Abstract
The WRKY family of transcription factors (TFs) play an intricate role in regulating the stress signaling pathways by autoregulation or may be by cross regulation through interaction with other proteins. Although WRKY TFs are considered to be plant specific, however, their presence has been reported from unicellular algae, slime mould, and gymnosperms. We have isolated the JcWRKY cDNA from an important biofuel crop Jatropha curcas growing in the wastelands of India. The JcWRKY gene has an ORF of 693 bp and encodes a 230 amino acids protein with estimated molecular mass of 25.25 kDa. JcWRKY shows close homology to FaWRKY1 and St-WRKY1. The JcWRKY contains seven potential phosphorylation sites, which might be involved in regulating its function. The transcript analysis revealed that the JcWRKY transcript gets upregulated in response to salinity, dehydration, salicylic acid (SA), methyl jasmonate (MeJa), and collar rot fungus Macrophomina. However, maximum expression is observed under SA, highlighting its role in enhancing systemic acquired resistance for disease tolerance. The JcWRKY recombinant protein showed binding to W-box of pathogenesis related-1 (PR-1) and iso1 (encoding isoamylase1) promoters. Overexpression of JcWRKY in Escherichia coli enhanced the growth of cells in NaCl, KCl, mannitol, sorbitol, SA, and MeJa treatments, indicating that it protects and promotes growth under ionic, osmotic, and chemical stresses. The enhancement in growth can be due to the regulation of stress responsive genes. Therefore, it can be used as an important gene for enhancing abiotic and biotic resistance in plants and to facilitate faster growth of E. coli cells under stress conditions for efficient expression.
Introduction
Plants are exposed to multifarious environmental conditions in the form of different abiotic and biotic stresses such as drought, light, temperature, insects, and microbes. The survival and productivity of all higher plants are dependent on their ability to adapt themselves to these varying adverse conditions. The response and adaptation of plants to the multigenic abiotic stress and the monogenic biotic stress involve an array of physiological and biochemical mechanisms. The abiotic stress being multigenic and qualitative is difficult to manipulate as compared to biotic stress and there exists significant cross talk among the different stress signaling pathways. Although significant advances have been made in understanding the mechanism of stress regulation at the physiological, biochemical, and molecular levels, however, the challenge to deploy the knowledge toward enhancing crop productivity still persists.
Plants are exposed to multiple stresses simultaneously, and have developed intricate mechanisms to integrate a wide range of tissue, developmental, and environmental signals to regulate complex patterns of gene expression established over a long period of evolution as sessile organisms (Wu et al., 2007). In plants, gene expression is largely controlled at the level of transcription by a diverse group of transcription factors (TFs). These TFs have widely expanded in plants probably due to significant variability and complexity of plant metabolism, as compared with other organisms (Shiu et al., 2005). Different TFs such as NAC, MYB, DREB, and WRKYs have been identified in plants, which play vital roles in plant growth, development, as well as abiotic and biotic stresses.
The WRKY genes comprise the largest family of plant-specific TFs, and play a broad role as positive and negative regulators in plant defense regulation, abiotic stresses (Agarwal et al., 2011), as well as growth and development (Ulker and Somssich, 2004). Although WRKY genes show evolution from unicellular to complex multicellular forms, however, to date, they are reported in large numbers in flowering plants. The first WRKY gene was identified in sweet potato and since then, WRKY proteins have been found in a large number of higher plants. WRKY proteins are defined primarily by the highly conserved 60 amino acid WRKY domain, comprising a highly conserved WRKY motif at the amino (N)-terminus and a novel metal chelating zinc finger at the carboxy (C)-terminus. The WRKYs are classified on the basis of the number of WRKY motifs present and the composition of the zinc finger motif. Group I contains two WRKY domains, while Group II and Group III possess a single WRKY domain. Group I and II have the same C2H2-type zinc finger motif (C-X4–5-C-X22–23-H-X-H), whereas Group III shows altered C2HC zinc finger motif (C-X7-C-X23-H-X-C). Group II is further divided into subgroups a–e on the basis of additional amino acid motifs present outside the WRKY domain. In plants, WRKY genes show functional diversity largely through the expression divergence, in contrast to sequence divergence (Babu et al., 2006). Interestingly, the Group III members comprising about 20% of the WRKY family in higher plants, evolved late in land plants. In Arabidopsis, nearly all Group III members respond to diverse biotic stresses (Dong et al., 2003; Kalde et al., 2003), suggesting that this group of genes plays an important role in plant adaptation. The Group III genes are greatly amplified in monocots (Zhang and Wang, 2005). Interestingly, rice WRKY genes of Group III are evolutionarily more active, as they evolved due to tandem and segmental gene duplication compared with those of Arabidopsis (Wu et al., 2005).
Therefore, WRKY TFs with a broad spectrum of functions, integrating and cross-talking with different signal transduction pathways are emerging as interesting TFs for providing multiple stress tolerance. Equally important is the isolation of these TFs from stress-tolerant plants like Jatropha curcas, an alternative nonfeed bioenergy resource being advocated for growing on wastelands. Jatropha native to tropical America belongs to the Euphorbiaceae family and is represented by 172 species, a plant with great social and economic importance, valued for its wide range of applications. Although a lot of enthusiasm and excitement exist over the potential of Jatropha, a number of uncertainties and criticism too persist, largely due to lack of scientific knowledge and failure in the ability to domesticate this crop. The productivity of Jatropha is largely hampered by abiotic factors like salinity, drought, and biotic factors, including fungi and viruses (Johnson et al., 2011). Among the different fungal diseases, collar rot causes severe loss for J. curcas plantations on the wasteland near Bhavnagar, Gujarat, India. It is important to isolate and characterize abiotic and biotic stress responsive TFs from an important biofuel crop Jatropha, so that they can be genetically engineered in Jatropha itself to upregulate a cascade of stress responsive genes to enhance abiotic stress and disease resistance. In this study, we have isolated the WRKY TF from Jatropha and studied its role in response to multiple stress tolerance.
Materials and Methods
Plant material and stress treatments
One-year-old plants of J. curcas (accession no. IC565735; CSMCRI), grown in plastic pots and maintained at 25°C±2°C, a 16-h photoperiod at a photon flux intensity of 200 μmol m−2 s−1 and 85% relative humidity, were used for the present study. Macrophomina phaseolina culture (ID no. 6022) was obtained from IARI (Indian Agricultural Research Institute).
