Abstract
Background: Bartonella species are important emerging pathogens in human and veterinary medicine. In the context of their daily activities, veterinary professionals have frequent animal contact and arthropod exposures. Detection of Bartonella spp. using traditional culture methods has been limited by poor sensitivity, making it difficult to determine the prevalence of infection in this population. We have developed a detection method combining enrichment culture and molecular amplification, which increases testing sensitivity.
Methods: We performed a cross-sectional study to determine the prevalence of detectable Bartonella spp. in the blood of veterinary personnel and nonveterinary control subjects. Bartonella was detected by enrichment blood culture with conventional PCR followed by DNA sequencing. Results were correlated with epidemiological variables and symptoms.
Results: We detected DNA from at least one Bartonella species in 32 (28%) of the 114 veterinary subjects. After DNA sequencing, the Bartonella species could be determined for 27 of the 32 infected subjects, including B. henselae in 15 (56%), B. vinsonii subsp. berkhoffii in seven (26%), B. koehlerae in six (22%), and a B. volans–like sequence in one (4%). Seventy percent of Bartonella-positive subjects described headache compared with 40% of uninfected veterinarians (p=0.009). Irritability was also reported more commonly by infected subjects (68% vs. 43%, p=0.04).
Conclusions: Our study supports an emerging body of evidence that cryptic Bartonella bloodstream infection may be more frequent in humans than previously recognized and may induce symptoms. Longitudinal studies are needed to determine the natural course and clinical features of Bartonella infection.
Key Words: : Bartonella, Diagnostic test, Veterinarian, Zoonosis, Bacteremia
Introduction
Bartonella species are important emerging pathogens in human and veterinary medicine. The genus Bartonella is currently comprised of 30 species of fastidious, Gram-negative bacteria that are highly adapted to one or more mammalian reservoir hosts (Kordick and Breitschwerdt 1995, Jacomo et al. 2002). Although cat scratch disease (caused by B. henselae), bacillary angiomatosis (B. henselae, B. quintana), and endocarditis (caused by eight Bartonella spp. to date) are the best recognized manifestations of bartonellosis, Bartonella spp. have been associated with varied clinical manifestations, including encephalitis, neuroretinitis, anterior uveitis, hemolytic anemia, thrombocytopenia, glomerulonephritis, pneumonia, and osteomyelitis (Hashkes et al. 1996, Dehio 1997, Jacobs and Schutze 1998, Tsukahara et al. 2000, Ayoub et al. 2002, Jacomo et al. 2002).
In apparently healthy reservoir mammals, including cats, wild canines, and rodents, Bartonella may produce prolonged or indefinite bacteremia, which can usually be detected using lysis centrifugation or freeze–thaw blood culture followed by PCR (Breitschwerdt and Kordick 2000). Recent evidence has demonstrated intraerythrocytic and endothelial localization of Bartonella, thereby providing a unique strategy for bacterial persistence and transmission (Dehio 1997, Dehio 2001, Rolain et al. 2002, Chomel et al. 2003). In sick nonreservoir animals, including humans, isolation or molecular detection of Bartonella infection is much more difficult using conventional techniques due to fastidious growth characteristics and low bacterial load (Breitschwerdt et al. 1999, Jacomo et al. 2002). In most instances, contemporary microbiological approaches for the isolation of Bartonella spp. from immunocompetent subjects with serological, pathological, or molecular evidence of infection have not been successful. Notable exceptions include PCR amplification of B. henselae DNA from the lymph nodes of people with cat scratch disease and the successful culture and PCR detection of several Bartonella spp. from the blood, serum, or heart valves of endocarditis patients (La Scola and Raoult 1999, Turner et al. 2005). Also, B. henselae has on rare occasion been isolated from the blood of children with cat scratch disease (Del Prete et al. 2000, Arvand and Schad 2006).
Recently, we have successfully combined two approaches to document chronic Bartonella infections in the blood of various animal species: enrichment culture in a special growth medium (Bartonella Alpha Proteobacteria Growth Medium [BAPGM]), followed by conventional or real-time PCR using Bartonella genus- and species-specific primers (Maggi and Breitschwerdt 2005, Maggi et al. 2005). This approach substantially improves the sensitivity of Bartonella detection in blood samples obtained from sick animals and humans, as compared with traditional culture methods. Previously, we have been able to detect and isolate Bartonella spp. from veterinary personnel with extensive animal exposure, many of whom were tested because of a history of chronic debilitating illnesses of unknown origin (Breitschwerdt et al. 2007, Breitschwerdt et al. 2008, Breitschwerdt et al. 2010). The enhanced sensitivity of this diagnostic approach now allows us to more fully investigate whether bacteremia with Bartonella spp. is more common in subjects with extensive animal contact than currently recognized so that we can begin to determine additional clinical phenotypes and assess epidemiological associations among patient populations. Here, we report a cross-sectional study in which the serological and molecular prevalences of Bartonella infection were investigated and bloodstream infection was correlated with clinical symptoms in a cohort of veterinary personnel.
