (Yasuda et al., 2010; Ueki et al., 2014b), were prepared separately, transiently expressed in COS-7 cells, and the resulting DNase I activities in both the lysates derived from the transfected cells and their conditioned medium were determined separately by the SRED method (Fig. 1). Generally, alterations in the activity induced by amino-acid substitution corresponding to each SNP in the lysates derived from the transfected cells were similar to those in the conditioned medium.
FIG. 1.
Effect of the amino-acid substitution derived from each nonsynonymous SNPs examined in this study on the DNase I activity. The DNase I activities in both (A) the lysates from cells transfected with each construct and (B) their conditioned medium were assayed by the SRED method (Nadano et al., 1993). Relative activities of amino-acid-substituted constructs corresponding to nonsynonymous SNPs to that of the wild-type DNase I were presented. The bar presents SD (n=4). SD, standard deviation; SNPs, single-nucleotide polymorphisms; SRED, single radial enzyme diffusion.
The levels of DNase I activity derived from the A157V, A193V, and T229M constructs were significantly lower than those of the wild-type enzyme. Obviously, substitution of the Ala residues at positions 157 and 193 in the DNase I protein reduced the activity to about 60% of that of the wild-type enzyme, and furthermore, substitution of the Thr residue at position 229 reduced the activity greatly to about 20%. It should be noted that the Y233C construct exhibited no DNase I activity under our assay conditions. Therefore, DNase I activity was found to be abolished by amino-acid substitutions resulting from SNP p.Tyr233Cys. On the other hand, when compared with the wild type, the activity levels of I166M and S221N constructs were significantly high; substitution of the Ile and Ser residues at positions 166 and 221, respectively, in the protein increased the DNase I activity about 1.5 fold. However, the levels of DNase I activity derived from the other amino-acid-substituted constructs were virtually the same as those of the wild type, indicating that substitution of the Ala, Met, Ala, Val, Val, Ser, Ala, and Ala residues at positions 81, 186, 232, 238, 247, 251, 259, and 260, respectively, in the DNase I protein exerted little effect on the activity. It was demonstrated that, among the amino-acid residues related to nonsynonymous SNPs examined in this study, each of the amino-acid residues at positions 157, 193, 229, and 233 are involved in expression of the activity, and that especially the residue at position 233 is indispensable.
Based on a compiled expression analysis of the amino-acid-substituted DNase I corresponding to each of the nonsynonymous SNPs in DNASE1, we were able to classify these SNPs into four classes according to the alterations in the enzyme activity levels brought about by the corresponding amino-acid substitution: those not affecting the activity, those elevating it, those abolishing it, and those reducing it. The 58 nonsynonymous SNPs in DNASE1 were sorted into 23 that did not affect the activity, 12 that abolished it, 14 that reduced it, and 9 that increased it (Table 1).
Table 1.
Summary on Evaluation of All the Nonsynonymous SNPs in DNASE1 as a Functional SNP; Genetic Distribution, Effect of the Corresponding Amino-Acid Substitution on the DNase I Activity, and Amino-Acid Multiple Alignment of Animal DNases I
| SNP | Exona | Genetic polymorphismb | MAFc | Heterozygosityc | Activityd | Amino-acid multiple alignment of 40 animal DNases Ie |
|---|---|---|---|---|---|---|
| SNPs not affecting the activity | ||||||
| rs8176927 p.Arg2Ser; c.6G>T |
2 | Only African | 0.0242 | 0.047 | 1.17±0.17 | In the signal sequence |
| rs61741279 p.Gly3Asp; c.8G>A |
2 | Mono-allelic | <0.0003 | 0.000 | 1.17±0.17 | In the signal sequence |
| rs145239050 p.Lys5Arg; c.14A>G |
2 | Mono-allelic | <0.0003 | 0.000 | 1.63±0.50 | In the signal sequence |
| rs148015097 p.Ala14Val; c.41C>T |
2 | Mono-allelic | <0.0003 | 0.000 | 1.10±0.35 | In the signal sequence |
| rs141673463 p.Ala26Thr; c.76G>A |
2 | Mono-allelic | <0.0003 | 0.000 | 1.14±0.50 | Not conserved; substituted by p.Ala26Thr; Gly (11), Cys, or Ser (4) |
| rs34907394 p.Glu35Asp; c.105G>C |
2 | Mono-allelic | <0.0003 | 0.000 | 1.23±0.28 | Not conserved; substituted by Asp (14), Met (2), Thr (2), Ala, Asn, Gln, Arg, or Val |
| rs140530129 p.Ala81Thr; c.241G>A |
2 | Mono-allelic | <0.0003 | 0.000 | 0.95±0.074 | Not conserved; substituted by Gly (3), Lys, Ile, Asp (6), Thr (5), Ser (18), or His |
| rs190768401 p.Arg95Gln; c.284G>A |
4 | Mono-allelic | <0.0003 | 0.000 | 1.14±0.39 | Well conserved in 35 species; substituted by His (3), Thr, or Ala |
| Not registrated p.Val114Met; c.340G>A |
5 | Mono-allelic | <0.0003 | 0.000 | 1.10±0.35 | Not conserved; substituted by Leu (15), Arg, Val (9), Thr (5), Ser, Ala (2), Met, or Gln |