To study the expression of the JcWRKY gene transcript, the 1-year-old seedlings were subjected to different stress treatments. Before giving the treatment, the seedlings were not watered for 3 days. The different stress treatments were as follows: (1) Salt treatment: The pot soil with seedlings was irrigated with 500 mL of 250 mM NaCl solution. (2) Methyl jasmonate (MeJa) treatment: The pot soil with seedlings was irrigated with 500 mL of 100 μM of MeJa (Sigma Aldrich) solution. (3) Salicylic acid (SA) treatment: The pot soil with seedlings was irrigated with 500 mL of 2.5 mM of SA solution. (4) Macrophomina treatment: Inoculum was prepared by growing the culture on potato dextrose agar plates at 26°C for 5 days. The fungal mat with fungal mycelia and microsclerotia was separated and macerated in sterile water. Microsclerotia were then counted in the suspension and the final inoculum concentration was made to 300±20 microsclerotia/mL in sterilized water. Each pot was irrigated with 500 mL of suspension. Another set of seedlings was maintained under control conditions. For all treatments, leaf tissue was collected after 12, 24, and 48 h of treatment and kept at −80°C until use.
Isolation of WRKY TF cDNA
The leaf tissue of J. curcas was ground under liquid nitrogen to a fine powder with cold mortar and pestle. Total RNA was isolated from the leaf tissues using Raflex TM solution I and solution II (GeNei) as per the manufacturer's protocol. First-strand cDNA synthesis was done using the cDNA synthesis kit (Invitrogen). The degenerate and oligo dT primers used are as follows: G2PAF1-5′GCWMGNGTNTCNGTNMGAGC3′, G2PAF2-5′CCWMGDGCHTAYTATMGATGC3′, G2PAF3-5′ AARCARGTDCARMGRTGY3′, PAOligodT-5′CAGACGAGAGTGTGGAGGACTGCTGCTGGTGTAGCTTTTTTTTTTTTTTTTTT3′, PAR1-5′CAG ACGAGAGTGTGGAGG3′, and PAR2 5′GACTGCTGCTGGTGTAGC3′. Using the above primers, amplicons of varying lengths were obtained. The fragments were cloned in the pJET 1.2 vector (MBI Fermentas) and sequenced. One of the clones was made full length by 5′ RACE (Invitrogen) with the following primers JcWRKYR1, 5′AACAAGTTGCATTTATTACGAGA3′, JcWRKYR2, 5′CAGCGTTGAACCTTCTTCTTCG3′, and JcWRKYR3, 5′AACCCTTCACCTGCTTCTGGCC3′. The clone was sequenced at Macrogen. The sequence was analyzed by the BLAST program provided by NCBI (National Center of Biotechnology Information (www.ncbi.nih.gov/). The WRKY amino acid sequences of different plants were obtained from the NCBI database and a phylogenetic tree of the amino acid sequences was constructed using the DNAMAN software. Secondary structure prediction was carried out by ExPASy tools (www.expasy.ch/tools/).
Expression analysis of the JcWRKY gene transcript by semiquantitative RT-PCR
Total RNA was extracted from J. curcas stress-treated (as mentioned above) and control leaves. RNA was then treated with DNaseI (MBI Fermentas), followed by first-strand cDNA synthesis using Superscript III Reverse transcriptase (Invitrogen). The expression of JcWRKY was studied. The reverse transcriptase PCR was carried out using the stress-treated and control cDNAs as template, gene-specific primers for JcWRKY (F-5′ATGCAGGGGGAATATGAGACATAC3′ and R-5′ TTACAATTTTGTAGACTCTATATC3′) and actin gene primers (F-5′TGAGTCACACTGTGCCAATT3′ and R-5′TGCAATACCAGGGAACATAG3′), with the following PCR conditions 94°C, 2 min 1 cycle; 94°C, 1 min; 54°C, 1 min and 72°C, 1 min 28 cycles, and last 72°C, 7 min for 1 cycle. The band intensities of both the WRKY and actin genes were quantified using Gene tool analysis software (Syngene) and plotted as relative value with respect to the actin gene band for each treatment separately. The experiment was repeated thrice independently, and the mean, standard deviation, and Student's t-test were performed using Microsoft Excel. Asterisks (*) denote standard deviation (SD), where p≤0.05.
Cloning of JcWRKY into an Escherichia coli expression vector, isolation and purification of the recombinant protein
The coding region of JcWRKY was PCR amplified from pJET1.2 (MBI Fermentas) using two primers (forward 5′CGCGGATCCATGCAGGGGGAATATGAG3′ and reverse 5′CCGCTCGAGTTACAATTTTGTAGACTC3′), with the flanking restriction sites of BamH1 in the forward primer and XhoI site in the reverse primer. The amplified product was gel purified, digested with BamHI and XhoI, and cloned into the BamHI and XhoI sites of pET-28a expression vector (Novagen). The recombinant plasmid and vector plasmid were transformed in the BL21 (DE3) star Escherichia coli strains (pET28a+JcWRKY). The BL21 (DE3) star cells transformed with the pET28a vector alone were used as control. The recombinant plasmid transformed in BL21 (DE3) star E. coli strains (pET28a+JcWRKY) was induced with 1 mM IPTG and cells were harvested after 2, 4, and 12 h of induction and the recombinant protein was purified under native condition to homogeneity using the Ni-NTA Fast Start Kit (Qiagen) following the manufacturer's protocol.
DNA probes and gel mobility shift assay
Two sets of complementary oligonucleotides, PR-1F 5′TTATTCAGCCATCAAAGTTGACCAATAATACC3′ and PR-1R-5′GGTATTATTGGTCAACTTTGATGGCTGAATAA3′, were synthesized for the W-box (TGAC) element derived from the parsley pathogenesis related-1 (PR-1) promoter (Rushton et al., 1996) and another iso1F 5′TCGCTAACCAGTGACTTCCACGTTTCATCATTTATT3′ iso1R5′AATAAATGATGAAACGTGGAAGTCACTGGTTAG CGA3′ for W-box from the iso1 promoter (Sun et al., 2003). Three micrograms of each complementary oligonucleotide belonging to W-box from two different promoters was annealed separately using the annealing buffer (100 mM Tris-HCl, 5 mM NaCl, and 10 mM EDTA) by incubating at 60°C for 5 min and the temperature was brought down to 37°C for 15 min. The electrophoretic mobility shift assay (EMSA) was carried out using the EMSA kit (Invitrogen) with an 80 ng probe and 500 to 1100 ng of purified protein at room temperature for 20 min. The reaction was terminated by adding 10×DNA loading dye and fractionated on nondenaturing 8% acrylamide gel in 0.5×TBE buffer. The protein electrophoresis unit was kept in an ice box to maintain low temperature of the running buffer. Before loading the experimental reactions, the gel was prerun at 120 V for 30 min and then the reactions were loaded and run at 120 V for 2 h. The gel was stained with ethidium bromide.
