Abstract
Objectives
We compared the proportion of conception by in vitro fertilization (IVF) and without IVF in fetuses with and without congenital heart disease (CHD).
Methods
This was a retrospective review of fetal echocardiograms at Columbia University from 2007-2010, to identify the mode of conception.
Results
Echocardiography was performed on 2,828 fetuses, and 2,761 (97.6%) had the method of conception documented. 9.5% were conceived via IVF. CHD was diagnosed in 22.4%, consisting predominantly of complex CHD. The proportion of IVF conception was lower in fetuses with CHD (6.9% CHD vs 10.3% no CHD, OR=0.65 [95% CI 0.46-0.92] p=0.01). IVF fetuses were conceived by elder mothers and were more likely part of a multiple gestation than those without IVF. In a multivariate model controlling for maternal age and multiple gestation, IVF was not associated with CHD diagnosis (OR=1.1 [95% CI 0.77-1.7], p=0.51).
Conclusion
At a tertiary referral center, fetuses with CHD were not more likely to be conceived by IVF after controlling for maternal age and multiple gestation. These results differ from those of several previous reports, which may be related to our study population, and the exclusion of isolated atrial shunts and patent ductus arteriosus, which are normal fetal findings.
Introduction
Whether there is an association between conception by in vitro fertilization (IVF) and congenital heart disease (CHD) is not clear. Multiple population-based studies have looked at the association between IVF and birth defects, with inconsistent results 1-7. Up to a 3-4 times higher rate of CHD has been reported in children conceived by IVF 2-5,7. However, many of these studies are hampered by low numbers of CHD cases and lack of appropriate control populations. Additionally, inclusion of minor anomalies such as patent ductus arteriosus (PDA), isolated secundum atrial septal defects (ASD's) and non-cardiac vascular abnormalities as CHD in many of these studies is another limitation. Based on potential increased risk, it has been suggested to refer all IVF pregnancies for fetal echocardiography.
We aimed to identify the mode of conception in fetuses referred for echocardiography at our institution, a tertiary care referral center with a high volume of prenatally diagnosed complex CHD (>150 cases per year), in order to compare the proportion of IVF conception in fetuses with CHD to fetuses with normal cardiac structure. We hypothesized IVF conception would be associated with CHD diagnosis.
Methods
This was a case-control study consisting of a retrospective chart review of all patients who underwent a fetal echocardiogram performed by a pediatric cardiologist at Columbia University Medical Center between 1/1/07 and 12/31/10. Approval was obtained from the Columbia University Medical Center Institutional Review Board. Subjects were identified using a fetal cardiology patient database and echocardiogram database. Maternal electronic medical records were also reviewed. Fetuses were classified as having CHD based on intracardiac structural anomalies detected on fetal echocardiogram, or as having a normal cardiac structure. Postnatal infant cardiac diagnoses were reviewed when available. Fetuses with cardiomyopathy or arrhythmia and otherwise normal intracardiac anatomy were classified as normal cardiac structure. Patent foramen ovale (PFO) and PDA are normal findings on a fetal echocardiogram, and thus neither were included as CHD. Method of conception was recorded for all subjects as documented in the patient database via patient self-reporting on a standard patient intake questionnaire, with confirmation based on physician documentation on pediatric echocardiogram and obstetric ultrasound reports. Conception was categorized as IVF (± intracytoplasmic sperm injection) or non-IVF. Data was not uniformly available on ovarian stimulation or intrauterine insemination, so in the absence of in vitro fertilization, these were categorized as non-IVF. Additional variables recorded included reason for referral for fetal echocardiogram, maternal age, multiple gestation, presence of twin-twin transfusion syndrome (TTTS), maternal diabetes status, and fetal or postnatal extracardiac and genetic anomalies.
Statistical analysis was performed using SPSS, version 18.0 (SPSS, Inc., 2009, Chicago, IL.) Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated by the Mantel-Haenszel method. Differences in means were compared using student's t-tests, with alpha level set at 0.05. Multivariate logistic regression analysis was used to explore predictors of CHD diagnosis.