| rs34923865 p.Tyr117Ser; c.590A>C |
5 | Only Caucasian | 0.0026 | 0.006 | 1.29±0.37 | Well conserved in 35 species; substituted by Phe (4) or Glu |
| rs144059899 p.Asp129Asn; c.385G>A |
5 | Mono-allelic | <0.0003 | 0.000 | 1.92±0.81 | Well conserved except reptiles (Gly or Thr) |
| rs76397583 p.Asn132Ser; c.395A>G |
5 | Mono-allelic | <0.0003 | 0.000 | 1.45±0.66 | Not conserved; substituted by Ser (20), Ile (3), Pro, Met (2), or Glu (4) |
| rs140745748 p.Val172Ile; c.514G>A |
6 | Mono-allelic | <0.0003 | 0.000 | 1.21±0.71 | Well conserved in 39 species; substituted by Ala |
| rs74892550 p.Val185Ile; c.544G>A |
7 | Mono-allelic | <0.0003 | 0.000 | 1.49±0.36 | Not conserved; substituted by Ile (23), Met (3), or Ala (4) |
| rs147093089 p.Met186Val; c.556A>G |
7 | Mono-allelic | <0.0003 | 0.000 | 0.78±0.41 | Not conserved; substituted by Val (3), Leu (11), Phe (3), or Ile (4) |
| rs34186031 p.Pro219Ser; c.655C>T |
7 | Mono-allelic | <0.0003 | 0.000 | 1.13±0.29 | Not conserved; substituted by Ser (3), Gly (7), or Thr (3) |
| rs146249371 p.Ala232Gly; c.695C>G |
7 | Mono-allelic | <0.0003 | 0.000 | 1.29±0.12 | Not conserved; substituted by Pro (8) |
| rs200149984 p.Val238Leu; c.712G>C |
8 | Mono-allelic | <0.0003 | 0.000 | 0.95±0.37 | Not conserved; substituted by Ala (15) or Leu |
| rs148684969 p.Val247Iler; c.739G>A |
8 | Mono-allelic | <0.0003 | 0.000 | 1.05±0.077 | Not conserved; substituted by Ile (8), Thr, Leu, Tyr, or Glu |
| rs200538894 p.Ser251Leu; c.752C>T |
8 | Mono-allelic | <0.0003 | 0.000 | 1.29±0.30 | Not conserved; substituted by Thr (6) or Met |
| rs142079857 p.Ala259Gly; c.777C>G |
8 | Mono-allelic | <0.0003 | 0.000 | 1.39±0.19 | Not conserved; substituted by Val (3), Thr (3), Lys (5), Glu (6), Gln (2), Ser (2), Arg, or Leu |
| rs201413861 p.Ala260Gly; c.779C>G |
8 | Mono-allelic | <0.0003 | 0.000 | 0.72±0.10 | Not conserved; substituted by Glu (4), Thr (5), Asp (3), Asn, Val (2), Ser, or Lys |
| rs8176924 p.Gly262Asp; c.785G>A |
8 | Mono-allelic | <0.0003 | 0.000 | 1.16±0.54 | Not conserved; substituted by Arg (2), Asn (7), Lys (5), His (4), or Asp (2) |
| SNPs abolishing the activity | ||||||
| rs121912990 p.Lys5Ter; c.13A>T |
2 | Mono-allelic | <0.0003 | 0.000 | n.d. | Nonsense substitution in the signal sequence |
| rs142318540 p.Gln60Arg; c.179A>G |
3 | Mono-allelic | <0.0003 | 0.000 | n.d. | Well conserved in 38species; substituted by Asp or Glu |
| rs8176928 p.Arg107Gly; c.319A>G |
4 | Mono-allelic | <0.0003 | 0.000 | n.d. | Well conserved in 38 species; substituted by Ile or Lys |
| rs150621329 p.Arg133Gln; c.398G>A |
5 | Mono-allelic | <0.0003 | 0.000 | n.d. | Conserved in all the species |
| rs150621329 p.Arg133Leu; c.398G>T |
5 | Mono-allelic | <0.0003 | 0.000 | n.d. | Conserved in all the species |
| rs149379211 p.Phe140Cys; c.419T>G |
5 | Mono-allelic | <0.0003 | 0.000 | n.d. | Conserved in all the species |
| rs142644209 p.Asp190His; c.568G>C |
7 | Mono-allelic | <0.0003 | 0.000 | n.d. | Conserved in all the species |
| rs146236198 p.Asn192Ile; c.575A>T |
7 | Mono-allelic | <0.0003 | 0.000 | n.d. | Conserved in all the species |
| rs8176940 p.Cys231Tyr; c.692G>A |
7 | Mono-allelic | <0.0003 | 0.000 | n.d. | Conserved in all the species |
| rs199826318 p.Tyr233Cys; c.698G>A |
7 | Mono-allelic | <0.0003 | 0.000 | n.d. | Conserved in all the species |
| rs139254891 p.Arg235Trp; c.703A>T |
7 | Mono-allelic | <0.0003 | 0.000 | n.d. | Conserved in all the species |
| rs201571412 p.Arg244Ter; c.730C>T |
8 | Mono-alleleic | <0.0003 | 0.000 | n.d. | Nonsense substitution |
| SNPs reducing the activity | ||||||
| rs77254040 p.Gln31Glu; c.91C>G |
2 | Mono-allelic | <0.0003 | 0.000 | 0.43±0.10 (p<0.001) | Not conserved; substituted by Arg (20), Lys (5), Glu (2), Ala, Asp, or Phe |
| rs143865851 p.Arg53Cys; c.157C>T |
3 | Mono-allelic | <0.0003 | 0.000 | 0.20±0.050 (p<0.001) | Well conserved in the 32species; substituted by Gln (2), Glu (2), Thr, Ala, Gly, or Met |
| rs144227093 p.Tyr54Cys; c.161A>T |
3 | Mono-allelic | <0.0003 | 0.000 | 0.39±0.10 (p<0.001) | Conserved in 39 species; substituted by His |
| rs45545238 p.Gln60His; c.180G>C |
3 | Mono-allelic | <0.0003 | 0.000 | 0.41±0.081 (p<0.001) | Well conserved in 38species; substituted by Asp or Glu |
| rs143058517 p.Val111Met; c.331G>A |
5 | Mono-allelic | <0.0003 | 0.000 | 0.24±0.019 (p<0.001) | Well conserved in all the species above reptile; substituted by Ala, Leu (4), Met, or Phe |
| rs138676148 p.Arg139Gly; c.415A>G |
5 | Mono-allelic | <0.0003 | 0.000 | 0.31±0.040 (p<0.001) | Not conserved; substituted by Lys (17), Trp, Met (7), or His |
| rs143407371 p.Ala157Val; c.470C>T |
6 | Mono-allelic | <0.0003 | 0.000 | 0.65±0.038 (p<0.05) | Not conserved; substituted by Ser (5) or Thr (14) |
| rs146238243 p.Pro159Leu; c.476C>T |