Functional analysis of E. coli cells transformed with JcWRKY under different stresses
Spot and liquid culture assay
The effect of NaCl, KCl, mannitol, sorbitol, SA, and MeJa was studied on the growth of E. coli cells containing the recombinant plasmid and vector plasmid with the help of spot and liquid culture assay. E. coli cells were grown in the Luria Bertani (LB) medium to an OD600 0.6. Thereafter, 1 mM IPTG was added and cells were grown for 12 h at 37°C, and then diluted to 10−2, 10−3, and 10−4. Ten microliters from each dilution was spotted on LB basal plates and on plates supplemented with 500 mM NaCl, 500 mM KCl, 400 mM mannitol, 100 mM sorbitol, 2.5 mM SA, and 150 μM MeJa. The experiment was repeated thrice independently. For liquid assay, E. coli cells were grown as for spot assay, diluted at OD600 to 0.6. Then, 400 μL cells were inoculated in 10 mL of LB basal medium and a medium containing 500 mM NaCl, 500 mM KCl, 400 mM mannitol, 1000 mM sorbitol, 2.5 mM SA, and 150 μM MeJa. The bacterial suspension was allowed to grow at 37°C at 160 rpm and harvested at every 2 h up to 12 h for growth measurements at OD600. The experiment was repeated thrice independently, and the mean, standard deviation, and Student's t-test were performed using Microsoft Excel. Asterisks (*) denote standard deviation (SD), where p≤0.05.
Results
Cloning and sequence analysis of JcWRKY TF cDNA from J. curcas
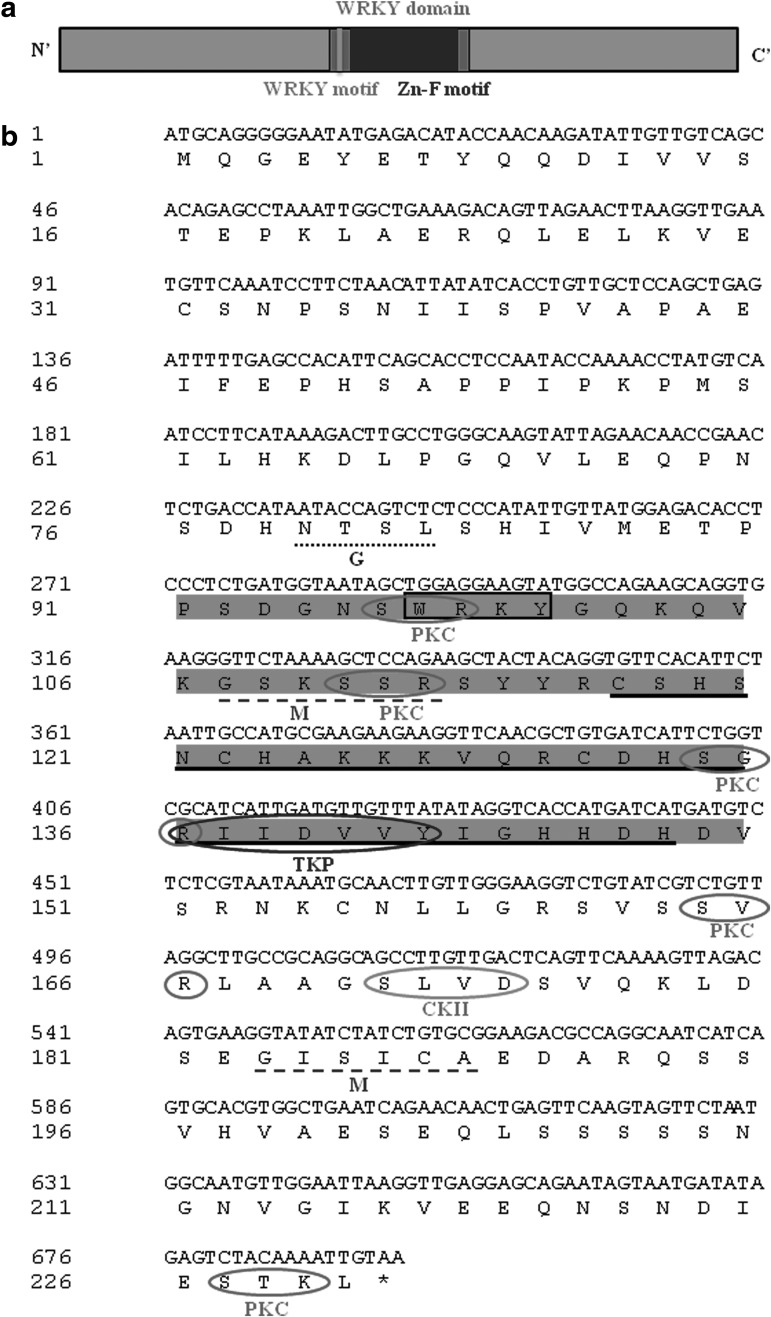
The JcWRKY clone of 533 bp was amplified using the degenerate primers. The JcWRKY clone had an open reading frame of 693 bp (NCBI acc no KC191643), encoding a protein of 230 amino acid, with a calculated molecular weight of 25.25 kDa and predicted pI of 5.13. The JcWRKY protein shows the presence of one WRKY domain with C2H2-type (C-X4-5-C-X22-23-H-X-H) configuration of one zinc finger-like motif (Fig. 1a, b); therefore, it gets classified in Group II WRKY proteins. The WRKY domain comprises of 60 amino acids extending from proline 91 to valine 150. The PROSITE analysis reveals the presence of N-glycosylation site (NTSL, 79–82), five protein kinase C (PKC) phosphorylation sites (SwR 96–98, SsR 110–112, SgR 134–136, SvR 164–166, StK 227–229), two N-myristoylation sites (GSksSR 107–112 and GIsiCA 183–188), tyrosine kinase phosphorylation site (136–142 RiiDvvY), Casein kinase II (CKII) phosphorylation site (SlvD 171–174), and microbodies C-terminal targeting signal (228–230 TKL). The deduced JcWRKY amino acid showed 46% identity to FaWRKY1 (Fragaria x ananassa) and St-WRKY1 (Solanum tuberosum). Phylogenetic relationships among some WRKY proteins show that JcWRKY forms an independent branch although it gets clustered with Group II WRKY proteins (Fig. 1c). The secondary structure of JcWRKY was predicted using the PSIPRED protein structure prediction server (ExPASy tools) and revealed the presence of five coils and one alpha-helix and three strands (Fig. 1d).
