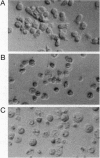
Abstract
Triggering of the Fas/APO-1 cell-surface receptor induces apoptosis through an uncharacterized chain of events. Exposure of Fas-sensitive cells to an agonist monoclonal antibody induced cell death and a 200-300% elevation in endogenous levels of the sphingolipid ceramide, a proposed intracellular mediator of apoptosis. In contrast, similar treatment of Fas-resistant cells caused insignificant changes in ceramide levels. Because resistant cell lines expressed the Fas antigen, these results indicate that these cells have a defect in the proximal signaling events leading to ceramide generation. Exposure of the resistant cell lines to a synthetic analog of ceramide induced apoptosis, thus bypassing Fas resistance and indicating that the signaling pathways downstream of ceramide were intact. Furthermore, activation of protein kinase C with the diacylglycerol analog phorbol 12-myristate 13-acetate significantly reduced Fas-induced cytotoxicity, suggesting opposing roles for ceramide and protein kinase C in regulation of apoptosis. These results provide evidence for ceramide as a necessary and sufficient lipid mediator of Fas-mediated apoptosis and suggest this process may be modulated via activation of additional signal-transduction pathways.
Full text
PDF
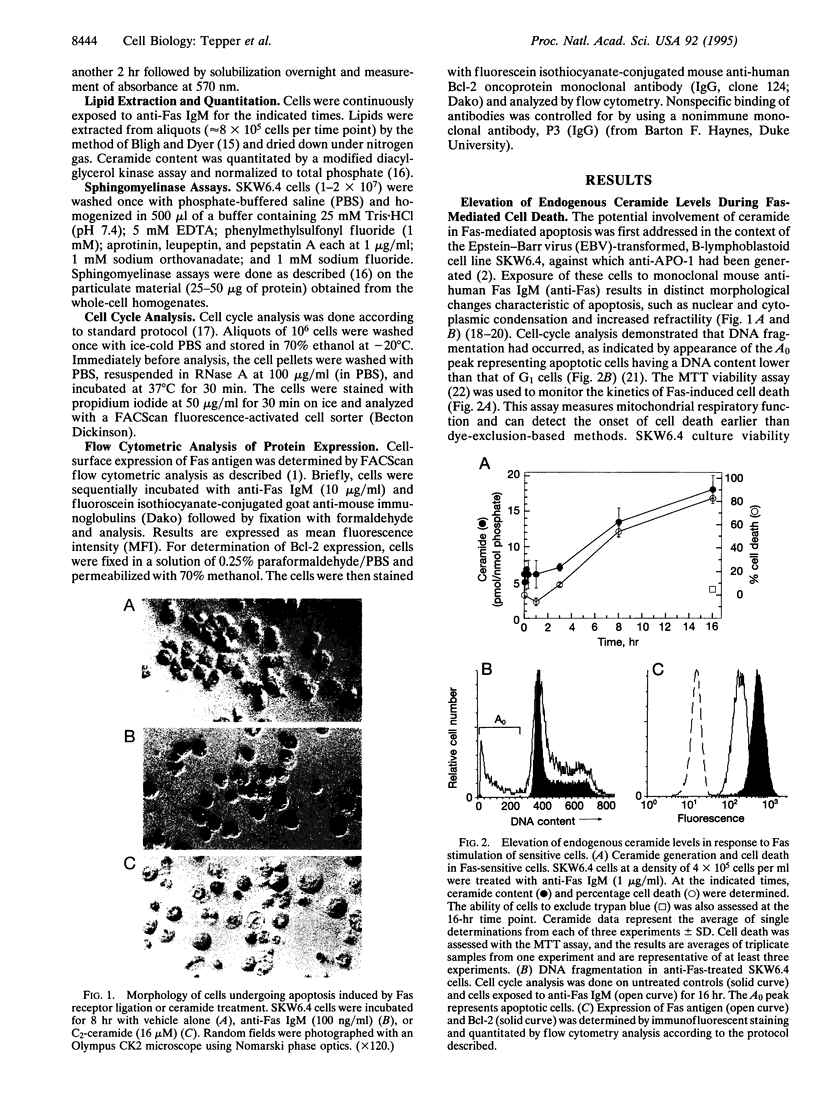
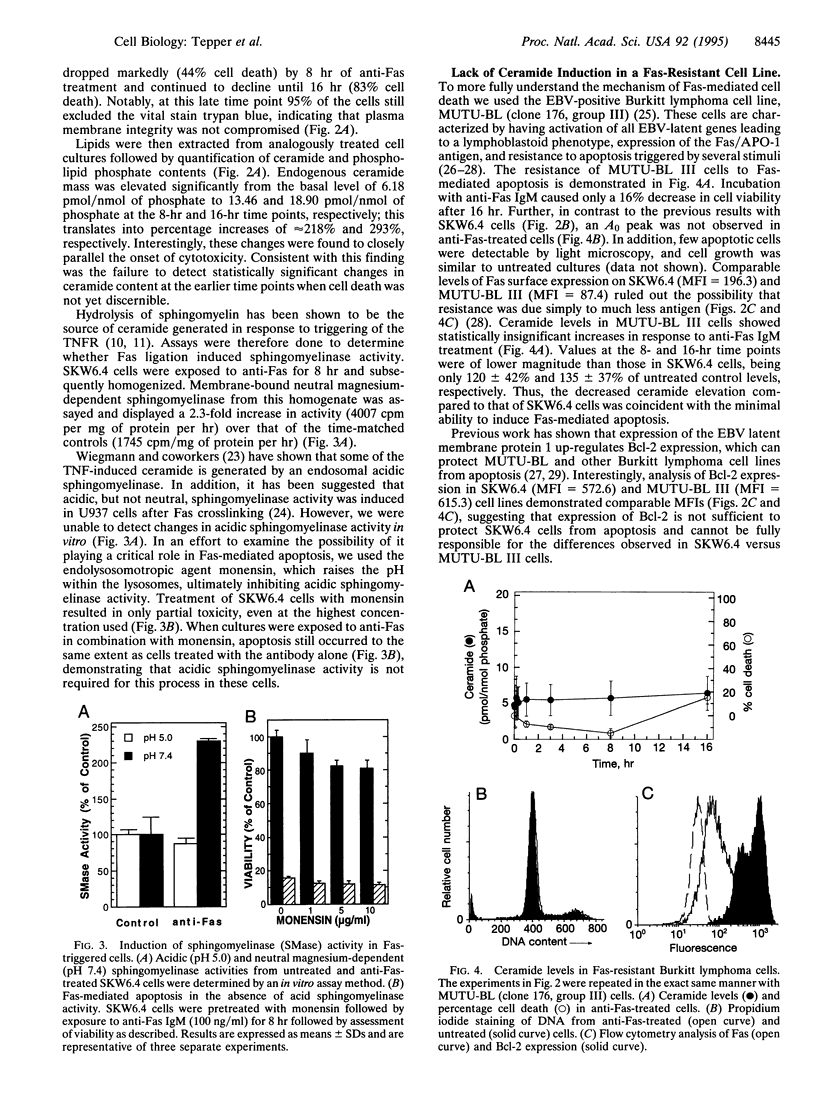
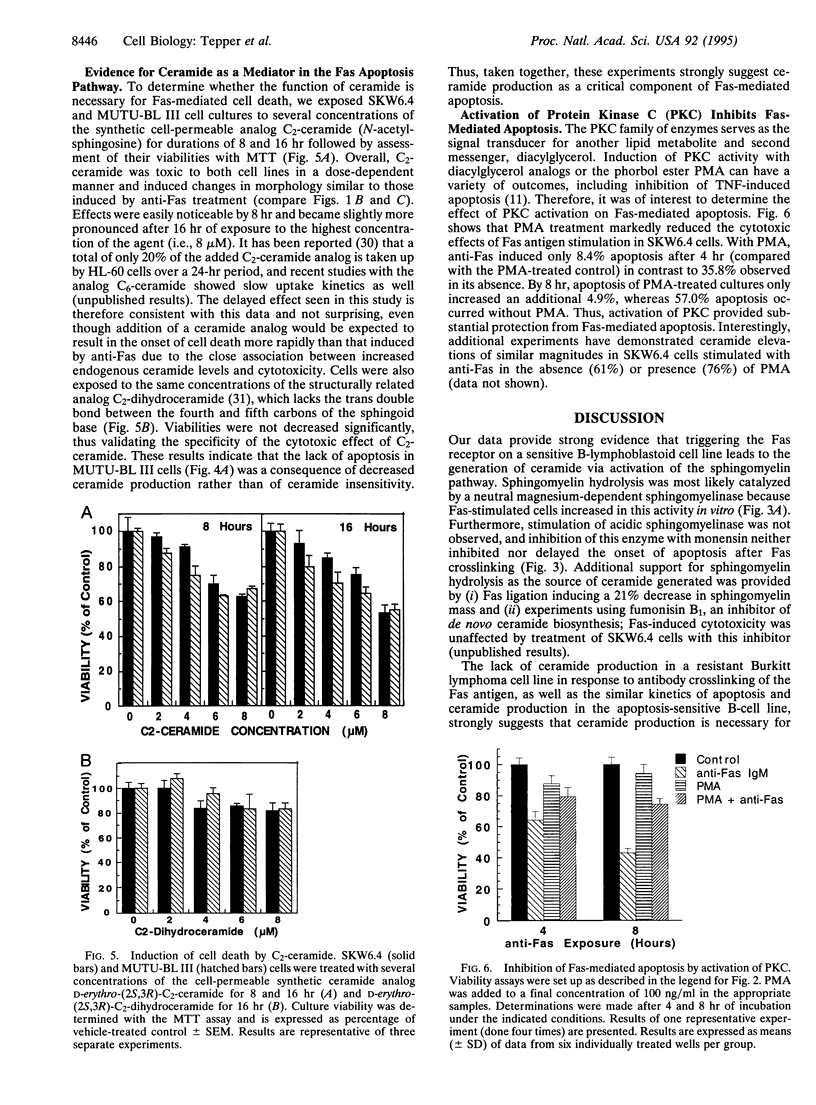

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bielawska A., Crane H. M., Liotta D., Obeid L. M., Hannun Y. A. Selectivity of ceramide-mediated biology. Lack of activity of erythro-dihydroceramide. J Biol Chem. 1993 Dec 15;268(35):26226–26232. [PubMed] [Google Scholar]