Materials and Methods
We performed a cross-sectional study to determine the prevalence of Bartonella spp. bacteremia in veterinary personnel, as detected by enrichment blood culture with conventional PCR followed by DNA sequencing of amplicons, the association of bacteremia with chronic clinical symptoms, and the potential epidemiological associations. Institutional Review Board approval for this study was received from both Duke University Medical Center and North Carolina State University.
Subject recruitment
Veterinary personnel
We recruited a convenience sample of veterinarians and veterinary technicians who were attendees at a national continuing education conference in Orlando, Florida in January, 2008. All attendees of the conference were notified of the opportunity to participate in the study in their meeting registration materials. In February, 2008, recruitment was extended to veterinary personnel affiliated with the North Carolina State University College of Veterinary Medicine.
Nonveterinary controls
We recruited a convenience sample of nonveterinary adult volunteers from among students and employees at Duke University Medical Center. These subjects were intended to serve as an unexposed population so that they could be appropriate negative controls for our laboratory methods. They were not recruited to compare specific risks associated with Bartonella infection.
Data and specimen collection
Both veterinary subjects and nonveterinary controls completed a standardized questionnaire that included demographic information, clinical symptoms experienced, as well as occupational and nonoccupational domestic and wild animal exposures, bite and scratch history, and travel history (see Tables 1 and 2). Approximately 10–12 mL of blood (5–6 mL of EDTA, 5–6 mL of serum separator) was collected at the time of enrollment. Aseptic technique was used using povidone-iodine or chlorhexidine decontamination of the skin. Venipuncture and specimen transport were performed by an experienced research nurse. Blood samples were transported by car to the Intracellular Pathogens Research Laboratory (IPRL) at North Carolina State University College of Veterinary Medicine, a Biosafety Level 3 (BSL3)-certified laboratory.
Table 1.
Exposures and Demographics of Veterinary and Control Subjects
| Controls (%) | Veterinarians (%) | p value | |
|---|---|---|---|
| Demographics and travel | |||
| Median age (years) | 28 | 47 | 0.00002 |
| Gender | |||
| Female | 18 (56.3) | 83 (72.8) | 0.08533 |
| Male | 14 (43.8) | 31 (27.2) | |
| US travel | |||
| Any | 29 (90.6) | 111 (97.4) | 0.11946 |
| Northeast | 22 (68.8) | 67 (58.8) | 0.41246 |
| Southwest | 13 (40.6) | 67 (58.8) | 0.07443 |
| Southeast | 17 (53.1) | 91 (79.8) | 0.00527 |
| Animal contact during travel | 10 (31.3) | 77 (67.5) | 0.00040 |
| Insect contact during travel | 15 (46.9) | 100 (87.7) | <0.00001 |
| International travel | |||
| Any | 23 (71.9) | 73 (64) | 0.52798 |
| Asia | 10 (31.3) | 17 (14.9) | 0.04268 |
| Australia | 2 (6.3) | 10 (8.8) | 1.00000 |
| Europe | 18 (56.3) | 53 (46.5) | 0.42384 |
| South America | 7 (21.9) | 18 (15.8) | 0.43174 |
| Other | 0 (0.0) | 10 (8.8) | 0.11814 |
| Animal contact during travel | 9 (28.1) | 44 (38.6) | 0.30592 |
| Insect contact during travel | 17 (53.1) | 59 (51.8) | 1.00000 |
| Animal scratches and bites | |||
| Dogs | 0.00004 | ||
| Daily | 1 (3.1) | 11 (9.6) | |
| Weekly to monthly | 2 (6.3) | 47 (41.2) | |