6 | Mono-allelic | <0.0003 | 0.000 | 0.27±0.10 (p<0.001) | Conserved in all the species |
| rs139424576 p.Asp167His; c.499G>C |
6 | Mono-allelic | <0.0003 | 0.000 | 0.52±0.073 (p<0.001) | Not conserved; substituted by Asn (7), Ser (2), or Gln |
| rs143371936 p.Ala193Val; c.578C>T |
7 | Mono-allelic | <0.0003 | 0.000 | 0.63±0.078 (p<0.05) | Well conserved in 39 species; substituted by Thr |
| rs148373909 p.Arg207Cys; c.619C>T |
7 | Mono-allelic | <0.0003 | 0.000 | 0.12±0.022 (p<0.001) | Well conserved in the higher species above chondrichthyes; substituted by Ser (2) or Glu |
| rs200620452 p.Thr229Met; c.686C>T |
7 | Mono-allelic | <0.0003 | 0.000 | 0.21±0.037 (p<0.001) | Not conserved; substituted by His, Ser, Asn (4), Ala, or Gly |
| rs1053874 p.Gln244Arg; c.731A>G |
8 | All populations | 0.4355 | 0.492 | 0.48±0.015 (p<0.001) | Not conserved; substituted by Arg (7), Leu (2), Met (5), Lys (4), or Thr |
| rs8176939 p.Ala246Pro; c.736G>C |
8 | Mono-allelic | <0.0003 | 0.000 | 0.16±0.060 (p<0.001) | Not conserved; substituted by Ser (7), Gly (8), Ile, or Asp |
| SNPs elevating the activity | ||||||
| rs147546841 p.Tyr46His; c.136T>C |
2 | Mono-allelic | <0.0003 | 0.000 | 1.85±0.63 (p<0.05) | Not conserved; substituted by Val, Phe (3), Ile (10), Ser, Leu (3), Arg (2), Met, or Thr (2) |
| rs143363152 p.Val70Leu; c.208G>T |
3 | Mono-allelic | <0.0003 | 0.000 | 1.57±0.22 (p<0.005) | Well conserved in the higher species above reptiles; substituted by Ile (7), Thr (6), Leu, or Met |
| rs141801594 p.Asp120Asn; c.358G>A |
5 | Mono-allelic | <0.0003 | 0.000 | 1.87±0.40 (p<0.05) | Well conserved in the 34 species; substituted by Asn, Lys, Ser (2), or Pro (2) |
| rs8176919 p.Gly127Arg; c.379G>A |
5 | Only African | 0.0094 | 0.018 | 2.11±0.52 (p<0.01) | Conserved except reptile (Ser) |
| rs139615062 p.Arg143Gln; c.428G>A |
5 | Mono-allelic | <0.0003 | 0.000 | 1.76±0.43 (p<0.05) | Not conserved; substituted by Pro (21), His, Leu (4), Lys (3), Asn, Ser (4), or Ala |
| rs1799891 p.Pro154Ala; c.460C>G |
6 | Mono-allelic | <0.0003 | 0.000 | 1.43±0.25 (p<0.05) | Well conserved in the 31 species; substituted by Ala (3), Ser (4), Val, or Gly |
| rs201942334 p.Ile166Met; c.498C>G |
6 | Mono-allelic | <0.0003 | 0.000 | 1.45±0.10 (p<0.05) | Not conserved; substituted by Met, Val (5), or Leu (2) |
| rs150933932 p.Ala168Val; c.503C>T |
6 | Mono-allelic | <0.0003 | 0.000 | 1.64±0.31 (p<0.05) | Well conserved in the 29 species; substituted by Ser (5), Gly, Ile, or Asn |
| rs138354028 p.Ser221Asn; c.662G>A |
7 | Mono-allelic | <0.0003 | 0.000 | 1.86±0.23 (p<0.01) | Not conserved; substituted by Thr (12), Asp (5), Lys, Asn (2), Ala, His, or Cys |
Exon, in which each SNP is located, is shown according to the numbering in Figure 3.
Populations exhibiting a genetic heterogeneity for the corresponding SNP among 16 different populations examined in this study are shown.
The MAF and heterozygosity of each SNP were calculated based on the total subjects (n=1752) examined in this study.
The values are expressed as relative activity of each amino-acid-substituted construct to that of the wild type, representing the mean±standard deviation (n=4). p-Values in parenthesis were calculated as differences between the activities of the substituted and wild type enzyme by means of the unpaired, Student's t-test.
Number in parenthesis is the number of species in which the corresponding amino acid is substituted.
n.d., the activity derived from the corresponding amino-acid-substituted construct could not be detected under our assay conditions.
MAF, minor allele frequency; SNPs, single-nucleotide polymorphisms.
Possible involvement of the amino-acid residue corresponding to each SNP in expression of the enzyme activity
Multiple alignment analysis of the amino-acid sequences of animal DNase I was performed for assessing the role of each SNP-related amino-acid residue in the DNase I protein (Table 1 and Supplementary Fig. S1); 40 animal DNases I surveyed from among Animalia organisms available on the genome database, in addition to vertebrate DNases I previously determined (Takeshita et al., 2001, 2003; Yasuda et al., 2004a, 2004b), were analyzed. It should be noted that the amino-acid residue at position 233 related to loss of the enzyme activity through amino-acid substitution derived from SNP p.Tyr233Cys was completely conserved among animal DNase I species, whereas the residues corresponding to all the SNPs not affecting the activity were not conserved. On the other hand, with regard to the amino-acid residues corresponding to each of the SNPs reducing or increasing the activity, those well conserved were weaved with those not conserved in the amino-acid sequence of animal DNases I, suggesting that the effect of the amino-acid substitutions resulting from these SNPs on the enzyme activity may have been attributable to the properties of individual amino acids.