- Brunner T., Mogil R. J., LaFace D., Yoo N. J., Mahboubi A., Echeverri F., Martin S. J., Force W. R., Lynch D. H., Ware C. F. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature. 1995 Feb 2;373(6513):441–444. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- Cifone M. G., De Maria R., Roncaioli P., Rippo M. R., Azuma M., Lanier L. L., Santoni A., Testi R. Apoptotic signaling through CD95 (Fas/Apo-1) activates an acidic sphingomyelinase. J Exp Med. 1994 Oct 1;180(4):1547–1552. doi: 10.1084/jem.180.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dbaibo G. S., Obeid L. M., Hannun Y. A. Tumor necrosis factor-alpha (TNF-alpha) signal transduction through ceramide. Dissociation of growth inhibitory effects of TNF-alpha from activation of nuclear factor-kappa B. J Biol Chem. 1993 Aug 25;268(24):17762–17766. [PubMed] [Google Scholar]
- Dhein J., Walczak H., Bäumler C., Debatin K. M., Krammer P. H. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95) Nature. 1995 Feb 2;373(6513):438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- Dobrowsky R. T., Kamibayashi C., Mumby M. C., Hannun Y. A. Ceramide activates heterotrimeric protein phosphatase 2A. J Biol Chem. 1993 Jul 25;268(21):15523–15530. [PubMed] [Google Scholar]
- Falk M. H., Trauth B. C., Debatin K. M., Klas C., Gregory C. D., Rickinson A. B., Calender A., Lenoir G. M., Ellwart J. W., Krammer P. H. Expression of the APO-1 antigen in Burkitt lymphoma cell lines correlates with a shift towards a lymphoblastoid phenotype. Blood. 1992 Jun 15;79(12):3300–3306. [PubMed] [Google Scholar]
- Finke J., Fritzen R., Ternes P., Trivedi P., Bross K. J., Lange W., Mertelsmann R., Dölken G. Expression of bcl-2 in Burkitt's lymphoma cell lines: induction by latent Epstein-Barr virus genes. Blood. 1992 Jul 15;80(2):459–469. [PubMed] [Google Scholar]
- Gregory C. D., Dive C., Henderson S., Smith C. A., Williams G. T., Gordon J., Rickinson A. B. Activation of Epstein-Barr virus latent genes protects human B cells from death by apoptosis. Nature. 1991 Feb 14;349(6310):612–614. doi: 10.1038/349612a0. [DOI] [PubMed] [Google Scholar]
- Gregory C. D., Rowe M., Rickinson A. B. Different Epstein-Barr virus-B cell interactions in phenotypically distinct clones of a Burkitt's lymphoma cell line. J Gen Virol. 1990 Jul;71(Pt 7):1481–1495. doi: 10.1099/0022-1317-71-7-1481. [DOI] [PubMed] [Google Scholar]