| Rarely or never | 29 (90.6) | 56 (49.1) | |
| Cats | 0.00000 | ||
| Daily | 0 (0.0) | 13 (11.4) | |
| Weekly to monthly | 5 (15.6) | 70 (61.4) | |
| Rarely or never | 27 (84.4) | 31 (27.2) | |
| Birds | 0.05299 | ||
| Daily | 0 (0.0) | 1 (0.9) | |
| Weekly to monthly | 0 (0.0) | 15 (13.2) | |
| Rarely or never | 32 (100.0) | 98 (86) | |
| Horses | 1.00000 | ||
| Daily | 0 (0.0) | 2 (1.8) | |
| Weekly to monthly | 0 (0.0) | 2 (1.8) | |
| Rarely or never | 32 (100.0) | 110 (96.5) | |
| Reptiles | 0.57636 | ||
| Weekly to monthly | 0 (0.0) | 4 (3.5) | |
| Rarely or never | 32 (100.0) | 110 (96.5) | |
| Other Animals | 0.00154 | ||
| No | 30 (93.8) | 97 (85.1) | |
| Yes | 0 (0.0) | 17 (14.9) | |
| Arthropod exposures | |||
| Fleas | <0.00001 | ||
| Daily | 0 (0.0) | 50 (43.9) | |
| Weekly to monthly | 1 (3.1) | 50 (43.9) | |
| Rarely or never | 31 (96.9) | 14 (12.3) | |
| Ticks | <0.00001 | ||
| Daily | 0 (0.0) | 23 (20.2) | |
| Weekly to monthly | 3 (9.4) | 69 (60.5) | |
| Rarely or never | 29 (90.6) | 22 (19.3) | |
| Biting Flies | 0.00001 | ||
| Daily | 0 (0.0) | 15 (13.2) | |
| Weekly to monthly | 3 (9.4) | 47 (41.2) | |
| Rarely or never | 29 (90.6) | 52 (45.6) | |
| Mosquitoes | 0.00123 | ||
| Daily | 2 (6.3) | 29 (25.4) | |
| Weekly to monthly | 15 (46.9) | 65 (57) | |
| Rarely or never | 15 (46.9) | 20 (17.5) | |
| Lice | 0.06915 | ||
| Weekly to monthly | 0 (0.0) | 12 (10.5) | |
| Rarely or never | 32 (100.0) | 102 (89.5) | |
| Other Arthropods | 0.69154 | ||
| No | 31 (96.9) | 105 (92.1) | |
| Animal exposures | |||
| Dogs | <0.00001 | ||
| Daily | 10 (31.3) | 105 (92.1) | |
| Weekly to monthly | 8 (25) | 6 (5.3) | |
| Rarely or never | 14 (43.8) | 3 (2.6) | |
| Cats | <0.00001 | ||
| Daily | 6 (18.8) | 102 (89.5) | |
| Weekly to monthly | 6 (18.8) | 8 (7) | |
| Rarely or never | 20 (62.5) | 4 (3.5) | |
| Birds | <0.00001 | ||
| Daily | 0 (0.0) | 27 (23.7) | |
| Weekly to monthly | 1 (3.1) | 31 (27.2) | |
| Rarely or never | 31 (96.9) | 56 (49.1) | |
| Horses | 0.00009 | ||
| Daily | 0 (0.0) | 16 (14) | |
| Weekly to monthly | 0 (0.0) | 24 (21.1) | |
| Rarely or never | 32 (100.0) | 74 (64.9) | |
| Reptiles | 0.00152 | ||
| Daily | 0 (0.0) | 3 (2.6) | |
| Weekly to monthly | 0 (0.0) | 26 (22.8) | |
| Rarely or never | 32 (100.0) | 85 (74.6) | |
| Cattle | 0.16859 | ||
| Daily | 0 (0.0) | 3 (2.6) | |
| Weekly to monthly | 0 (0.0) | 11 (9.6) | |
| Rarely or never | 32 (100.0) | 100 (87.7) | |
| Goats | 0.06423 | ||
| Daily | 0 (0.0) | 6 (5.3) | |
| Weekly to monthly | 0 (0.0) | 12 (10.5) | |
| Rarely or never | 32 (100.0) | 96 (84.2) | |
| Poultry | 0.10107 | ||
| Daily | 0 (0.0) | 3 (2.6) | |
| Weekly to monthly | 0 (0.0) | 12 (10.5) | |
| Rarely or never | 32 (100.0) | 99 (86.8) | |
| Swine | 0.74165 | ||
| Daily | 0 (0.0) | 2 (1.8) | |
| Weekly to monthly | 0 (0.0) | 4 (3.5) | |
| Rarely or never | 32 (100.0) | 108 (94.7) | |
| Sheep | 0.13164 | ||
| Daily | 0 (0.0) | 4 (3.5) | |
| Weekly to monthly | 0 (0.0) | 10 (8.8) | |
| Rarely or never | 32 (100.0) | 100 (87.7) | |
| Wild Animals | 0.00008 | ||
| Daily | 0 (0.0) | 2 (1.8) | |
| Weekly to monthly | 0 (0.0) | 35 (30.7) | |
| Rarely or never | 32 (100.0) | 77 (67.5) | |
| Other Animals | 0.00007 | ||
| No | 30 (93.8) | 74 (64.9) | |
| Yes | 1 (3.1) | 40 (35.1) | |
| Duration of Animal Exposure | <0.00001 | ||
| ≤10 years | 32 (100.0) | 2 (1.8) | |
| >10 years | 0 (0.0) | 112 (98.2) | |
Table 2.