FIG. 1.
(a) Schematic representation of JcWRKY protein. The characteristic domains found in WRKY proteins are indicated to show their relative positions along the protein. Zn-F stands for zinc finger motif. (b) The sequence represents 690 bp cDNA of JcWRKY and its amino acid sequence. Highlighted parts represent the WRKY domain. Box represents the WRKY motif, the zinc finger motif is underlined with solid lines. The residues targeted for phosphorylation are circled with different color solid lines and indicated by PKC, TKP, and CKII. The N-myristoylation sites (M) are underlined with dashed lines and the N-glycosylation site (G) is underlined with a dotted line. (c) Relationship among WRKY proteins is represented by the phylogenetic tree of amino acid sequences constructed using DNAMAN software. Scale represents the branch length. The sequences used are from JcWRKY (KC191643), FaWRKY1 (EU727547), StWRKY1 (AJ278507), VvWRKY1 (AY585679), AtWRKY6 (AF331712), HvWRKY38 (AA548544), OsWRKY24 (BK005027), CaWRKY2 (DQ402421), TcWRKY53 (EF053036), AtWRKY25 (NM_128578), AtWRKY38 (NM_ 122163), AtWRKY62 (NM_120268), and AtWRKY53 (NM_118512). (d) Secondary structure prediction by ExPASy tools.
Differential expression analysis of WRKY TFs under different stress conditions
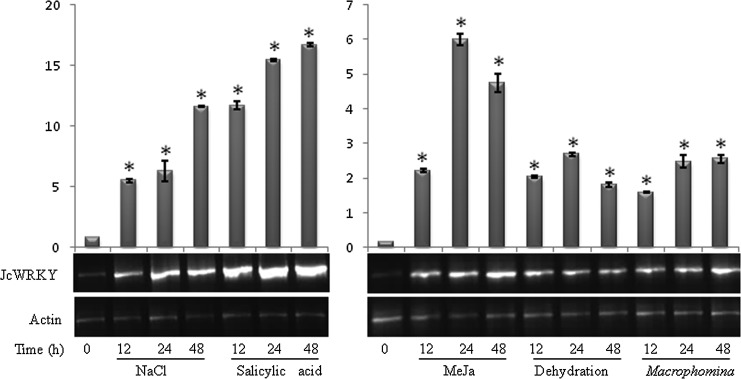
To study the transcript expression of JcWRKY in response to various stress conditions, semiquantitative RT-PCR was performed (Fig. 2). The Student's t-test showed significant variation with respect to control (0 h) in response to all the treatments for JcWRKY transcript expression. The JcWRKY gene showed increased transcript accumulation in response to all the stresses, showing maximum expression with SA ranging from 14-fold to 21-fold as compared with control, during 12–48 h of duration. The minimum expression is observed in case of Macrophomina treatment ranging from 10- to 16-fold, increasing with increasing time duration from 12 to 48 h.
FIG. 2.
Transcript analysis of JcWRKY gene in response to 250 mM NaCl, 2.5 mM salicylic acid (SA), 100 μM methyl jasmonate (MeJa), dehydration, and Macrophomina fungus for 12, 24, and 48 h of duration. The 0-h time point served as the control. Graphs over each gel figure show the relative value of JcWRKY against the actin gene to show the quantitative increase in the expression levels. The asterisk (*) denotes significant variation of JcWRKY transcript at specific time point with respect to control (0 h) in response to the treatments at p≤0.05.
DNA-binding property of JcWRKY to specific cis element
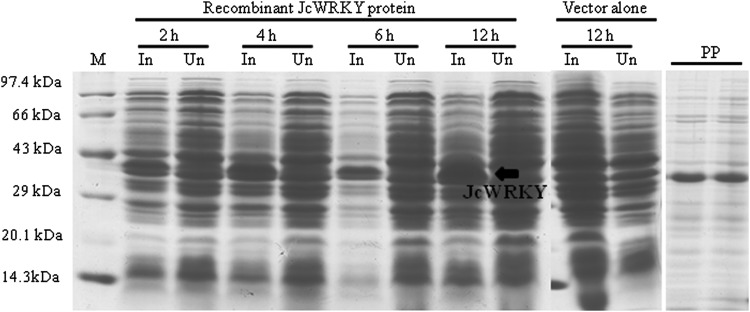
The 6X His-Tag fused JcWRKY recombinant protein was induced with 1 mM IPTG for different time periods. The protein showed maximum induction at 12 h; therefore, the protein was induced for 12 h and purified to near homogeneity (Fig. 3). The purified recombinant protein (without thrombin cleavage) was used to study JcWRKY protein–W-box DNA interactions by EMSAs. The recombinant protein showed binding to both the W-box from PR-1 and iso1 promoters (Fig. 4b, c). The strength of binding increased on increasing the amount of recombinant protein from 600 to 1100 ng with an 80 ng probe. The heterologous probe (MCS of pBSK+) did not bind to the recombinant protein (Fig. 4a).
FIG. 3.
SDS-PAGE analysis of induced and uninduced JcWRKY recombinant protein in Escherichia coli BL21 (DE3) star cells. M marker, Lanes 1, 3, 5, 7 induced protein with 2, 4, 6, and 12 h of induction. Arrow represents the induced recombinant JcWRKY protein. Lanes 2, 4, 6, 8 uninduced protein with 2, 4, 6, 12 h of growth. Lanes 9, 10 induced and uninduced vector alone, respectively. Lanes PP represent purified recombinant protein.
FIG. 4.
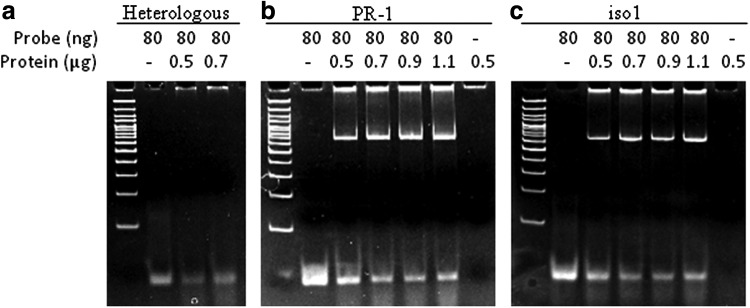
Electrophoretic mobility shift assay (EMSA) study showing binding of JcWRKY protein with heterologous probe (a) and W-box elements of pathogenesis related-1 (PR-1) promoter (b) and iso1 promoter (c).