- Haimovitz-Friedman A., Kan C. C., Ehleiter D., Persaud R. S., McLoughlin M., Fuks Z., Kolesnick R. N. Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis. J Exp Med. 1994 Aug 1;180(2):525–535. doi: 10.1084/jem.180.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson S., Rowe M., Gregory C., Croom-Carter D., Wang F., Longnecker R., Kieff E., Rickinson A. Induction of bcl-2 expression by Epstein-Barr virus latent membrane protein 1 protects infected B cells from programmed cell death. Cell. 1991 Jun 28;65(7):1107–1115. doi: 10.1016/0092-8674(91)90007-l. [DOI] [PubMed] [Google Scholar]
- Itoh N., Nagata S. A novel protein domain required for apoptosis. Mutational analysis of human Fas antigen. J Biol Chem. 1993 May 25;268(15):10932–10937. [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Ishii A., Yonehara M., Mizushima S., Sameshima M., Hase A., Seto Y., Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991 Jul 26;66(2):233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- Jayadev S., Linardic C. M., Hannun Y. A. Identification of arachidonic acid as a mediator of sphingomyelin hydrolysis in response to tumor necrosis factor alpha. J Biol Chem. 1994 Feb 25;269(8):5757–5763. [PubMed] [Google Scholar]
- Jayadev S., Liu B., Bielawska A. E., Lee J. Y., Nazaire F., Pushkareva MYu, Obeid L. M., Hannun Y. A. Role for ceramide in cell cycle arrest. J Biol Chem. 1995 Feb 3;270(5):2047–2052. doi: 10.1074/jbc.270.5.2047. [DOI] [PubMed] [Google Scholar]
- Ju S. T., Panka D. J., Cui H., Ettinger R., el-Khatib M., Sherr D. H., Stanger B. Z., Marshak-Rothstein A. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995 Feb 2;373(6513):444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. Y., Linardic C., Obeid L., Hannun Y. Identification of sphingomyelin turnover as an effector mechanism for the action of tumor necrosis factor alpha and gamma-interferon. Specific role in cell differentiation. J Biol Chem. 1991 Jan 5;266(1):484–489. [PubMed] [Google Scholar]