Self-Reported Symptoms and Medical History of Veterinary Subjects
| Bartonella negative | Bartonella positive | ||
|---|---|---|---|
| Clinical features | (Total %) | (Total %) | p value |
| Fatigue | 44 (64.7) | 21 (72.4) | 0.4911 |
| Chronic fatigue | 32 (47.8) | 15 (53.6) | 0.6571 |
| Sleepiness | 33 (45.8) | 14 (48.3) | 0.8295 |
| Insomnia | 27 (39.7) | 15 (55.6) | 0.1770 |
| Irritability | 31 (43.7) | 19 (67.9) | 0.0440 |
| Headache | 29 (40.3) | 21 (70) | 0.0088 |
| Memory problems | 27 (38.6) | 12 (41.4) | 0.8241 |
| Confusion | 10 (15.2) | 4 (13.8) | 1.0000 |
| Disorientation | 5 (7.6) | 4 (13.8) | 0.4484 |
| Eye pain | 10 (14.5) | 5 (17.2) | 0.7629 |
| Vision impairment | 13 (18.8) | 8 (27.6) | 0.4195 |
| Balance problems | 14 (20.9) | 6 (20.7) | 1.0000 |
| Arthralgia | 40 (56.3) | 20 (69) | 0.2692 |
| Muscle pain | 31 (47.7) | 18 (62.1) | 0.2643 |
| Muscle weakness | 30 (54.5) | 12 (54.5) | 1.0000 |
| Tremor | 16 (22.9) | 5 (17.2) | 0.6005 |
| Numbness | 27 (40.3) | 11 (39.3) | 1.0000 |
| Paralysis | 3 (7.5) | 0 (0.0) | 0.5540 |
| Excretory dysfunction | 13 (19.7) | 6 (20.7) | 1.0000 |
| Shortness of breath | 18 (26.9) | 8 (27.6) | 1.0000 |
| Poor appetite | 6 (9.1) | 3 (10.3) | 1.0000 |
| Weight loss | 2 (3) | 2 (6.9) | 0.5832 |
| Depression | 14 (21.2) | 10 (35.7) | 0.1954 |
| Syncope | 3 (4.7) | 3 (10.3) | 0.3715 |
| Other | 6 (23.1) | 2 (18.2) | 1.0000 |
| Unable to perform activities of daily living | 8 (13.8) | 6 (20.7) | 0.2373 |
| Unable to perform job activities | 11 (17.7) | 6 (20.7) | 0.4838 |
| Treated with corticosteroidsa | 20 (31.7) | 11 (40.7) | 0.4713 |
| Treated with antibioticsa | 22 (34.9) | 16 (59.3) | 0.0385 |
| Specialty evaluation last 5 years | 46 (59.7) | 21 (70) | 0.3788 |
This refers to treatments with either corticosteroids or antibiotics that the patients had received by their own medical providers within the previous 12 months. These treatments were not provided within this study. The clinical rationale for these prior treatments was not collected as part of this study.
Specimen processing and diagnostic testing
Patient EDTA-anticoagulated blood samples and sera were stored for approximately 24 h at 4°C until processed by one investigator (R.G. Maggi) in the Intracellular Pathogens Laboratory. Using standard operating procedures, we screened for Bartonella spp. in DNA extracted from EDTA-anticoagulated blood, enrichment liquid culture of patient blood, and from blood agar plate colony isolates, if obtained after subculture of the previously enriched blood samples (see Fig. 1) (Maggi et al. 2011).
FIG. 1.
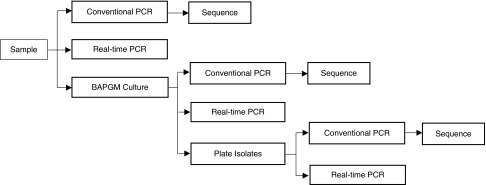
Specimen processing for Bartonella detection.
Growth medium
Enrichment culture of blood samples was performed as previously described (Maggi, Duncan 2005). An aliquot of 1 mL of EDTA whole blood was inoculated into 10 mL of BAPGM, after which the cultures were maintained at 35°C in a 5% CO2, water-saturated atmosphere. After 7-day culture, a 1-mL aliquot of pre-enrichment culture was inoculated onto blood agar plates and incubated as described. Plates were checked for colony formation at 7, 14, and 21 days after plating.
Conventional PCR analysis
Bartonella spp. and strain classification was performed using primers designed to amplify two consensus sequences in the Bartonella 16S–23S intergenic spacer region as described previously (Maggi and Breitschwerdt 2005). Amplicon size obtained from the 16S–23S ITS region is species dependent, allowing a preliminary species identification based upon amplicon size. Two sets of oligonucleotides, 325s and 1100as and 438s and 1000as, were used as forward and reverse primers, respectively, for the amplification of Bartonella spp. DNA at the genus level. Additionally, as previously reported (Breitschwerdt et al. 2011), PCR screening for B. koehlerae was performed using species-specific oligonucleotides Bkoehl-1s and Bkoehl1125as as forward and reverse primers, respectively. Amplification of the ITS region at both genus and species (B. koehlerae) levels were performed in a 25-μL final volume reaction containing 12.5 μL of Tak-Ex® Premix (Fisher Scientific), 0.25 μL of 30 μM of each forward and reverse primer (IDT® DNA Technology), 7.3 μL of molecular-grade water, and 5 μL of DNA from each sample tested. PCR negative controls were prepared using 5 μL of dH2O (when testing isolates from plates), 5 μL of DNA from blood of a healthy dog, or 5 μL of DNA extracted from uninoculated BAPGM-negative controls (when testing BAPGM enrichment cultures). Positive controls for PCR were prepared by serial dilution (using dog blood DNA) of genomic DNA from B. henselae (Houston I strain type) down to 0.001 pg/μL (equivalent to 0.5 bacteria/μL).
Conventional PCR was performed in an Eppendorf Mastercycler EPgradient® under the following conditions—a single hot-start cycle at 95°C for 2 min followed by 55 cycles of denaturing at 94°C for 15 s, annealing at 66°C for 15 s, and extension at 72°C for 18 s. Amplification was completed by an additional cycle at 72°C for 1 min, and products were analyzed by 2% agarose gel electrophoresis with detection using ethidium bromide under ultraviolet light. Amplicon products were sequenced to establish species and ITS strain identification. All PCR and uninoculated BAPGM enrichment controls remained negative throughout the study period.