Expression of JcWRKY protein promotes the growth of E. coli cells under stress conditions
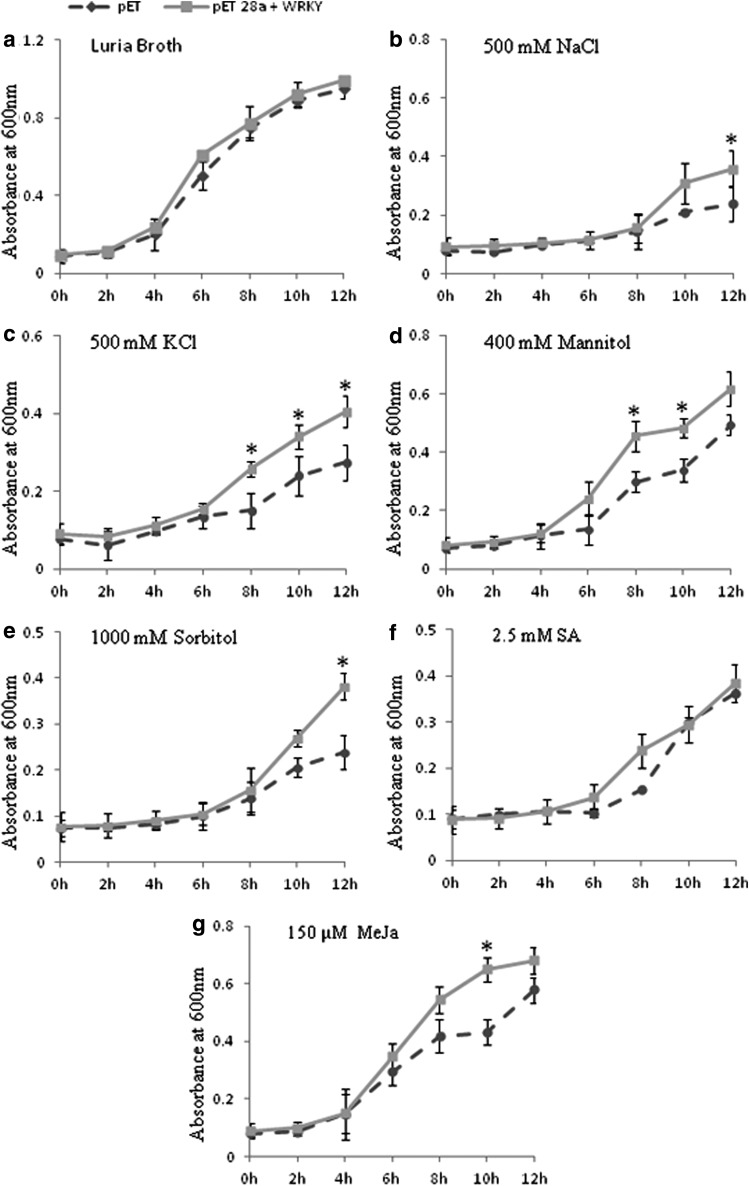
To evaluate the protective properties of JcWRKY to different stress treatments, the growth of E. coli cells containing the plasmid JcWRKY and vector pET28a was analyzed both quantitatively (liquid assay in a broth, Fig. 5) and qualitatively (spot assay on a semisolid medium, Fig. 6). The recombinant and wild-type cells showed similar growth under control conditions in both semisolid and broth media. However, the recombinant cells showed enhanced growth under stress conditions as compared with wild-type cells. In response to ionic stress in the NaCl and KCl supplemented media, the growth of recombinant cells was significantly higher at 12 h with NaCl and at 8, 10, and 12 h with KCl. Similarly, osmotic stress imposed by mannitol (8 and 10 h) and sorbitol (12 h) treatments imparted a significantly higher growth compared with wild-type cells. The secondary signaling molecules, involved in biotic stress regulation like SA and MeJa, were also used to study their effect on E. coli growth; both the treatments inhibited the growth of wild-type cells, however, significant growth was observed at 10 h with MeJa treatment.
FIG. 5.
The growth analysis of E. coli cells harboring JcWRKY recombinant plasmid and pET28a alone in the Luria Bertani (LB) liquid medium and with different supplements. (a) LB medium. (b) 500 mM NaCl. (c) 500 mM KCl. (d) 1,000 mM sorbitol. (e) 400 mM mannitol. (f) 2.5 mM SA. (g) 150 μM MeJa. OD600 was recorded at a 2-h interval up to 12 h and mean values are represented in graph. The asterisk (*) denotes significant variation at specific time points between JcWRKY recombinant plasmid and pET28a alone at p≤0.05.
FIG. 6.
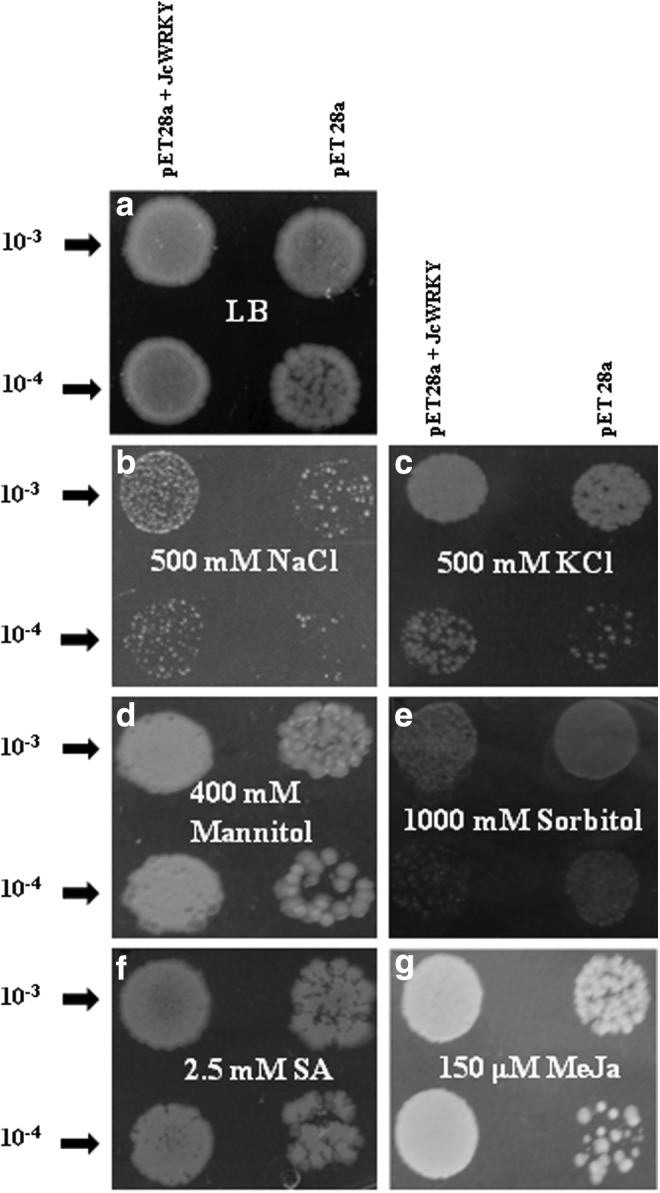
The growth analysis of E. coli cells containing JcWRKY recombinant plasmid and pET28a alone in the LB medium (solid) with different supplements. (a) LB only. (b) 500 mM NaCl. (c) 500 mM KCl. (d) 1,000 mM sorbitol. (e) 400 mM mannitol. (f) 2.5 mM SA. (g) 150 μM MeJa.