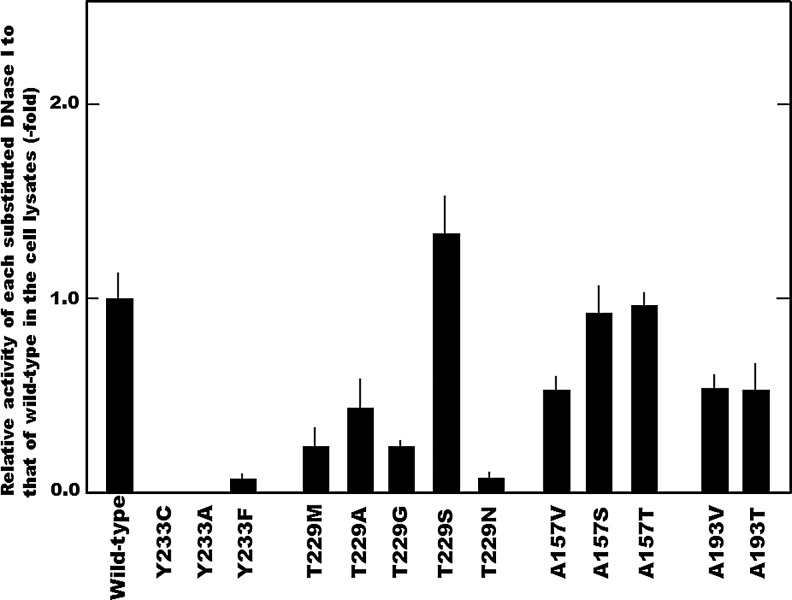
Next, in order to demonstrate the potential involvement of each amino-acid residue corresponding to the functional SNPs in the enzymatic activity, a series of amino-acid-substituted DNase I related to each SNP were examined (Fig. 2). Previously, we reported that SNP p.Arg207Cys in DNASE1 gives rise to an additional Cys residue between the two essential Cys residues, assuming that the new Cys residue at position 207 might form an aberrant disulfide bond with Cys 195 or Cys 231, inducing structural instability through loss of the essential disulfide bond and thereby drastically reducing or abolishing the activity (Baron et al., 1998; Yasuda et al., 1999; Chen et al., 2004). However, the substitution of Tyr233 by Ala (T233A) abrogated the DNase I activity completely, in the same manner as that by Cys (T233C), and furthermore, substitution by Phe (T229F) drastically reduced the activity to about 10% of that of the wild-type DNase I. Since the Tyr residue at position 233 in the protein is completely conserved in animal DNase I species, and only the phenyl group cannot sufficiently serve as an alternative to the Tyr residue, abolishment of the activity caused by the amino-acid substitution corresponding to SNP p.Tyr233Cys is attributable to disappearance of the Thr residue itself, and not to the appearance of a Cys residue. With regard to SNP p.Thr229Met, which caused reduction of the activity, DNase I in which Thr229 was substituted by Ala (T229A), Gly (T229G), or Asn (T229N), which are situated at the corresponding sites in the DNase Is of other species, exhibited low levels of activity similar to those of the T229M construct; whereas replacement by Ser (T229S) had little effect on the activity. These findings indicate that hydroxyl amino acids at position 229 might be involved in the expression of full activity. When the Ala residue at position 157 was substituted by Thr or Ser, which occur in the DNase Is of other species, the activity of each substituent (A157T and A157S, respectively) was similar to that of the wild type, suggesting that hydroxy amino-acid residues at position 157 might be more suitable than an Ala residue for expression of the activity. Furthermore, since Ala193 is almost completely conserved in animal DNase I species and substitution by Thr (A193T), which is present in fish DNase I (Yasuda et al., 2004b), reduced the activity to a level similar to that of A193V, the Ala residue at position 193 would appear to be essential for the activity. Pan et al. (1998) have proposed that the amino-acid residues in the DNase I protein can be classified into four groups based on their different functional roles; 4 residues are absolutely essential for catalysis, 3 are crucial acidic residues serving as ligands for metal ion chelation, 7 are critical components of DNA-interacting positions encircling the active site, and 13 residues interact with DNA distantly from the active site. However, no amino-acid residue corresponding to the SNPs affecting the enzyme activity examined in this study belong to these groups. Furthermore, in order to ascertain whether reduction of the activity in these functional SNPs was ascribed to protein instability of each substituted DNase I, we examined relative thermal stability of the A157V, A193V, and T229M substituents; compared with thermal stability of the wild-type DNase I, relative thermal stability of each was 1.2, 1.0 and 1.0, respectively. It seems plausible that reduction of the activity induced by the amino-acid substitutions resulting from these SNPs was not at least due to protein instability of the corresponding substituent.
FIG. 2.
Involvement of the amino-acid residues related to each functional SNPs examined in this study on the DNase I activity. Relative activities of amino-acid-substituted constructs related to functional SNPs to that of the wild-type DNase I are presented. The DNase I activity in the cells transfected with each construct was assayed by the SRED method (Nadano et al., 1993). The bar present SD (n=4).
Genotype distribution of the 14 nonsynonymous SNPs in DNASE1 for 14 different populations worldwide
A simple and novel genotyping procedure was developed using PCR-RFLP for all of the 14 nonsynonymous SNPs in DNASE1, with those for other SNPs having been previously developed (Yasuda et al., 2010; Ueki et al., 2014b). Since the substitution sites corresponding to nine SNPs in DNASE1 neither suppressed nor created any known restriction enzyme recognition sites, we employed a mismatched PCR-amplification method for genotyping (Yasuda et al., 1995b): Incorporation of a deliberate mismatch close to the 3′-terminus of a PCR primer enables the creation of a recognition site for each enzyme. After digestion of the amplified DNA fragment for each SNP, the appearance of the expected product, as shown in Supplementary Table S1, derived from the respective alleles in each SNP, enabled us to determine the genotypes easily.