Sequencing analysis
PCR amplicon sequence analysis was performed using a commercial company (Eton Biosciences, Research Triangle Park, NC). Chromatogram evaluation and sequence alignment were performed using Contig-Express and AlignX softwares (Vector NTI Suite 10.1, Invitrogen Corp., Carlsbad, CA). Bacteria species and strain were defined by comparing similarities with other sequences deposited in the GenBank database using the Basic Local Alignment Search Tool (Blast v. 2.0)
Bartonella IFA serological testing
Bvb, Bh, and Bk antibodies were determined in the IPRL using cell culture grown bacteria as antigens and following standard immunofluorescent antibody assay (IFA) techniques. Canine isolates of Bvb genotype I (NCSU 93CO-01 Tumbleweed, ATCC type strain #51672; Breitschwerdt et al. 1995), Bvb genotype II (NCSU 95CO-08, Winnie; Kordick and Breitschwerdt 1998), and Bvb genotype III (NCSU 06CO-01 Klara; Cadenas et al. 2008) and feline isolates of Bh H-1 strain (NCSU 93FO-23 Cisco), B. henselae SA2 strain (NCSU 95FO-099, Missy), and Bk (NCSU 09FO-01, Trillium) colonies were passed from agar plate grown cultures into Bartonella-permissive cell lines, i.e., the DH82 (a canine monocytoid) cell line for Bh strains H-1 and SA2, Bvb I and B. koehlerae and Vero cells (a mammalian fibroblast cell line) for Bvb II and III to obtain antigens for IFA testing. For each antigen, heavily infected cell cultures were spotted onto 30-well Teflon-coated slides (Cel-Line/Thermo Scientific), air-dried, acetone-fixed, and stored frozen. Fluorescein conjugated goat anti-human IgG (Cappel, ICN) was used to detect bacteria within cells using a fluorescent microscope (Carl Zeiss Microscopy, LLC, Thornwood, NY). Serum samples diluted in phosphate-buffered saline (PBS) solution containing normal goat serum, Tween-20, and powdered nonfat dry milk to block nonspecific antigen binding sites were screened at dilutions of 1:16 to 1:64. All sera that were reactive at a titer of 1:64 were further tested with two-fold dilutions out to 1:8192. To avoid confusion with possible nonspecific binding found at low dilutions, a cutoff of 1:64 was selected as a seroreactive titer.
Data analysis
Questionnaire data for the study were collected on paper forms and entered into a Microsoft Access database. Data entry was validated by comparing the electronic records with the information on the forms. Associations of demographic, risk factor, and exposure variables were assessed with medians and interquartile ranges for continuous variables and with counts and rates in contingency tables for categorical data. To assess the statistical significance of these associations, we used the Kruskal–Wallis test for continuous measures and the Fisher exact test for cross-classifications of categorical variables. All analyses were performed using SAS v. 9.2 or 9.3 (Cary, NC).
Results
Subject recruitment
We enrolled a total of 114 veterinary personnel. Their mean age was 47 years, and 73% were women (Table 1). Seventy-nine (69%) were veterinarians, 29 (25%) were veterinary technicians, and the remainder were veterinary students or employed by veterinary practices. For comparison to the laboratory results derived from veterinary personnel, 32 nonveterinary, healthy control subjects were recruited from among the medical and nursing staff at Duke University Medical Center. As compared with the veterinary subjects, these control subjects controls were younger (mean age 32 vs. 46 years, p<0.001) and included more males (43% vs. 27%, p=0.085).
As expected, the veterinary personnel had extensive animal contact as compared with controls (Table 1). Approximately 90% of the veterinary subjects had daily contact with dogs and cats, and more than half reported dog or cat scratches at least monthly. The vast majority of control subjects reported no animal exposure, and only one had daily animal contact. Exposure to ticks, fleas, and biting flies were reported significantly more frequent among veterinary subjects, in addition to an increased exposure to lice that did not reach statistical significance.
Bartonella detection
We detected DNA from at least one Bartonella species in 32 (28%) of the 114 veterinary subjects. After sequencing the PCR amplicons, we were able to speciate the detected Bartonella in 27 of the 32 infected subjects. Of these, 15 (56%) had B. henselae, seven (26%) had B. vinsonii subsp. berkhoffii, six (22%) had B. koehlerae, and one (4%) had a B. volans–like sequence. Two subjects were co-infected with two Bartonella species, one with B. vinsonii subsp. berkhoffii plus B. henselae and one with B. koehlerae plus B. henselae. None of the control subjects had Bartonella detected in their blood or in BAPGM enrichment cultures (p≤0.0001), and no isolates were obtained.