Results
Echocardiograms were performed on 2,828 fetuses from 2,542 pregnancies during the study period. Of these, 2,480 (97.6%) pregnancies had their method of conception documented, accounting for 2,761 (97.6%) of fetuses. Those without a documented method of conception were excluded from subsequent analyses. Reasons for maternal referral for fetal echocardiogram are shown in Figure 1. Suspicion of CHD was the most common reason for referral. IVF was not often the primary reason for referral to our center during the study period. CHD was diagnosed prenatally in 605 fetuses, and an additional 14 fetuses had postnatal findings of a ventricular septal defect (VSD), giving a total number of 619/2,761 (22.4%) fetuses with CHD in the study group. The details of the cardiac diagnoses are shown in Figure 2. Of all fetuses with and without CHD, 263 (9.5%) were the result of an IVF conception.
Figure 1.
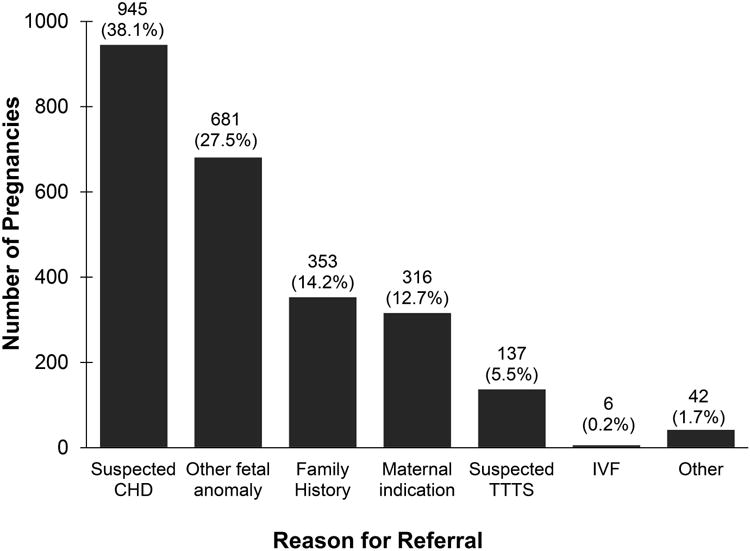
Reason for referral for fetal echocardiogram, by pregnancy, total N = 2,480. CHD = congenital heart disease, TTTS = twin-twin transfusion syndrome, IVF = in vitro fertilization
Figure 2.
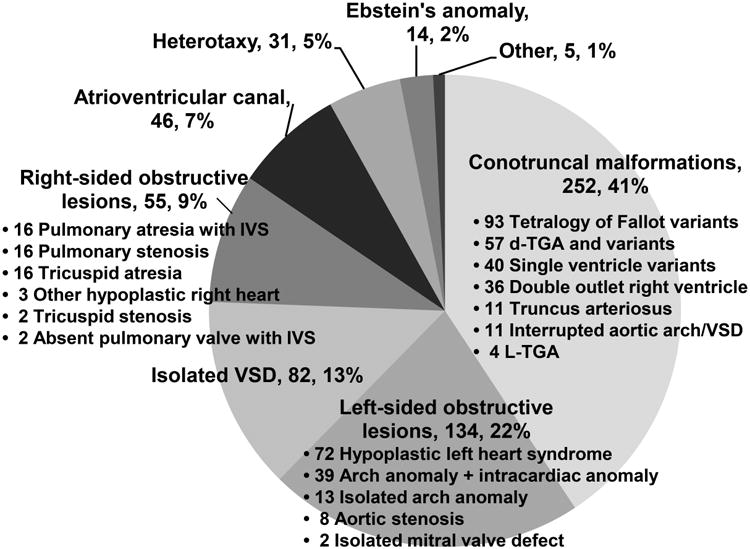
Breakdown of types of congenital heart disease (n, %) seen in the study population, total N = 619. Note that acquired pulmonary valve lesions secondary to twin-twin transfusion syndrome were not included. IVS = intact ventricular septum, TGA = transposition of the great arteries, VSD = ventricular septal defect
Baseline characteristics of the fetuses with CHD are noted in Table 1. 6.9% of fetuses with CHD were the concepts in an IVF cycle, compared with 10.3% with normal cardiac structure (OR = 0.65 [95% CI 0.46-0.92], p = 0.01). Of note, all 14 fetuses with a postnatally diagnosed VSD were not conceived in an IVF cycle. Fetuses with CHD were less likely to be a part of a multiple gestation (OR = 0.31 [95% CI 0.23-0.42], p <0.001). There was no difference in mean maternal age. Characteristics of fetuses conceived in an IVF cycle are shown in Table 2. IVF fetuses had a higher mean maternal age and were more likely to be the product of a multiple gestation. In a multivariate model controlling for maternal age and multiple gestation, IVF was not associated with CHD diagnosis (OR = 1.1 [95% CI 0.77-1.7], p=0.51). Conversely, multiple gestation continued to have a negative association with CHD (OR = 0.3 [95% CI 0.22-0.41], p <0.001).