- Köhler H. R., Dhein J., Alberti G., Krammer P. H. Ultrastructural analysis of apoptosis induced by the monoclonal antibody anti-APO-1 on a lymphoblastoid B cell line. Ultrastruct Pathol. 1990 Nov-Dec;14(6):513–518. doi: 10.3109/01913129009076138. [DOI] [PubMed] [Google Scholar]
- Lynch D. H., Watson M. L., Alderson M. R., Baum P. R., Miller R. E., Tough T., Gibson M., Davis-Smith T., Smith C. A., Hunter K. The mouse Fas-ligand gene is mutated in gld mice and is part of a TNF family gene cluster. Immunity. 1994 May;1(2):131–136. doi: 10.1016/1074-7613(94)90106-6. [DOI] [PubMed] [Google Scholar]
- Mathias S., Dressler K. A., Kolesnick R. N. Characterization of a ceramide-activated protein kinase: stimulation by tumor necrosis factor alpha. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10009–10013. doi: 10.1073/pnas.88.22.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Obeid L. M., Linardic C. M., Karolak L. A., Hannun Y. A. Programmed cell death induced by ceramide. Science. 1993 Mar 19;259(5102):1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- Oehm A., Behrmann I., Falk W., Pawlita M., Maier G., Klas C., Li-Weber M., Richards S., Dhein J., Trauth B. C. Purification and molecular cloning of the APO-1 cell surface antigen, a member of the tumor necrosis factor/nerve growth factor receptor superfamily. Sequence identity with the Fas antigen. J Biol Chem. 1992 May 25;267(15):10709–10715. [PubMed] [Google Scholar]
- Okazaki T., Bielawska A., Bell R. M., Hannun Y. A. Role of ceramide as a lipid mediator of 1 alpha,25-dihydroxyvitamin D3-induced HL-60 cell differentiation. J Biol Chem. 1990 Sep 15;265(26):15823–15831. [PubMed] [Google Scholar]
- Singer G. G., Abbas A. K. The fas antigen is involved in peripheral but not thymic deletion of T lymphocytes in T cell receptor transgenic mice. Immunity. 1994 Aug;1(5):365–371. doi: 10.1016/1074-7613(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Strum J. C., Small G. W., Pauig S. B., Daniel L. W. 1-beta-D-Arabinofuranosylcytosine stimulates ceramide and diglyceride formation in HL-60 cells. J Biol Chem. 1994 Jun 3;269(22):15493–15497. [PubMed] [Google Scholar]