Serology
Of the veterinary subjects, 18 (16%) had insufficient serum volume for serological testing. Of the 96 subjects with evaluable results from both serology and by enrichment culture/PCR-based detection, 23 had detectable Bartonella by enrichment culture/PCR and 73 had tested negative. Overall, 42 of 96 (44%) veterinary personnel had detectable Bartonella antibodies with an IFA titer of 1:64 or greater to at least one Bartonella species antigen. However, only 9 (39%) of the 23 subjects with detectable Bartonella DNA by PCR had detectable Bartonella antibodies. Thirty-four of 73 (47%) subjects who lacked detectable Bartonella DNA were seropositive. Of the healthy, nonveterinarian volunteers, only one individual had a 1:64 B. henselae antibody titer. There was no seroreactivity to B. koehlerae, or B. vinsonii subsp. berkhoffii.
Epidemiological associations
Neither age nor gender was associated with Bartonella infection among veterinary personnel. No specific type of animal or arthropod exposure was associated with Bartonella infection. A history of travel to Asia was more common among infected subjects (33% vs. 9%, p=0.006) (Table 2). Animal contact during travel, however, was not more common.
Clinical findings
In all, 110 of 114 veterinary subjects had complete clinical questionnaires (96%). Eighty-six of 110 (78%) had at least one positive symptom from the clinical questionnaire and 80 (73%) reported two or more symptoms. Bartonella was detected by enrichment culture/PCR in 28 (33%) subjects who had at least one symptom and 27 (34%) of those who had at least two symptoms (Table 2). Only two subjects (7%) who had detectable Bartonella were asymptomatic. Two or more symptoms were found in 90% of subjects with detectable Bartonella as compared with 71% of negative subjects (p=0.04). Most symptoms were equally common among infected and uninfected veterinary personnel. However, 70% of Bartonella-positive subjects described recurrent headache compared with 40% of negative subjects (p=0.009). Irritability was also more common (68% vs. 43%, p=0.04). The combination of headache and irritability was found in 16 (73%) infected subjects compared with 19 (37%) of uninfected subjects (p=0.005). Subjects with Bartonella were more likely to have received antibiotics in the previous year (60% vs. 35%, p=0.04), but were not more likely to have received corticosteroids or to have seen a medical subspecialist.
Discussion
Zoonotic infections are among the occupational hazards of veterinary medicine, potentially making veterinary personnel an ideal population in which to study the clinical spectrum of Bartonella infection. Although Bartonella spp. are well-known human pathogens, the insensitivity of traditional culture methods has left many unanswered questions, including whether bartonellosis is more common, has more diverse clinical features, or is more persistent than has been previously recognized.
In this study, we have demonstrated that a novel, sensitive enrichment culture method combined with molecular detection identified bloodstream infection with Bartonella spp. in 28% of tested veterinarians and veterinary personnel. The specificity of this culture method is supported by the lack of any PCR positive results among 32 nonveterinary volunteer subjects with limited animal exposure. Because B. henselae is the predominant flea-transmitted Bartonella spp. found in both cats and dogs, it is not surprising that B. henselae was the predominant species detected by PCR and enrichment blood culture in veterinary personnel, comprising 56% of isolates. B. vinsonii subsp. berkhoffii and B. koehlerae, both emerging pathogens, were also common, accounting for 28% and 22% of the positive subjects, respectively.
Among these veterinary personnel, the only specific exposure significantly associated with Bartonella infection was a history of travel to Asia. One-third of infected subjects had traveled to Asia, compared with 9% of uninfected subjects. Kosoy et al., using cell culture or BAPGM enrichment culture/PCR methodologies, found Bartonella DNA in 14 of 261 Thai patients with acute febrile illnesses (Kosoy et al. 2010). In contrast to the results from Thailand, where most patients were infected with a rodent Bartonella species, most of the veterinary personnel in this study were exposed to flea-transmitted Bartonella spp. that infect pet and stray cats and dogs. In a separate study, 27% of 336 Thai patients with fever had serologically confirmed or probable bartonellosis (Kosoy et al. 2010). This result was unexpected, and as such, our study did not address specific destinations within Asia, nor whether our subjects had experienced febrile illnesses during travel.
Of critical interest when evaluating our findings is whether Bartonella infection, when detected by our method, is associated with clinical illness. The increased frequency of headache and irritability among subjects with positive culture/PCR results suggest that not only are these individuals symptomatic, they also share certain clinical features. It is also noteworthy that subjects found to have positive cultures were more likely to have received antibiotics in the past year. An alternative interpretation is that infected subjects had increased health-seeking behaviors compared with uninfected subjects, and that their overall utilization of health care resources may have been higher. A longitudinal prospective study or follow-up of our veterinary cohort may illustrate whether our minimally symptomatic patients with Bartonella infection continued to have ongoing symptoms and increased health care utilization.