The distribution of the genotype and allele frequencies for 14 nonsynonymous SNPs in DNASE1 was determined in 14 different populations, including three ethnic groups (n=1752) (Supplementary Table S2). Consequently, in addition to the other nonsynonymous SNPs in DNASE1 previously examined in the same populations (Yasuda et al., 2010; Fujihara et al., 2011; Ueki et al., 2014b), these results enabled us to clarify the genetic distribution of all of the 58 nonsynonymous SNPs in DNASE1 that have already been registered in the database.
All of the 14 nonsynonymous SNPs in DNASE1 examined in this study were found to be distributed in a mono-allelic manner; only the predominant allele for each SNP was found in all of the subjects in our study populations. In the NCBI dbSNP, no frequency data are available for p.Ala154Val, p.Ser221Asn, p.Thr229Met, p.Tyr233Cys, p.Ser251Leu, and p.Ala259Gly. Since only the predominant allele was observed in each of these 6 SNPs in the 14 different populations, it was clarified that these SNPs exhibited extremely low genetic diversity with a heterozygosity of 0.000, meaning that the minor allele frequency for each of these SNPs would be less than 0.0003 on the whole. Therefore, also considering our previous study (Yasuda et al., 2010; Ueki et al., 2014b), we were able to demonstrate that among the 58 nonsynonymous SNPs in DNASE1, only 4 SNPs (p.Arg2Ser, p.Tyr117Ser, p.Gly127Arg, and p.Arg244Gln) exhibited genetic heterozygosity in some or all of the populations examined, whereas the others were found to be distributed in a mono-allelic manner; all of the subjects were genotyped as homozygous for the predominant allele in the latter SNPs.
The compiled results for all the nonsynonymous SNPs in DNASE1 examined in this study, in addition to those clarified in our previous studies (Yasuda et al., 2010; Fujihara et al., 2011; Ueki et al., 2014b), are summarized in Table 1. It is worth noting that, for the 12 SNPs abolishing the activity, each of the minor alleles producing a loss-of-function variant was not distributed worldwide. Therefore, these activity-abolishing SNPs in DNASE1 may, in general, exert no influence on the levels of DNase I activity at the population level. Thus, with regard to these nonsynonymous SNPs, it could be assumed that DNASE1 is generally well conserved, thereby avoiding any marked reduction of the enzyme activity in the human populations.
Discussion
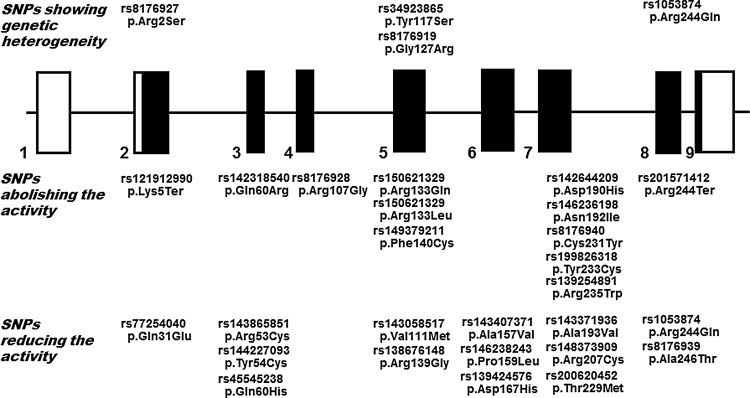
In the present study, we have extensively expanded our genetic and functional characterization of nonsynonymous SNPs in the human DNASE1 that is potentially relevant to SLE. Therefore, all of the 58 nonsynonymous SNPs reported or registered in the database, and likely give rise to alterations in the levels of in vivo DNase I activity through amino-acid substitution, could be evaluated and it could be checked as to whether these SNPs might serve as a functional SNP. To our knowledge, this study is the first that has comprehensively clarified the distribution of all of the nonsynonymous SNPs in DNASE1 in different worldwide populations, and also the effect of each SNP on the enzyme activity. It is worth noting that a compiled expression analysis of the amino-acid-substituted DNase I corresponding to each of these nonsynonymous SNPs demonstrated that they could be sorted into 23 SNPs not affecting the activity, 12 that abolished it, 14 that reduced it, and 9 that increased it. Thus, among all the nonsynonymous SNPs in DNASE1, SNPs showing functionality or genetic polymorphism could be identified (Fig. 3).
FIG. 3.
Identification of SNPs showing functionality or genetic polymorphism among all the nonsynonymous SNPs in DNASE1. The genomic structure of the human DNASE1 is presented; exons are shown by solid boxes, in which solid and clear boxes correspond to the translated and untranslated regions of the mRNA, respectively (Yasuda et al., 1995a). Each ID number in the NCBI database is shown. According to alterations in the levels of DNase I activity resulting from the corresponding amino-acid substitution, 58 nonsynonymous SNPs in DNASE1 could be classified into 23 SNPs not affecting the activity, 9 that increased it, 12 that abolished it, and 14 that reduced it; each SNP of the latter two groups, along with those showing a genetic polymorphism, are shown at the site of exons located.