Discussion
The WRKY TFs form a complex network in the stress-regulated signal transduction pathways. Interestingly, a single WRKY TF is involved in regulating different stresses both as negative and positive regulators through protein–protein interaction, autoregulation, by binding to W-box of their own promoters, and also through cross-regulation by binding to the W-box of other promoters (Rushton et al., 2010). The JcWRKY TF shows the presence of seven potential phosphorylation sites, indicating that phosphorylation might be playing an important role in regulating its activity. The activity of WRKY TFs is modulated by phosphorylation (Agarwal et al., 2011) as WRKY TFs act downstream of various MAPKs in regulating plant gene activation. The diversification of this gene family in plants occurred in response to pressures imposed by environmental factors and plant pathogens and it plays an important role in both abiotic and biotic stress signaling (Agarwal et al., 2011). The microarray analysis of the Arabidopsis root reveals upregulation of 18 WRKY genes and downregulation of eight WRKY genes in response to salinity stress (Jiang and Deyholos, 2006); similarly, in soybean, 39% of WRKY genes were induced by abiotic stresses (Zhou et al., 2008). The JcWRKY showed accumulation in response to salinity and dehydration stress and also with both SA and MeJa, the two important endogenous defense signaling molecules involved in systemic acquired resistance (SAR) and disease resistance. Interestingly, JcWRKY showed induction with M. phaseolina, the causative agent of collar rot in Jatropha. Earlier, we reported the induction of JcPR-10a gene in response to the Macrophomina fungus and that the recombinant JcPR-10a protein inhibits the growth of this fungus (Agarwal et al., 2013). Similar reports on different WRKY TFs on imparting fungal stress tolerance have been published. The FaWRKY1 from strawberry showed induction with Colletotrichum acutatum infection, treatments with elicitors, and also by wounding (Villarejo et al., 2009). Its Arabidopsis sequence homologue, AtWRKY75, is reported in regulating phosphate starvation responses (Devaiah et al., 2007) and its overexpression in Arabidopsis leads to oxidative burst and suppression of hyphal growth of Sclerotinia sclerotiorum (Chen et al., 2013). St-WRKY1, another Group II WRKY TF showed induction in response to Erwinia carotovora. However, no induction was detected on treatment with SA, MeJa, ethylene, or wounding (Dellagi et al., 2000). VvWRKY1 expression was upregulated by SA, ethylene, hydrogen peroxide, wounding, and its overexpression resulted in reduced fungal susceptibility (Marchive et al., 2007). Marè et al. (2004) report the transcript accumulation of Hv-WRKY38 in response to low temperature and dehydration.
The WRKY proteins bind specifically to a cis-element TTGAC(C/T), termed the W-box. The W-box is found in the promoters of a large number of plant defense-related genes (Eulgem et al., 2000), including WRKY itself. The elicitor response element (W-box) in tobacco class I chitinase gene, CHN50 promoter, is recognized by WRKY proteins (Yang et al., 1999). A recent microarray study revealed that the W-box is an important element in the promoter element of defense-related genes, including PR-1, a marker gene for SAR (Maleck et al., 2000). Chromo immunoprecipitation studies revealed that the PcWRKY1 protein binds to the W-boxes of its own promoter as well as with the promoter of PcWRKY3 (Turck et al., 2004); similarly, AtWRKY33 also regulates its own expression (Mao et al., 2011). JcWRKY showed binding to the W-box element of the PR-1 promoter and iso1 promoter. The PR-1 gene is a marker gene for SAR, whereas the barley iso1 gene is sugar inducible (Sun et al., 1999), suggesting the involvement of JcWRKY in defense as well as the sugar signaling pathway.
The overexpression of JcWRKY enhanced the growth of E. coli cells under different stress treatments. Recently, some plant genes and TFs have been analyzed for their stress tolerance potential employing the E. coli heterologous expression system. The SbSI-1, a novel salt inducible gene from S. brachiata cells, showed tolerance to desiccation and salinity stress in E. coli cells (Yadav et al., 2012). ThPOD3 from Tamarix hispida could confer drought tolerance in E. coli (Guo et al., 2010). A cytoplasmic Hsp70 (PgHsc70) from Pennisetum glaucum showed protective in vitro chaperone activity against damage caused by heat and salinity, when expressed in E. coli (Reddy et al., 2010). Liu and Zheng (2005) reported that PM2 a Group III LEA protein from soybean enhanced salt tolerance to E. coli cells, further, the duplication of the amino acid 129–262, (22-mer repeating region) lead to much further survival ratios. Similarly, other LEA genes, PM11 (Group I) and PM30 (Group III) also showed increased salt stress tolerance in E. coli (Lan et al., 2005). Earlier, the mangrove allene oxide cyclase (AOC) gene enhanced the salt tolerance of E. coli, yeast, and tobacco cells (Yamada et al., 2002). We have earlier studied that the JcPR-10a protein retards the growth of E. coli cells under stress conditions (Agarwal et al., 2013). Similarly, the role of SbDREB2A (Gupta et al., 2010) and MuNAC4 TFs (Pandurangaiah et al., 2013) also showed the stress tolerance in using the E. coli heterologous system.