Interpretation of our results is complicated by the high rate of symptoms in our study population. While 44% of individuals with adequate serum volumes for IFA testing were seropositive and 28% were infected with Bartonella spp., 76% acknowledged at least one symptom on our questionnaire. Symptoms were far less common among our nonveterinary controls, but this population was significantly younger, and there may have been ascertainment bias toward sicker veterinary subjects. Chronic symptoms are common in the general population. Up to 20% of subjects in the general population report chronic fatigue, nearly half suffer moderate or severe chronic pain, and one-quarter describe some degree of cognitive dysfunction (Chen 1986, Croft et al. 1993, Buchwald et al. 1995, Luo et al. 2005). With such a high background rate of symptoms, it is difficult to establish causality without a longitudinal study design, a large study cohort, and age- and sex-matched controls. Additionally, we cannot exclude that these findings may be an artifact of the multiple comparisons. In our study, however, all but two culture-positive patients had at least one symptom, whereas 24 of 80 patients with negative cultures were asymptomatic. This supports the possibility that Bartonella infection, as detected by our method, is associated with symptoms.
Historically, the microbiological documentation of Bartonella in the blood has been possible almost exclusively in patients with endocarditis. Our cohort, on the other hand, was not nearly as ill as the typical endocarditis patient and lacked the physical signs and symptoms associated with that disease on physical exam. That human patients may tolerate low-grade Bartonella bacteremia is consistent with abundant animal data. A wide variety of feral, domestic, and agricultural mammals, including pet cats and dogs, have been found to have bloodstream carriage of various Bartonella spp. (Breitschwerdt and Kordick 2000). Asymptomatic Bartonella bacteremia is very common in domestic cats: In 10 studies conducted in numerous geographic regions, 167 of 457 total cats were found to have Bartonella spp. bacteremia (range 9.1%–89.5%). The duration of continuous bacteremia in some animals, when tested sequentially, has been greater than a year for both B. henselae and B. vinsonii subsp. berkhoffii.
An important question raised by this study is the prevalence of subacute Bartonella infection in the nonveterinary human population. Approximately 39% of American households own at least one dog and 33% own at least one cat, comprising tens of millions of persons with close domestic animal exposure (Oksi et al. 1995). It remains unexplored whether Bartonella bacteremia is a common occurrence for pet owners or persons in the pet industry.
Conclusions
In conclusion, our study supports an emerging body of evidence that human Bartonella bloodstream infection may be more frequent and more persistent than previously recognized. Further studies that test subjects at independent, blinded laboratories will be necessary to verify the findings in our study. Longitudinal studies of infected patients will help determine the natural course of Bartonella infection as detected by this method, if bacteremia is relapsing or sustained, and what clinical features are associated with Bartonella spp. infections. Current research does not address whether treatment is necessary or effective for patients with Bartonella infection as detected by our method. Finally, investigations into other populations, such as pet owners and persons employed by the pet care industry, will provide insight into the prevalence of Bartonella infection in these risk groups.
Acknowledgments
Dr. Lantos was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number KL2TR001115. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This work was supported by the Southeastern Center for Emerging Biological Threats. Additionally, Brandy Ferguson was supported by a training grant from the Clinical and Translational Science Awards consortium.
We thank the executive board of the North American Veterinary Conference for facilitating blood sample collection, Barbara Hegarty for production of the cell culture grown Bartonella spp. antigens used in this study, and Julie Bradley for performing the serological testing.
Author Disclosure Statement
Ricardo G. Maggi is Chief Technical Officer, Galaxy Diagnostics, Research Triangle Park, NC. Edward B. Breitschwerdt is Chief Scientific Officer, Galaxy Diagnostics, Research Triangle Park, NC. No competing financial interests exist for the remaining authors.
References
- Arvand M, Schad SG. Isolation of Bartonella henselae DNA from the peripheral blood of a patient with cat scratch disease up to 4 months after the cat scratch injury. J Clin Microbiol 2006; 44:2288–2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoub EM, McBride J, Schmiederer M, Anderson B. Role of Bartonella henselae in the etiology of Henoch-Schonlein purpura. Pediatr Infect Dis J 2002; 21:28–31 [DOI] [PubMed] [Google Scholar]