Possible implications of the nonsynonymous SNPs in DNASE1 in relation to the etiology of SLE
Patients with SLE harbor mutations resulting in a lack or reduction of DNase I activity (Yasutomo et al., 2001; Dittmar et al., 2009), and similarly, DNase I-deficient mice develop SLE (Napirei et al., 2000). Furthermore, it has been reported that levels of serum DNase I activity are lower in SLE patients than in healthy subjects (Chitrabamrung et al., 1981; Sallai et al., 2005; Dittmar et al., 2007; Martinea-Valle et al., 2009). These findings suggest that, since DNase I is implicated in the clearance of apoptotic and/or necrotic cell debris, loss and/or functional deficiency of DNase I could result in failure to clear debris that might serve as the origin of nucleosomes, which can elicit an immune response resulting in autoimmune dysfunction. Furthermore, Hakkim et al. (2010) suggested that insufficient neutrophil extracellular trap (NET) degradation by DNase I would enable NETs to persist and, thus, foster the presentation of chromatin-associated self-antigens, a process which may promote SLE. Therefore, functional SNPs in DNASE1 causing loss of function or marked reduction of the enzyme activity should be considered as significant genetic factors that can lead to autoimmune dysfunction. In our studies, we were able to identify many of the functional SNPs in DNASE1 affecting the enzyme activity, irrespective of whether they showed a polymorphic distribution worldwide; in particular, 14 activity-reducing and 12 activity-abolishing SNPs were confirmed to be functional. Thus, it could be assumed that a minor allele of SNPs p.Lys5Ter, p.Gln60Arg, p.Arg107Gly, p.Arg133Gln, p.Arg133Leu, p.Phe140Cys, p.Asp190His, p.Asn192Ile, p.Cys231Tyr, p.Tyr233Cys, p.Arg235Trp, and p.Arg244Ter in DNASE1, producing a loss-of-function DNase variant, might be a genetic risk factor for SLE.
Especially, in the 12 SNPs abolishing the enzyme activity, each of the minor alleles producing a loss-of-function variant was not distributed worldwide. These findings indicate that these activity-abolishing SNPs in DNASE1 may, in general, exert no influence on the levels of DNase I activity in human populations. However, it could be assumed that a minor allele of functional SNPs producing a loss-of-function DNase variant might be a genetic risk factor for autoimmune diseases, despite its remarkably low genetic heterogeneity. These findings may have clinical implications in relation to the prevalence of SLE. In this context, in order to clarify any clinical association of these functional SNPs in DNASE1 with the incidence of autoimmune diseases, it will be necessary to examine the distribution of each SNP, along with levels of in vivo DNase I activity, in SLE-patient groups.
Comparison of DNASE1 with other members of the DNase family in terms of nonsynonymous SNPs
So far, several members of DNASE1 and protein family displaying a high similarity in their nucleotide and amino-acid sequences to the original DNase I have been identified and characterized (Rodriguez et al., 1997; Shiokawa and Tanuma, 2001). We have been conducting genetic and expression analyses of nonsynonymous SNPs in the DNase family genes in order to evaluate whether these SNPs may be functionally implicated in various diseases, including autoimmunity (Table 2). Our studies have demonstrated that almost all of the nonsynonymous SNPs in the genes encoding other members of the human DNase family, DNase I-like 1 (Ueki et al., 2010a, 2014a), I-like 2 (Ueki et al., 2013), and I-like 3 (Ueki et al., 2009, 2014b), along with DNase II (Ueki et al., 2010b; Kimura-Kataoka et al., 2013), exhibit a mono-allelic distribution in the same study populations, similar to DNase I. These findings enable us to conclude that the human DNase family has, on the whole, been well conserved at the protein level during the evolution of human populations. It is especially noteworthy that, other than only polymorphic SNP p.Arg244Gln affecting the activity in DNASE1 (Yasuda et al., 2010), all of the SNPs affecting the DNase activity were distributed in a mono-allelic manner worldwide. With regard to the nonsynonymous SNPs potentially resulting in alterations of in vivo DNase activity, the human DNase family genes show markedly low genetic diversity, enabling sufficient levels of in vivo activity derived from the DNase family to be retained in human populations as a whole.
Table 2.
Summary on Nonsynonymous Single-Nucleotide Polymorphisms Altering DNase Activity or Showing Genetic Polymorphism in the Genes Encoding Human DNase Family
| SNPsa | |||||||
|---|---|---|---|---|---|---|---|
| DNase family | Total nonsynonymous SNPs | Not affecting the activity | Reducing the activity | Abolishing the activity | Elevating the activity | SNPs showing genetic polymorphismb | Reference |
| DNase I | 58 | 23 | 14 | 12 | 9 | 4 | This study |
| Yasuda et al. (2010) | |||||||
| Ueki et al. (2014b) | |||||||
| DNase I-like 1 | 22 | 16 | 3 | 2 | 1 | 1 | Ueki et al. (2010a) |
| Ueki et al. (2014a) | |||||||
| DNase I-like 2 | 6 | 1 | 4 | 1 | 0 | 0 | Ueki et al. (2010a) |
| Ueki et al. (2013) | |||||||
| DNase I-like 3 | 25 | 18 | 5 | 2 | 0 | 1 | Ueki et al. (2009) |
| Ueki et al. (2014b) | |||||||
| DNase II | 15 | 6 | 5 | 4 | 0 | 1 | Ueki et al. (2010b) |
| Kimura-Kataoka et al. (2014) | |||||||
Total numbers of SNPs classified into each category are presented.
Genetic polymorphism for each SNP was examined in our study populations.
As shown in Table 2, compared with other members of the human DNase family (6–25 sites), DNASE1 contains many more nonsynonymous SNPs (58 loci). Furthermore, similar to DNase I-like 2, DNASE1 contains a higher ratio of SNPs, resulting in reduction/abolishment of the enzyme activity through amino-acid substitution than is the case in the other members; however, the DNase I-like 2 gene has markedly fewer nonsynonymous SNPs. Therefore, it has been clarified that, among the human DNase family genes, DNASE1 is able to tolerate the generation of nonsynonymous SNPs, and that the amino-acid substitutions resulting from the SNPs easily create alterations in the levels of enzyme activity.
Supplementary Material
Acknowledgments
DNA samples of bloodstain samples of the Ovambo and Turkish populations were kindly provided by B. Brinkmann. Blood samples of Korean and Mongolian populations were kindly provided K. Shiwaku. This study was supported in part by Grants in Aid from Japan Society for the Promotion of Science (22249023 to T.Y. and 25460864 to M.U.).
Disclosure Statement
No competing financial interests exist.