Table 1.
Characteristics of fetuses with congenital heart disease compared to those with normal cardiac structure.
| Parameter | CHD (N = 619) | Normal Cardiac Structure (N = 2,142) | Unadjusted OR [95% CI] | P value |
|---|---|---|---|---|
| Mean maternal age (years) | 30.7 ± 6.4 | 30.9 ± 6.4 | N/A | 0.88 |
| IVF Conception | 43 (6.9%) | 220 (10.3%) | 0.65 (0.46 – 0.92) | 0.01 |
| Multiple Gestation | 56 (9.0%) | 520 (24.3%) | 0.31 (0.23 – 0.42) | <0.001 |
CHD = congenital heart disease, IVF = in vitro fertilization
Table 2. Characteristics of fetuses who were products of in vitro fertilization, compared to those from conception without in vitro fertilization.
| Parameter | IVF (N = 263) | Non-IVF (N = 2,498) | Unadjusted OR [95% CI] | P value |
|---|---|---|---|---|
| Mean maternal age (years) | 35.4 ± 5.2 | 30.3 ± 6.3 | N/A | <0.001 |
| Multiple Gestation | 180 (68.4%) | 396 (15.9%) | 11.5 (8.7 – 15.3) | <0.001 |
| CHD | 43 (16.3%) | 576 (23.1%) | 0.65 (0.46 – 0.92) | 0.01 |
CHD = congenital heart disease, IVF = in vitro fertilization
For the pregnancies conceived by IVF, the breakdown of reasons for referral for fetal echocardiogram mirrored those conceived without IVF, with the exception that a significantly greater percentage were referred for diagnosis of TTTS (12.1% IVF vs 4.9% no IVF, OR = 2.7 [95% CI 1.6-4.4], p<0.001). Additional analysis to control for suspected TTTS as the reason for fetal echocardiography referral, along with maternal age and multiple gestation, showed no association between IVF and CHD (OR = 0.91 [95% CI 0.61-1.4], p=0.65).
When analysis was done considering CHD cases by pregnancy instead of by fetus, there were 616 out of 2,480 pregnancies affected by CHD (due to 3 sets of twins where both fetuses had CHD). In this analysis, IVF conception accounted for 7% of the pregnancies with CHD and 7% of pregnancies with normal cardiac structure. Again, after controlling for multiple gestation and maternal age, there was no association between CHD and IVF (OR = 1.2 [ 95% CI 0.82-1.8], p=0.32).
Separate analysis of the singleton population showed IVF conception to account for 3.4% of singletons with normal cardiac structure and 4.4% of singletons with CHD, with no association between CHD and IVF after controlling for maternal age (OR = 1.2 [95% CI 0.71-1.9], p=0.53). Analysis of multiple gestation pregnancies showed IVF conception to account for 35% of pregnancies affected with CHD and 30% of pregnancies with normal cardiac structure, which was not a significant difference after controlling for maternal age (OR = 1.5 [95% CI 0.75-3.0], p=0.23).
A breakdown of types of cardiac lesions in IVF fetuses compared with those conceived without IVF is displayed in Table 3. After controlling for maternal age and multiple gestation, no significant differences were seen in any subtype of CHD.
Table 3.