- Breitschwerdt EB, Kordick DL. Bartonella infection in animals: Carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. Clin Microbiol Rev 2000; 13:428–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitschwerdt EB, Kordick DL, Malarkey DE, Keene B, et al. . Endocarditis in a dog due to infection with a novel Bartonella subspecies. J Clin Microbiol 1995; 33:154–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitschwerdt EB, Atkins CE, Brown TT, Kordick DL, et al. . Bartonella vinsonii subsp. berkhoffii and related members of the alpha subdivision of the Proteobacteria in dogs with cardiac arrhythmias, endocarditis, or myocarditis. J Clin Microbiol 1999; 37:3618–3626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitschwerdt EB, Maggi RG, Duncan AW, Nicholson WL, et al. . Bartonella species in blood of immunocompetent persons with animal and arthropod contact. Emerg Infect Dis 2007; 13:938–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitschwerdt EB, Maggi RG, Nicholson WL, Cherry NA, et al. . Bartonella sp. bacteremia in patients with neurological and neurocognitive dysfunction. J Clin Microbiol 2008; 46:2856–2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitschwerdt EB, Maggi RG, Lantos PM, Woods CW, et al. . Bartonella vinsonii subsp. berkhoffii and Bartonella henselae bacteremia in a father and daughter with neurological disease. Parasites Vectors 2010; 3:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitschwerdt EB, Mascarelli PE, Schweickert LA, Maggi RG, et al. . Hallucinations, sensory neuropathy, and peripheral visual deficits in a young woman infected with Bartonella koehlerae. J Clin Microbiol 2011; 49:3415–3417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchwald D, Umali P, Umali J, Kith P, et al. . Chronic fatigue and the chronic fatigue syndrome: Prevalence in a Pacific Northwest health care system. Ann Int Med 1995; 123:81–88 [DOI] [PubMed] [Google Scholar]
- Cadenas MB, Bradley J, Maggi RG, Takara M, et al. . Molecular characterization of Bartonella vinsonii subsp. berkhoffii genotype III. J Clin Microbiol 2008; 46:1858–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MK. The epidemiology of self-perceived fatigue among adults. Prev Med 1986; 15:74–81 [DOI] [PubMed] [Google Scholar]
- Chomel BB, Kasten RW, Sykes JE, Boulouis HJ, et al. . Clinical impact of persistent Bartonella bacteremia in humans and animals. Ann NY Acad Sci 2003; 990:267–278 [DOI] [PubMed] [Google Scholar]
- Croft P, Rigby AS, Boswell R, Schollum J, et al. . The prevalence of chronic widespread pain in the general population. J Rheumatol 1993; 20:710–713 [PubMed] [Google Scholar]
- Dehio C. Pathogenesis of Bartonella (Rochalimaea) infections. Bull Inst Pasteur 1997; 95:195–207 [Google Scholar]
- Dehio C. Bartonella interactions with endothelial cells and erythrocytes. Trends Microbiol 2001; 9:279–285 [DOI] [PubMed] [Google Scholar]
- Del Prete R, Fumarola D, Ungari S, Fumarola L, et al. . Polymerase chain reaction detection of Bartonella henselae bacteraemia in an immunocompetent child with cat-scratch disease. Eur J Pediatr 2000; 159:356–359 [DOI] [PubMed] [Google Scholar]
- Hashkes PJ, Trabulsi A, Passo MH. Systemic cat-scratch disease presenting as leukocytoclastic vasculitis. Pediatr Infect Dis J 1996; 15:93–95 [DOI] [PubMed] [Google Scholar]
- Jacobs RF, Schutze GE. Bartonella henselae as a cause of prolonged fever and fever of unknown origin in children. Clin Infect Dis 1998; 26:80–84 [DOI] [PubMed] [Google Scholar]
- Jacomo V, Kelly PJ, Raoult D. Natural history of Bartonella infections (an exception to Koch's postulate). Clin Diagn Lab Immunol 2002; 9:8–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordick DL, Breitschwerdt EB. Intraerythrocytic presence of Bartonella henselae. J Clin Microbiol 1995; 33:1655–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordick DL, Breitschwerdt EB. Persistent infection of pets within a household with three Bartonella species. Emerg Infect Dis 1998; 4:325–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosoy M, Bai Y, Sheff K, Morway C, et al. . Identification of Bartonella infections in febrile human patients from Thailand and their potential animal reservoirs. Am J Trop Med Hyg 2010; 82:1140–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Scola B, Raoult D. Culture of Bartonella quintana and Bartonella henselae from human samples: A 5-year experience (1993 to 1998). J Clin Microbiol 1999; 37:1899–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo N, Johnson JA, Shaw JW, Feeny D, et al. . Self-reported health status of the general adult U.S. population as assessed by the EQ-5D and Health Utilities Index. Med Care 2005; 43:1078–1086 [DOI] [PubMed] [Google Scholar]
- Maggi RG, Breitschwerdt EB. Potential limitations of the 16S–23S rRNA intergenic region for molecular detection of Bartonella species. J Clin Microbiol 2005; 43:1171–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi RG, Duncan AW, Breitschwerdt EB. Novel chemically modified liquid medium that will support the growth of seven bartonella species. J Clin Microbiol 2005; 43:2651–2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi RG, Mascarelli PE, Pultorak EL, Hegarty BC, et al. . Bartonella spp. bacteremia in high-risk immunocompetent patients. Diagn Microbiol Infect Dis 2011; 71:430–437 [DOI] [PubMed] [Google Scholar]
- Oksi J, Uksila J, Marjamaki M, Nikoskelainen J, et al. . Antibodies against whole sonicated Borrelia burgdorferi spirochetes, 41-kilodalton flagellin, and P39 protein in patients with PCR- or culture-proven late Lyme borreliosis. J Clin Microbiol 1995; 33:2260–2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolain JM, Foucault C, Guieu R, La Scola B, et al. . Bartonella quintana in human erythrocytes. Lancet 2002; 360:226–228 [DOI] [PubMed] [Google Scholar]
- Tsukahara M, Tsuneoka H, Iino H, Murano I, et al. . Bartonella henselae infection as a cause of fever of unknown origin. J Clinical Microbiol 2000; 38:1990–1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JW, Pien BC, Ardoin SA, Anderson AM, et al. . A man with chest pain and glomerulonephritis. Lancet 2005; 365:2062. [DOI] [PubMed] [Google Scholar]