References
- Baron W.F., Pan C.Q., Spence S.A., Tyan A.M., Lazarus R.A., and Baker K.P. (1998). Cloning and characterization of an actin-resistant DNase I-like endonuclease secreted by macrophages. Gene 215,291–301 [DOI] [PubMed] [Google Scholar]
- Bodańo A., González A., Ferreiros-Vidal I., Balada E., Ordi J., Carreira P., Gómez-Reino J.J., and Conde C. (2006). Association of a non-synonymous single-nucleotide polymorphism of DNASE1 with SLE susceptibility. Rheumatology 45,819–823 [DOI] [PubMed] [Google Scholar]
- Chitrabamrung S., Ribin R.L., and Tan E.M. (1981). Serum deoxyribonuclease I and clinical activity in systemic lupus erythematosus. Rheumatol Int 1,55–60 [DOI] [PubMed] [Google Scholar]
- Chen W.-J., Lee I.-S., Chen C.-Y., and Liao T.-H. (2004). Biological functions of the disulfides in bovine pancreatic deoxyribonuclease I. Protein Sci 13,875–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counis M.F., and Torriglia A. (2000). DNases and apoptosis. Biochem Cell Biol 78,405–414 [PubMed] [Google Scholar]
- Dittmar M., Bischofs-Matheis N., Poppe R., and Kahaly G.J. (2009). A novel mutation in the DNASE1 gene is related with protein instability and decreased activity in thyroid autoimmunity. J Antoimmun 32,7–13 [DOI] [PubMed] [Google Scholar]
- Dittmar M., Poppe R., Bischofs C., Fredenhagen C, Kanitz M., and Kahaly G.J. (2007). Impaired deoxyribonuclease I activity in monoglandular and polyglandular autoimmunity. Exp Clin Endocrinol Diabetes 115,387–391 [DOI] [PubMed] [Google Scholar]
- Fujihara J., Ueki M., Yasuda T., Iida R., Soejima M., Koda Y., Kimura K., Kato H., Panduro A., Tongu M., and Takeshita H. (2011). Functional and genetic survey of all known single-nucleotide polymorphisms within the human deoxyribonuclease I gene in wide-ranging ethnic groups. DNA Cell Biol 30,205–217 [DOI] [PubMed] [Google Scholar]
- Hakkim A., Fürnrohr B.G., Amann K., Laube B., Abed U.A., Brinkmann V., Herrmann M., Voll R.E., and Zychlinsky A. (2010). Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci USA 107,9813–9818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedberg A., Mortensen E.S., and Rekvig O.P. (2011). Chromatin as a target antigen in human and murine lupus nephritis. Arthritis Res Ther 13,214–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura-Kataoka K., Ueki M., Takeshita H., Fujihara J., Iida R., Kato H., and Yasuda T. (2013). Seven non-synonymous single nucleotide polymorphisms (SNPs) in the genes encoding human deoxyribonuclease II may serve as a functional SNP potentially implicated in autoimmune dysfunction. Electrophoresis 34,3361–3369 [DOI] [PubMed] [Google Scholar]
- Martinea-Valle F., Balada E., Ordi-Ros J., Bujan-Rivas S., Sellas-Fernadez A., and Vilardell-Tarres M. (2009). DNase I activity in patients with systemic lupus erythematosus: relationship with epidemiological, clinical, immunological and therapeutical features. Lupus 18,418–423 [DOI] [PubMed] [Google Scholar]
- Mizuta R., Mizuta M., Araki S., Shiokawa D., Tanuma S., and Kitamura D. (2006). Action of apoptotic endonuclease DNase gamma on naked DNA and chromatin substrates. Biochem Biophys Res Commun 345,560–567 [DOI] [PubMed] [Google Scholar]
- Nadano D., Yasuda T., and Kishi K. (1993). Measurement of deoxyribonuclease I activity in human tissues and body fluids by a single radial enzyme diffusion method. Clin Chem 39,448–452 [PubMed] [Google Scholar]
- Napirei M., Gültekin A., Kloeckl T., Möröy T., Frostegård J., and Mannherz H.G. (2006). Systemic lupus-erythematosus: deoxyribonuclease I in necrotic chromatin disposal. Int J Biochem Cell Biol 38,297–306 [DOI] [PubMed] [Google Scholar]
- Napirei M., Karsunky H., Zevnik B., Stephan H., Mannherz H.G., and Möröy T. (2000). Feature of systemic lupus erythematosus in Dnase 1-deficien mice. Nat Genet 25,177–181 [DOI] [PubMed] [Google Scholar]
- Pan C.Q., Ulmar J.S., Herzka A., and Lazarus R.A. (1998). Mutation analysis of human DNase I at the DNA binding interface: implications for DNA recognition, catalysis, and metal ion dependence. Protein Sci 7,628–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A.M., Rodin D., Nomura H., Morton C.C., Weremowicz S., and Schneider M.C. (1997). Identification, lovalization, and expression of two novel human genes similar to deoxyribonuclease I. Genomics 42,507–513 [DOI] [PubMed] [Google Scholar]
- Sallai K., Nagy E., Derfalvy B., Müzes G., and Gergely A. (2005). Antinucleosome antibodies and decreased deoxyribonuclease I activity in sera of patients with systemic lupus erythematosus. Clin Diagn Lab Immunol 12,56–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H.D., Park B.L., Kim L.H., Lee H.-S., Kim T.-Y., and Bae A.-C. (2004). Common DNase I polymorphism associated autoantibody production among systemic lupus erythermatosus. Hum Mol Genet 13,2343–2350 [DOI] [PubMed] [Google Scholar]
- Shiokawa D., and Tanuma S. (2001). Characterization of human DNase I family endonuclease and activation of DNase gamma during apoptosis. Biochemistry 40,143–152 [DOI] [PubMed] [Google Scholar]