- Takahashi T., Tanaka M., Brannan C. I., Jenkins N. A., Copeland N. G., Suda T., Nagata S. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994 Mar 25;76(6):969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- Tartaglia L. A., Ayres T. M., Wong G. H., Goeddel D. V. A novel domain within the 55 kd TNF receptor signals cell death. Cell. 1993 Sep 10;74(5):845–853. doi: 10.1016/0092-8674(93)90464-2. [DOI] [PubMed] [Google Scholar]
- Taylor I. W. A rapid single step staining technique for DNA analysis by flow microfluorimetry. J Histochem Cytochem. 1980 Sep;28(9):1021–1024. doi: 10.1177/28.9.6157714. [DOI] [PubMed] [Google Scholar]
- Telford W. G., King L. E., Fraker P. J. Evaluation of glucocorticoid-induced DNA fragmentation in mouse thymocytes by flow cytometry. Cell Prolif. 1991 Sep;24(5):447–459. doi: 10.1111/j.1365-2184.1991.tb01173.x. [DOI] [PubMed] [Google Scholar]
- Trauth B. C., Klas C., Peters A. M., Matzku S., Möller P., Falk W., Debatin K. M., Krammer P. H. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989 Jul 21;245(4915):301–305. doi: 10.1126/science.2787530. [DOI] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R., Brannan C. I., Copeland N. G., Jenkins N. A., Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992 Mar 26;356(6367):314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- Wiegmann K., Schütze S., Machleidt T., Witte D., Krönke M. Functional dichotomy of neutral and acidic sphingomyelinases in tumor necrosis factor signaling. Cell. 1994 Sep 23;78(6):1005–1015. doi: 10.1016/0092-8674(94)90275-5. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Yonehara S., Ishii A., Yonehara M. A cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J Exp Med. 1989 May 1;169(5):1747–1756. doi: 10.1084/jem.169.5.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]