Types of congenital heart disease seen in fetuses conceived with and without in vitro fertilization, with adjusted odds ratio given after controlling for maternal age and multiple gestation.
| Type of CHD | N (%) of Non-IVF fetuses (total N = 2,498) | N (%) of IVF fetuses (total N = 263) | Adjusted OR [95% CI] | P value |
|---|---|---|---|---|
| Conotruncal defects | 228 (9.1%) | 24 (9.1%) | 1.6 (0.94 – 2.6) | 0.08 |
| Left-sided obstructive lesions | 127 (5.1%) | 7 (2.7%) | 1.0 (0.45 – 2.4) | 0.93 |
| Isolated ventricular septal defect | 80 (3.2%) | 2 (0.8%) | 0.28 (0.06 – 1.2) | 0.08 |
| Right-sided obstructive lesions | 50 (2%) | 5 (1.9%) | 1.4 (0.51 – 4.0) | 0.50 |
| Atrioventricular canal defects | 44 (1.8%) | 2 (0.8%) | 0.68 (0.15 – 3.0) | 0.61 |
| Heterotaxy | 28 (1.1%) | 3 (1.1%) | 3 (0.67 – 14) | 0.15 |
| Ebstein's anomaly | 14 (0.6%) | 0 | -- | -- |
| Other | 5 (0.2%) | 0 | -- | -- |
CHD = congenital heart disease, IVF = in vitro fertilization
Discussion
This study demonstrates that among our patients referred for fetal echocardiography, fetuses with CHD were not more likely to have been conceived by IVF, which was contrary to our initial hypothesis and to several previous studies.
Though controversy exists in the literature, many studies report an association between IVF conception and CHD 2-5,7. It has also been suggested that parental factors rather than the infertility treatment may underlie this association 8. However, many of these studies include milder forms of CHD such as PDA, PFO, and small ASD's. IVF has also been associated with preterm labor, preterm birth, low birth weight, and multiple gestation 2,3,9,10. This has potential to confound the diagnosis of certain types of CHD in the neonatal period. A PDA and PFO are normal structures in fetal life and can continue to be detected in the neonatal period, especially among premature infants 11. Because the foramen ovale is normally patent in a newborn and often appears stretched, it is not easy to distinguish a true secundum ASD from a stretched PFO in neonates. Additionally, small muscular VSD's of clinical insignificance are more likely to be detected in the neonatal period and may be more common in premature newborns 12,13.
Because a PDA and atrial shunting in the form of a PFO are normal fetal structures, they are not future persistent anomalies that can be ruled-out when screening is done during fetal life. Therefore, if postnatal echocardiography revealed an isolated PDA or PFO versus small secundum ASD in our study subjects, they were classified as having a normal cardiac structure. Primum ASD's were included as CHD under the subtype of atrioventricular canal defects. No isolated sinus venosus ASD's were diagnosed prenatally during the study period. Our finding that the IVF and non-IVF populations had no difference in the number of more complex types of CHD such as conotruncal, left ventricular outflow tract, and right ventricular outflow tract defectsis similar to part of the findings of Reefhuis et al. 6. They performed a large case-control study using a birth defect registry in the United States, and found no association between assisted reproductive technology and complex cardiac defects, but found significance in isolated atrial and ventricular septal defects in the subgroup of singletons. Our study differs in the finding of no significant difference in isolated VSD's in the IVF population.
According to data from the US Center for Disease Control, roughly 60,000 infants are born as a result of IVF in the United States annually, which accounts for 1.4% of live births in the US14. IVF has been listed as an indication for fetal echocardiography by the American Society of Echocardiography, as well as by the American Institute of Ultrasound in Medicine 15,16. Referral for fetal echocardiography may not be completely benign, as it has been associated with increased levels of maternal anxiety which in turn may have effects on the fetal brain and postnatal cognitive development 17,18. While we continue to support the recommendation for referral of all IVF pregnancies for fetal echocardiogram, and indeed all patients with IVF conception at our institution continue to be referred for fetal echocardiography, our results suggest the chance of diagnosing complex CHD in this population remains low. This information may be helpful for the fetal echocardiographer and referring obstetrician. It may even reassure the IVF patient who could experience anxiety related to the need for this additional screening test.