- Takeshita H., Yasuda T., Iida R., Nakajima T., Mori S., Mogi K., Kaneko Y., and Kishi K. (2001). Amphibian DNases I are characterized by a C-terminal end with a unique, cysteine-rich stretch and by the insertion of a serine residue into the Ca2+-binding site. Biochem J 357,473–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita H., Yasuda T., Nakajima T., Mogi K., Kaneko Y., Iida R., and Kishi K. (2003). A single amino acid substitution of Leu130Ile in snake DNases I contributes to the acquisition of thermal stability: a clue to the molecular evolutionary mechanism from cold-blooded to warm-blooded vertebrates. Eur J Biochem 70,307–314 [DOI] [PubMed] [Google Scholar]
- Tsukumo S., and Yasutomo K. (2004). DNase I in pathogenesis of systemic lupus erythematosus. Clin Immnol 113,14–18 [DOI] [PubMed] [Google Scholar]
- Ueki M., Fujihara J., Kimura K., Takeshita H., Iida R., and Yasuda T. (2013). Five non-synonymous SNPs in the gene encoding human deoxyribonuclease I-like 2 implicated in terminal differentiation of keratinocytes reduce or abolish its activity. Electrophoresis 34,456–462 [DOI] [PubMed] [Google Scholar]
- Ueki M., Fujihara J., Takeshita H., Kimura K., Iida R., Nakajima T., Kominato Y., Yuasa I., and Yasuda T. (2010a). Genetic and expression analysis of all non-synonymous single nucleotide polymorphisms in the human deoxyribonuclease I-like 1 and 2 genes. Electrophoresis 31,2063–2069 [DOI] [PubMed] [Google Scholar]
- Ueki M., Kimura K., Fujihara J., Takeshita H., Iida R., and Yasuda T. (2014a). Evaluation of all nonsynonymous single-nucleotide polymorphisms in the gene encoding human deoxyribonuclease I-like 1, possibly implicated in the blocking of endocytosis-mediated foreign gene transfer. DNA Cell Biol 33,79–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki M., Kimura K., Takeshita H., Fujihara J., Iida R., Sano R., Nakajima T, Kominato Y, Kawai Y, and Yasuda T. (2014b). Evaluation of all non-synonymous single nucleotide polymorphisms (SNPs) in the genes encoding human deoxyribonuclease I and I-like 3 as a functional SNP potentially implicated in autoimmunity. FEBS J 281,376–390 [DOI] [PubMed] [Google Scholar]
- Ueki M., Takeshita H., Fujihara J., Iida R., Yuasa I., Kato H., Panduro A., Nakajima T., Kominato Y., and Yasuda T. (2009). Caucasian-specific allele in non-synonymous single nucleotide polymorphisms of the gene encoding deoxyribonuclease I-like 3, potentially relevant to autoimmunity, produces an inactive enzyme. Clin Chim Acta 407,20–24 [DOI] [PubMed] [Google Scholar]
- Ueki M., Takeshita H., Fujihara J., Kimura K., Iida R., Yuasa I., Nakajima T., Kominato Y., and Yasuda T. (2010b). Genetic and expression analysis of all 7 non-synonymous single nucleotide polymorphisms in the human deoxyribonuclease II gene, with potential relevance to autoimmunity. Clin Chim Acta 411,92–98 [DOI] [PubMed] [Google Scholar]
- Yasuda T., Kishi K., Yanagawa U., and Yoshida A. (1995a). Structure of the human deoxyribonuclease I (DNase I) gene: identification of the nucleotide substitution that generates its classical genetic polymorphism. Ann Hum Genet 59,1–15 [DOI] [PubMed] [Google Scholar]
- Yasuda T., Nadano D., Tenjo E., Takeshita H., Sawazaki K., Nakanaga M., and Kishi K. (1995b). Genotyping of human deoxyribonuclease I polymorphism by the polymerase chain reaction. Electrophoresis 16,1889–1893 [DOI] [PubMed] [Google Scholar]
- Yasuda T., Iida R., Ueki M., Kominato Y., Nakajima T., Takeshita H., Kobayashi T., and Kishi K. (2004a). Molecular evolution of shark and other vertebrate DNases I. Eur J Biochem 271,4428–4435 [DOI] [PubMed] [Google Scholar]
- Yasuda T., Takeshita H., Iida R., Kogure S., and Kishi K. (1999). A new allele, DNASE1*6, of human deoxyribonuclease I polymorphism encodes an Arg to Cys substitution responsible for its instability. Biochem Biophys Res Commun 260,280–283 [DOI] [PubMed] [Google Scholar]
- Yasuda T., Takeshita H., Iida R., Ueki M., Nakajima T., Kaneko Y., Mogi K., Kominato Y., and Kishi K. (2004b). A single amino acid substitution can shift the optimum pH of DNase I for enzyme activity: biochemical and molecular analyses of the piscine DNase family. Biochim Biophys Acta 1672,174–183 [DOI] [PubMed] [Google Scholar]
- Yasuda T., Ueki M., Takeshita H., Fujihara J., Kimura K., Iida R., Tsubota E., Soejima M., Koda Y., Kato H., and Panduro A. (2010). A biochemical and genetic study on all non-synonymous single nucleotide polymorphisms of the gene encoding human deoxyribonuclease I potentially relevant to autoimmunity. Int J Biochem Cell Biol 42,1216–1225 [DOI] [PubMed] [Google Scholar]
- Yasutomo K., Horiuchi T., Kagami H., Tsukamoto H., Hashimura C., Urushihara M., and Kuroda Y. (2001). Mutation of DNASE1 in people with systemic lupus erythematosus. Nat Genet 28,313–314 [DOI] [PubMed] [Google Scholar]
- Valle F.M., Balada E., Ordi-Ros J., and Vilardell-Tarres M. (2008). DNase I and systemic lupus erythematosus. Autoimmun Rev 7,359–363 [DOI] [PubMed] [Google Scholar]
- Wu J.-R., and Zeng R. (2012). Molecular basis for population variation: From SNPs to SAPs. FEBS Lett 586,2841–2845 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.