The enhanced growth of JcWRKY harboring E. coli cells can be attributed to its binding to the promoter of either stress-responsive bacterial genes or to some other TFs; otherwise, might be acting directly as protein enhancing/favoring growth under stressful conditions. Although the WRKY TF family is considered to be plant specific (Eulgem et al., 2000), they have also been found in nonplant species, for example, Giardia lamblia, a primitive protozoan, Dictyostelium discoideum, a slime mold (Glockner et al., 2002; Pan et al., 2009), Chlamydomonas reinhardtii, unicellular green algae (Rushton et al., 2010), and gymnosperm Pinus monticola (Liu and Ekramoddoullah, 2009). Babu et al. (2006), suggest that a probable evolutionary pathway for emergence of the WRKY-GCM1 superfamily was from classical C2H2 zinc fingers (Znf) through an intermediate that was structurally close to the BED Zn-finger, where an α-helix of the zinc finger is altered to the C-terminal β-strand. C2H2 zinc finger-containing prokaryotic transcriptional regulator Ros from Agrobacterium tumefaciens has been functionally characterized indicating its existence in prokaryotes (Esposito et al., 2006). The WRKY TF family shows the evolution from simple unicellular to more complex multicellular forms as flowering plants, as flowering plants are reported to have the largest number of WRKY members compared with pine, fern, and moss. Yamasaki et al. (2008) proposed that during the course of evolution, ancestral Group I WRKY proteins of primitive eukaryotes, expanded as GCM (Glial Cells Missing), TFs, and WRKYs (Group I, II, III) and NAC from plant families. These studies suggest that the regulatory network shows some similarity in both eukaryotes and prokaryotes, getting more specified in function depending on the need for survival with the changing environment. Thus, the JcWRKY TF interacts with the transcriptional machinery or functions as a direct protein in the prokaryotic system, thereby regulating stress enhancement in E. coli cells. This system provides significant improvement in the growth of cells and can find application in recombinant protein production under stress conditions.
Acknowledgments
The financial assistance from the Department of Science and Technology (DST-Fast Track Scheme for Young Scientists) and the Council of Scientific and Industrial Research (CSIR-Scientists Pool Scheme), New Delhi, India is duly acknowledged.
Disclosure Statement
No competing financial interests exist.
References
- Agarwal P., Reddy M.P., and Chikara J. (2011). WRKY: its structure, evolutionary relationship, DNA-binding selectivity, role in stress tolerance and development of plants. Mol Biol Rep 38,3883–3896 [DOI] [PubMed] [Google Scholar]
- Agarwal P., Bhatt V., Singh R., Das M., Sopory S.K., and Chikara J. (2013). Pathogenesis-related gene, JcPR-10a from Jatropha curcas exhibit RNase and antifungal activity. Mol Biotechnol 2,412–425 [DOI] [PubMed] [Google Scholar]
- Babu M.M., Iyer L.M., Balaji S., and Aravind L. (2006). The natural history of the WRKY–GCM1 zinc fingers and the relationship between transcription factors and transposons. Nucleic Acids Res 22,6505–6520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Liu J., Lin G., Wang A., Wang Z., and Lu G. (2013). Overexpression of AtWRKY28 and AtWRKY75 in Arabidopsis enhances resistance to oxalic acid and Sclerotinia sclerotiorum. Plant Cell Rep 10,1589–1599 [DOI] [PubMed] [Google Scholar]
- Dellagi A., Helibronn J., Avrova A.O., Montesano M., Palva E.T., Stewart H.E., Toth I.K., Cooke D.E., Lyon G.D., and Birch P.R. (2000). A potato gene encoding a WRKY-like transcription factor is induced in interactions with Erwinia carotovora subsp. atroseptica and Phytophthora infestans and is coregulated with class I endochitinase expression. Mol Plant Microbe Interact 13,1092–1101 [DOI] [PubMed] [Google Scholar]
- Devaiah B.N., Karthikeyan A.S., and Raghothama K.G. (2007). WRKY75 transcription factor is a modulator of phosphate acquisition and root development in Arabidopsis. Plant Physiol 143,1789–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J., Chen C., and Chen Z. (2003). Expression profiles of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol Biol 51,21–37 [DOI] [PubMed] [Google Scholar]
- Esposito S., Baglivo I., Malgieri G., Russo L., Zaccaro L., D'Andrea L.D., Mammucari M., Di Blasio B., Isernia C., Fattorusso R., and Pedone P.V. (2006). A novel type of zinc finger DNA binding domain in the Agrobacterium tumefaciens transcriptional regulator Ros. Biochemistry 45,10394–10405 [DOI] [PubMed] [Google Scholar]
- Eulgem T., Rushton P.J., Robatzek S., and Somssich I.E. (2000). The WRKY superfamily of plant transcription factors. Trends Plant Sci 5,199–206 [DOI] [PubMed] [Google Scholar]
- Glockner G., et al. (2002). Sequence and analysis of chromosome 2 of Dictyostelium discoideum. Nature 418,79–85 [DOI] [PubMed] [Google Scholar]
- Gupta K., Agarwal P.K., Reddy M.K., and Jha B. (2010). SbDREB2A, an A-2 type DREB transcription factor from extreme halophyte Salicornia brachiata confers abiotic stress tolerance in Escherichia coli. Plant Cell Rep 29,1131–1137 [DOI] [PubMed] [Google Scholar]
- Guo X.H., Jiang J., Wang B.C., Li H.Y., Wang Y.C., Yang C.P., and Liu G.F. (2010). ThPOD3, a truncated polypeptide from Tamarix hispida, conferred drought tolerance in Escherichia coli. Mol Bio Rep 3,1183–1190 [DOI] [PubMed] [Google Scholar]
- Johnson T.S., Eswaran N., and Sujatha M. (2011). Molecular approaches to improvement of Jatropha curcas Linn.as a sustainable energy crop. Plant Cell Rep 30,1573–1591 [DOI] [PubMed] [Google Scholar]
- Kalde M., Barth M., Somssich I.E., and Lippok B. (2003). Members of the Arabidopsis WRKY group III transcription factors are part of different plant defence signalling pathways. Mol Plant Microbe Interact 16,295–305 [DOI] [PubMed] [Google Scholar]
- Lan Y., Cai D., and Zheng Y.Z. (2005). Expression in Escherichia coli of three different soybean late embryogenesis abundant (LEA) genes to investigate enhanced stress tolerance. Acta Bot Sin 47,613–621 [Google Scholar]
- Liu J.J., and Ekramoddoullah A.K. (2009). Identification and characterization of the WRKY transcription factor family in Pinus monticola. Genome 52,77–88 [DOI] [PubMed] [Google Scholar]