Our study has limitations. Data on the mode of conception was retrieved from standardized clinical patient intake questionnaires and medical professional confirmation was not always available. Though obstetric records were also reviewed, availability was inconsistent as not all patients who underwent echocardiography saw an obstetrician at our institution. Data on whether IVF included intracytoplasmic sperm injection was not available for all subjects and therefore was not analyzed. Data on in utero insemination and ovulation induction was not available for all subjects and was not analyzed; subjects who received these therapies but did not undergo IVF were classified as non-IVF. Furthermore, patients underwent IVF at multiple different centers, therefore access to our center's reproductive endocrinology database was not informative.
As we are a major pediatric cardiac surgical center, our study population is skewed towards fetuses with complex cardiac defects, sent in by referring physicians, many of them pediatric cardiologists, for evaluation and subsequent delivery at our institution. Our Maternal Fetal Medicine division also evaluates a high volume of fetuses with noncardiac anomalies, who also undergo fetal echocardiography. We found an overall prevalence of IVF of 9.5% in our study population, which is higher than the 3.4% of IVF births reported for the state of New York, though New York does have a higher IVF birth rate than the 1.4% reported for the United States as a whole14. The relatively high percentage of multiple gestation fetuses (20.9%) in our series may be related tothe higher IVF rate observed. Additionally, all fetuses undergoing echocardiography were included in this study, whether or not the pregnancy carried to term, which is another distinction from other reports on this topic. Bias in our results may be introduced if terminations are more likely among women who did not conceive with IVF. However the same would be true for any study that uses postnatal diagnosis of CHD.
Our Maternal Fetal Medicine division is a referral center for twin-twin transfusion syndrome (TTTS), and therefore pregnancies referred specifically because of monochorionic-diamniotic twin gestation constitute a population screened by fetal echocardiography which may have a higher IVF rate and lower rate of CHD. However, controlling for multiple gestation did not change the lack of association between IVF and CHD. Additionally, statistical analysis controlling for type of multiple gestation and for reason for referral also demonstrated no association between IVF and CHD. Recipient twins from pregnancies affected by TTTS are at risk for developing cardiomyopathy and subsequent acquired pulmonary outflow tract defects as a consequence of the hemodynamic alterations of TTTS. Because these lesions do not constitute an intrinsic cardiac defect, they were not included as CHD for the purposes of this study.
In conclusion, among fetuses undergoing echocardiography at our tertiary care referral center, fetuses diagnosed with CHD were not more likely to be the product of an IVF conception after controlling for maternal age and multiple gestation. This lack of association, which differs from several prior reports, may be related to the nature of our study population and the exclusion of isolated atrial shunts and patent ductus arteriosus, which are normal findings on a fetal echocardiogram.
What's already known about this topic?
Several studies have reported a higher rate of congenital heart disease (CHD) in children conceived by in vitro fertilization (IVF). The existing studies include normal fetal findings such as patent foramen ovale (PFO), patent ductus arteriosus (PDA), as well as noncardiac vascular anomalies, as CHD.
Recommendations have been made for all IVF pregnancies to undergo fetal echocardiography.
What does this study add?
We found no association between IVF conception and CHD diagnosis in our population, which consisted of fetuses undergoing echocardiography at a tertiary care center.
Acknowledgments
Grant Disclosure: I.A. Williams received support from Grant No. 1K23HD061601 from the NICHD/NIH. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of the NIH.
