Abstract
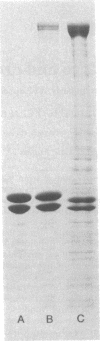
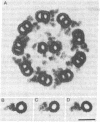
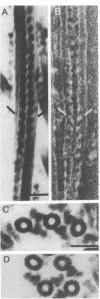
Dynein isolated from Chlamydomonas flagellar axonemes binds to microtubules assembled in vitro from 6S brain tubulin dimers. The dynein arms bind periodically along the length of the microtubules with a center-to-center spacing of 24 nm, equal to the periodicity of dynein arms on intact axonemes. The arms project from the in vitro assembled microtubules at an angle of approximately 55 degrees, thereby defining microtubule polarity. Dynein cosediments with microtubules through a sucrose gradient, as demonstrated by electron microscopy, gel electrophoresis, and ATPase analysis. In addition, dynein induces crossbridging between adjacent microtubules. Darkfield microscopy reveals that microtubules containing dynein are aggregated into large bundles; electron microscopy indicates that microtubules of the same polarity are crossbridged by a regular array of arms. Viewed by darkfield microscopy, addition of ATP to crossbridged microtubules causes their disaggregation; electron microscopy shows that the majority of these microtubules are no longer crossbridged. These observations are applicable to the determination of microtubule polarity and directionality of microtubule assembly in situ and suggest a role for dynein in cytoplasmic microtubule-based cellular movements.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen C., Borisy G. G. Structural polarity and directional growth of microtubules of Chlamydomonas flagella. J Mol Biol. 1974 Dec 5;90(2):381–402. doi: 10.1016/0022-2836(74)90381-7. [DOI] [PubMed] [Google Scholar]
- Amos L. A. Arrangement of high molecular weight associated proteins on purified mammalian brain microtubules. J Cell Biol. 1977 Mar;72(3):642–654. doi: 10.1083/jcb.72.3.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodgood R. A., Rosenbaum J. L. Initiation of brain tubulin assembly by a high molecular weight flagellar protein factor. J Cell Biol. 1976 Oct;71(1):322–331. doi: 10.1083/jcb.71.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cande W. Z., Snyder J., Smith D., Summers K., McIntosh J. R. A functional mitotic spindle prepared from mammalian cells in culture. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1559–1563. doi: 10.1073/pnas.71.4.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cande W. Z., Wolniak S. M. Chromosome movement in lysed mitotic cells is inhibited by vanadate. J Cell Biol. 1978 Nov;79(2 Pt 1):573–580. doi: 10.1083/jcb.79.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons B. H., Gibbons I. R. Properties of flagellar "rigor waves" formed by abrupt removal of adenosine triphosphate from actively swimming sea urchin sperm. J Cell Biol. 1974 Dec;63(3):970–985. doi: 10.1083/jcb.63.3.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY H. E. ELECTRON MICROSCOPE STUDIES ON THE STRUCTURE OF NATURAL AND SYNTHETIC PROTEIN FILAMENTS FROM STRIATED MUSCLE. J Mol Biol. 1963 Sep;7:281–308. doi: 10.1016/s0022-2836(63)80008-x. [DOI] [PubMed] [Google Scholar]
- Huang B., Piperno G., Luck D. J. Paralyzed flagella mutants of Chlamydomonas reinhardtii. Defective for axonemal doublet microtubule arms. J Biol Chem. 1979 Apr 25;254(8):3091–3099. [PubMed] [Google Scholar]
- Kim H., Binder L. I., Rosenbaum J. L. The periodic association of MAP2 with brain microtubules in vitro. J Cell Biol. 1979 Feb;80(2):266–276. doi: 10.1083/jcb.80.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin T. I., Morales M. F. Application of a one-step procedure for measuring inorganic phosphate in the presence of proteins: the actomyosin ATPase system. Anal Biochem. 1977 Jan;77(1):10–17. doi: 10.1016/0003-2697(77)90284-6. [DOI] [PubMed] [Google Scholar]
- Margolis R. L., Wilson L. Opposite end assembly and disassembly of microtubules at steady state in vitro. Cell. 1978 Jan;13(1):1–8. doi: 10.1016/0092-8674(78)90132-0. [DOI] [PubMed] [Google Scholar]
- Neal M. W., Florini J. R. A rapid method for desalting small volumes of solution. Anal Biochem. 1973 Sep;55(1):328–330. doi: 10.1016/0003-2697(73)90325-4. [DOI] [PubMed] [Google Scholar]
- Rosenbaum J. L., Binder L. I., Granett S., Dentler W. L., Snell W., Sloboda R., Haimo L. Directionality and rate of assembly of chick brain tubulin onto pieces of neurotubules, flagellar axonemes, and basal bodies. Ann N Y Acad Sci. 1975 Jun 30;253:147–177. doi: 10.1111/j.1749-6632.1975.tb19198.x. [DOI] [PubMed] [Google Scholar]
- Satir P. Studies on cilia. 3. Further studies on the cilium tip and a "sliding filament" model of ciliary motility. J Cell Biol. 1968 Oct;39(1):77–94. doi: 10.1083/jcb.39.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloboda R. D., Dentler W. L., Rosenbaum J. L. Microtubule-associated proteins and the stimulation of tubulin assembly in vitro. Biochemistry. 1976 Oct 5;15(20):4497–4505. doi: 10.1021/bi00665a026. [DOI] [PubMed] [Google Scholar]
- Summers K. E., Gibbons I. R. Adenosine triphosphate-induced sliding of tubules in trypsin-treated flagella of sea-urchin sperm. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3092–3096. doi: 10.1073/pnas.68.12.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M., Tonomura Y. Binding of 30s dynein with the B-tubule of the outer doublet of axonemes from Tetrahymena pyriformis and adenosine triphosphate-induced dissociation of the complex. J Biochem. 1978 Dec;84(6):1339–1355. doi: 10.1093/oxfordjournals.jbchem.a132256. [DOI] [PubMed] [Google Scholar]
- Telzer B. R., Rosenbaum J. L. Cell cycle-dependent, in vitro assembly of microtubules onto pericentriolar material of HeLa cells. J Cell Biol. 1979 Jun;81(3):484–497. doi: 10.1083/jcb.81.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten M. D., Lockwood A. H., Hwo S. Y., Kirschner M. W. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975 May;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witman G. B., Plummer J., Sander G. Chlamydomonas flagellar mutants lacking radial spokes and central tubules. Structure, composition, and function of specific axonemal components. J Cell Biol. 1978 Mar;76(3):729–747. doi: 10.1083/jcb.76.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]