- Liu Y., and Zheng Y. (2005). PM2, a group 3 LEA protein from soybean, and its 22-mer repeating region confer salt tolerance in Escherichia coli. Biochem Biophys Res Commun 331,325–332 [DOI] [PubMed] [Google Scholar]
- Maleck K., Levine A., Eulgem T., Morga A., Schmid J., Lawton K., Dangl J.L., and Dietrich R.A. (2000). The transcriptome of Arabidopsis thaliana during systemic acquired resistance. Nat Genet 26,403–410 [DOI] [PubMed] [Google Scholar]
- Mao G.H., Meng X.Z., Liu Y.D., Zheng Z.Y., Chen Z.X., and Zhang S.Q. (2011). Phosphorylation of a WRKY transcription factor by two pathogen-responsive MAPKs drives phytoalexin biosynthesis in Arabidopsis. Plant Cell 23,1639–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchive C., Mzid R., Deluc L., Barrieu F., Pirrello J., Gauthier A., Corio-Costet M.F., Regad F., Cailleteau B., Hamdi S., and Lauvergeat V. (2007). Isolation and characterization of a Vitis vinifera transcription factor, VvWRKY1, and its effect on responses to fungal pathogens in transgenic tobacco plants. J Exp Bot 58,1999–2010 [DOI] [PubMed] [Google Scholar]
- Marè C., Mazzucotelli E., Crosatti C., Francia E., Stanca A.M., and Cattivelli L. (2004). Hv-WRKY38: a new transcription factor involved in cold- and drought-response in barley. Plant Mol Biol 3,399–416 [DOI] [PubMed] [Google Scholar]
- Pan Y.J., Cho C.C., Kao Y.Y., and Sun C.H. (2009). A novel WRKY-like protein involved in transcriptional activation of cyst wall protein genes in Giardia lamblia. J Biol Chem 284,17975–17988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandurangaiah M., Reddy E.K., Rao G.L., Sivakumar M., Sudhakarbabu O., Nareshkumar A., Ramya M., Kirankumar V., Veeranagamallaiah G., and Sudhakar C. (2013). Cloning and expression analysis of MuNAC4 transcription factor protein from horsegram (Macrotyloma uniflorum (Lam.) Verdc.) conferred salt stress tolerance in Escherichia coli. Acta Physiol Planta 35,139–146 [Google Scholar]
- Reddy P.S., Mallikarjuna G., Kaul T., Chakradhar T., Mishra R.N., Sopory S.K., and Reddy M.K. (2010). Molecular cloning and characterization of gene encoding for cytoplasmic Hsc70 from Pennisetum glaucum may play a protective role against abiotic stresses. Mol Gene Genomics 3,243–254 [DOI] [PubMed] [Google Scholar]
- Rushton P.J., Somssich I.E., Ringler P., and Shen Q.J. (2010). WRKY transcription factors. Trends Plant Sci 15,247–258 [DOI] [PubMed] [Google Scholar]
- Rushton P.J., Torres J.T., Parniske M., Wernet P., Hahlbrock K., and Somssich I.E. (1996). Interaction of elicitor-induced DNA-binding proteins with elicitor response elements in the promoters of parsley PR1 genes. EMBO J 15,5690–5700 [PMC free article] [PubMed] [Google Scholar]
- Shiu S.H., Shih M.C., and Li W.H. (2005). Transcription factor families have much higher expansion rates in plants than in animals. Plant Physiol 139,18–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C., Palmqvist S., Olsson H., Boren M., Ahlandsberg S., and Jansson C. (2003). A novel WRKY transcription factor, SUSIBA2, participates in sugar signaling in barley by binding to the sugar-responsive elements of the iso1 promoter. Plant Cell 15,2076-2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C., Sathish P., Ahlandsberg S., and Jansson C. (1999). Analyses of isoamylase gene activity in wild-type barley indicate its involvement in starch synthesis. Plant Mol Biol 40,431–443 [DOI] [PubMed] [Google Scholar]
- Turck F., Zhou A., and Somssich I.E. (2004). Stimulus-dependent, promoter-specific binding of transcription factor WRKY1 to its native promoter and the defense related gene PcPR1-1 in parsley. Plant Cell 16,2573–2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulker B., and Somssich I.E. (2004). WRKY transcription factors: from DNA binding towards biological function. Curr Opin Plant Biol 5,491–498 [DOI] [PubMed] [Google Scholar]
- Villarejo E.S., Maldonado A.M., Amil-Ruiz F., de Los Santos B., Romero F., Pliego-Alfaro F., Munoz-Blanco J., and Caballero J.L. (2009). Evidence for a positive regulatory role of strawberry (Fragaria x ananassa) Fa WRKY1 and At WRKY75 proteins in resistance. J Exp Bot 60,3043–3065 [DOI] [PubMed] [Google Scholar]
- Wu G., Shao H.B., Chu L.Y., and Cai J.W. (2007). Insights into molecular mechanisms of mutual effect between plants and the environment. Agron Sustain Dev 27,69–78 [Google Scholar]
- Wu K.L., Guo Z.J., Wang H.H., and Li J. (2005). The WRKY family of transcription factors in rice and Arabidopsis and their origins. DNA Res 12,9–26 [DOI] [PubMed] [Google Scholar]
- Yadav N.S., Jha B., Agarwal P.K., Singh D., and Deo R. (2012). A novel salt-inducible gene SbSI-1 from Salicornia brachiata confers salt and desiccation tolerance in E coli. Mol Biol Rep 39,1943–1948 [DOI] [PubMed] [Google Scholar]
- Yamada A., Sekifuchi M., Mimura T., and Oze ki Y. (2002). The role of plant CCTa in salt- and osmotic-stress tolerance. Plant Cell Physiol 43,1043–1048 [DOI] [PubMed] [Google Scholar]
- Yamasaki K., Kigawa T., Inoue M., Watanabe S., Tateno M., Seki M., Shinojaki K., and Yokoyama S. (2008). Structures and evolutionary origins of plant-specific transcription factor DNA-binding domains. Plant Physiol Biochem 3,394–401 [DOI] [PubMed] [Google Scholar]
- Yang P., Wang Z., Fan B., Chen C., and Chen Z. (1999). A pathogen- and salicylic acid-induced WRKY DNA-binding activity recognizes the elicitor response element of the tobacco class I chitinase gene promoter. Plant J 18,141–149 [Google Scholar]
- Jiang Y., and Deyholos M.K. (2009). Functional characterization of Arabidopsis NaCl-inducible WRKY25 and WRKY33 transcription factors in abiotic. Plant Mo Biol 69,91–10 [DOI] [PubMed] [Google Scholar]
- Zhang Y., and Wang L. (2005). The WRKY transcription factor superfamily: its origin in eukaryotes and expansion in plants. BMC Evol Biol 5,1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q.Y., Tian A.G., Zou H.F., Xie Z.M., Lei G., Huang J., Wang C.M., Wang H.W., Zhang J.S., and Chen S.Y. (2008). Soybean WRKY-type transcription factor genes, GmWRKY13, GmWRKY21, and GmWRKY54, confer differential tolerance to abiotic stresses in transgenic Arabidopsis plants. Plant Biotech J 6,486–503 [DOI] [PubMed] [Google Scholar]