Footnotes
Conflicts of Interest: None declared
References
- 1.Ericson A, Kallen B. Congenital malformations in infants born after IVF: a population-based study. Hum Reprod. 2001 Mar;16(3):504–509. doi: 10.1093/humrep/16.3.504. [DOI] [PubMed] [Google Scholar]
- 2.Hansen M, Kurinczuk JJ, Bower C, Webb S. The risk of major birth defects after intracytoplasmic sperm injection and in vitro fertilization. N Engl J Med. 2002 Mar 7;346(10):725–730. doi: 10.1056/NEJMoa010035. [DOI] [PubMed] [Google Scholar]
- 3.Koivurova S, Hartikainen AL, Gissler M, Hemminki E, Sovio U, Jarvelin MR. Neonatal outcome and congenital malformations in children born after in-vitro fertilization. Hum Reprod. 2002 May;17(5):1391–1398. doi: 10.1093/humrep/17.5.1391. [DOI] [PubMed] [Google Scholar]
- 4.Anthony S, Buitendijk SE, Dorrepaal CA, Lindner K, Braat DD, den Ouden AL. Congenital malformations in 4224 children conceived after IVF. Hum Reprod. 2002 Aug;17(8):2089–2095. doi: 10.1093/humrep/17.8.2089. [DOI] [PubMed] [Google Scholar]
- 5.Olson CK, Keppler-Noreuil KM, Romitti PA, et al. In vitro fertilization is associated with an increase in major birth defects. Fertil Steril. 2005;84(5):1308–1315. doi: 10.1016/j.fertnstert.2005.03.086. [DOI] [PubMed] [Google Scholar]
- 6.Reefhuis J, Honein MA, Schieve LA, Correa A, Hobbs CA, Rasmussen SA. Assisted reproductive technology and major structural birth defects in the United States. Hum Reprod. 2009 Feb;24(2):360–366. doi: 10.1093/humrep/den387. [DOI] [PubMed] [Google Scholar]
- 7.Wen SW, Leader A, White RR, et al. A comprehensive assessment of outcomes in pregnancies conceived by in vitro fertilization/intracytoplasmic sperm injection. Eur J Obstet Gynecol Reprod Biol. 2010 Jun;150(2):160–165. doi: 10.1016/j.ejogrb.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 8.Davies MJ, Moore VM, Willson KJ, et al. Reproductive technologies and the risk of birth defects. N Engl J Med. 2012 May 10;366(19):1803–1813. doi: 10.1056/NEJMoa1008095. [DOI] [PubMed] [Google Scholar]
- 9.Births in Great Britain resulting from assisted conception, 1978-87. MRC Working Party on Children Conceived by In Vitro Fertilisation. BMJ. 1990 May 12;300(6734):1229–1233. doi: 10.1136/bmj.300.6734.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen VM, Wilson RD, Cheung A. Pregnancy outcomes after assisted reproductive technology. J Obstet Gynaecol Can. 2006 Mar;28(3):220–250. doi: 10.1016/S1701-2163(16)32112-0. [DOI] [PubMed] [Google Scholar]
- 11.Connuck D, Sun JP, Super DM, et al. Incidence of patent ductus arteriosus and patent foramen ovale in normal infants. Am J Cardiol. 2002 Jan 15;89(2):244–247. doi: 10.1016/s0002-9149(01)02214-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roguin N, Du ZD, Barak M, Nasser N, Hershkowitz S, Milgram E. High prevalence of muscular ventricular septal defect in neonates. J Am Coll Cardiol. 1995 Nov 15;26(6):1545–1548. doi: 10.1016/0735-1097(95)00358-4. [DOI] [PubMed] [Google Scholar]
- 13.Paladini D, Palmieri S, Lamberti A, Teodoro A, Martinelli P, Nappi C. Characterization and natural history of ventricular septal defects in the fetus. Ultrasound Obstet Gynecol. 2000 Aug;16(2):118–122. doi: 10.1046/j.1469-0705.2000.00202.x. [DOI] [PubMed] [Google Scholar]
- 14.Sunderam S, Kissin DM, Flowers L, et al. Assisted reproductive technology surveillance--United States, 2009. MMWR Surveill Summ. 2012 Nov 2;61(7):1–23. [PubMed] [Google Scholar]
- 15.Rychik J, Ayres N, Cuneo B, et al. American Society of Echocardiography guidelines and standards for performance of the fetal echocardiogram. J Am Soc Echocardiogr. 2004 Jul;17(7):803–810. doi: 10.1016/j.echo.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 16.AIUM practice guideline for the performance of fetal echocardiography. J Ultrasound Med. 2011 Jan;30(1):127–136. doi: 10.7863/jum.2011.30.1.127. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg KB, Monk C, Glickstein JS, et al. Referral for fetal echocardiography is associated with increased maternal anxiety. J Psychosom Obstet Gynaecol. 2010 Jun;31(2):60–69. doi: 10.3109/01674821003681472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buss C, Davis EP, Hobel CJ, Sandman CA. Maternal pregnancy-specific anxiety is associated with child executive function at 6-9 years age. Stress. 2011 Nov;14(6):665–676. doi: 10.3109/10253890.2011.623250. [DOI] [PMC free article] [PubMed] [Google Scholar]


