Abstract
Secretion of proinflammatory cytokines by LPS activated endothelial cells contributes substantially to the pathogenesis of sepsis. However, the mechanism involved in this process is not well understood. In the present study, we determined the role of a nonreceptor proline-rich tyrosine kinase, Pyk2, in LPS-induced IL-8 (CXCL8) production in endothelial cells. First, we observed a marked activation of Pyk2 in response to LPS. Furthermore, inhibition of Pyk2 activity in these cells by transduction with the catalytically inactive Pyk2 mutant, transfection with Pyk2-specific small interfering RNA, or treatment with Tyrphostin A9 significantly blocked LPS-induced IL-8 production. The supernatants of LPS-stimulated cells exhibiting attenuated Pyk2 activity blocked transendothelial neutrophil migration in comparison to the supernatants of LPS-treated controls, thus confirming the inhibition of functional IL-8 production. Investigations into the molecular mechanism of this pathway revealed that LPS activates Pyk2 leading to IL-8 production through the TLR4. In addition, we identified the p38 MAPK pathway to be a critical step downstream of Pyk2 during LPS-induced IL-8 production. Taken together, these results demonstrate a novel role for Pyk2 in LPS-induced IL-8 production in endothelial cells.
The endothelium lines the inner surface of blood vessels and functions as an interactive barrier between blood and tissue. Exposed to blood flow, it is the primary target for inflammatory agents during local and systemic inflammation (1, 2). Endothelial cells express chemokines that initiate the activation and recruitment of circulating leukocytes at sites of tissue inflammation (3, 4). The bacterial endotoxin, LPS, an essential component of the surface of Gram-negative bacteria (5), has potent proinflammatory properties by acting on many cell types including endothelial cells (3, 6). High levels of LPS are a major cause of Gram-negative septic shock, in which LPS induces numerous changes, including up-regulation of adhesion molecules as well as procoagulant activity, enhanced endothelial permeability, and secretion of proinflammatory mediators by the endothelium (3, 4, 7). Among these activities, secretion of IL-8 (CXCL8) by LPS-activated endothelial cells contributes substantially to inflammatory responses (8). IL-8 displays a chemotactic activity for neutrophils, which are the first line of immune cells to be recruited to infected areas (9, 10). IL-8 also activates neutrophils to generate several toxic products, such as arachidonic acid metabolites. Thus, during septicemia, IL-8 participates in a series of cellular events that severely damage the endothelium and surrounding tissues. However, the precise mechanism of LPS-induced signaling that leads to IL-8 secretion in endothelial cells is poorly understood. Elucidation of this pathway may be of immense significance in the design of anti-inflammatory therapies that act by regulating chemokine responses leading to septic shock-related events.
The proline-rich kinase 2, Pyk2, also known as RAFTK or CAKβ, is a cytoplasmic tyrosine kinase related to focal adhesion kinase (FAK)3 (11). However, unlike FAK, Pyk2 exhibits a more restricted tissue expression pattern primarily in epithelial cells, neuronal cells, fibroblasts, hematopoietic cells, and endothelial cells (11–13). Pyk2 has been shown to be activated in response to a broad range of stimuli, including extracellular signals that elevate intracellular Ca2+ concentration, agonists of G protein-coupled receptors, and engagement of Ag receptors on T cells, B cells, and mast cells (12, 14 –16). Pyk2 is also activated in response to inflammatory cytokines, stress signals, and integrin-mediated cell adhesion (12, 17, 18). It is fast emerging as a critical “platform” kinase that couples several receptors (including integrin and chemokine receptors) with a variety of downstream effectors, thus regulating various functions such as cell adhesion, migration, proliferation, and survival (11). Although the expression of Pyk2 has been identified in endothelial cells, very few reports have focused on its role in these cells. In view of recent studies that link the nonreceptor tyrosine kinase Pyk2 with inflammation (19, 20), our present study evaluated its role in mediating IL-8 secretion in LPS-stimulated endothelial cells.
Materials and Methods
Reagents, cells, and culture conditions
LPS and cyclohexamide were obtained from Sigma-Aldrich. The Pyk2 inhibitor (Tyrphostin A9), p38 MAPK inhibitor (SB203580), ERK kinase inhibitor (PD98059), Pam3Cys (TLR2 agonist), and polymyxin B were obtained from Calbiochem. Py99, Pyk2, phospho-Pyk2, and phospho-FAK Abs were obtained from BioSource International, whereas TLR2, TLR4, IL-8, phospho-ERK, ERK, phospho-p38, and p38 Abs were obtained from Santa Cruz Biotechnology. Isotype controls were purchased from BD Transduction Laboratories. HUVEC were purchased from Clonetics. Cells were grown at 37°C in 5% CO2 in endothelial growth medium EGM2-MV containing 2% FBS, 12 μg/ml bovine brain extract, 10 ng/ml human recombinant epidermal growth factor, 1 μg/ml hydrocortisone, and GA-1000 (1 μg/ml gentamicin and amphotericin B), according to the recommendations of the supplier. The human monocytic THP-1 cell line obtained from the American Type Culture Collection was cultured in RPMI 1640 medium supplemented with 10% FBS at 37°C in 5% CO2 atmosphere.
Stimulation
In all experiments, HUVEC were grown to 80% confluency in 6-well assay plates. The cells were stimulated with LPS in the presence of 0.5% FBS. In the case of inhibitor treatments (Tyrphostin A9, SB203580, PD98059), HUVEC were pretreated with inhibitor for 1 h after which they were stimulated with LPS for various time periods. The supernatant was used for the IL-8 or transendothelial migration (TEM) assays, and the cell lysates were used for the Western blotting and immunoprecipitation analyses. THP-1 cells were differentiated into macrophage-like cells by treating with 100 nM 1α,25-dihydroxy-vitamin D3 (Biomol) for 3 days. Vitamin D3-differentiated THP-1 cells were incubated with either Pam3Cys or LPS in the presence or absence of TLR2 and TLR4 blocking Abs, respectively, for 24 h. After stimulation, the culture supernatants were collected and the levels of IL-8 determined by ELISA.
Recombinant adeno-associated virus (AAV) transduction
High-efficiency gene delivery of the dominant negative Pyk2 mutant, Pyk2K457A (Pyk2MT), or a control gene, β-galactosidase (β-Gal), was accomplished using an recombinant AAV-based method. The AAV vectors were prepared as previously described (21). Briefly, the kinase-inactive Pyk2 mutant or the same vector encoding β-Gal cDNA (as a control for nonspecific effects of viral infection) were inserted between a CMV-immediate early promoter and an SV40 fragment (providing an intron as well as a polyadenylation function) in one of our lab’s standard AAV vector plasmids, pACP. Each gene cassette was framed between AAV2 inverted terminal repeats. The vectors were then packaged in AAV2 virions using a three-plasmid transient cotransfection system consisting of the vector plasmids pXX2 and pXX6. Cell lysates were collected 2 days after transfection, and the virions were isolated. The vector preps were then dialyzed before use. Genomic titers of the preparations, as determined by real-time PCR were ~1011 copies/ml. Before being exposed to the virus, the HUVEC were cultured overnight in complete medium. HUVEC were transduced by application of the AAV in a minimal amount of serum-free medium for 90 min at 37°C in a cell culture incubator. Equal volumes of complete endothelial basal medium (EBM) containing 4% serum were added to the cells to achieve a final serum concentration of 2%. The cells were finally cultured for 36 h before being used for the experiments described later. After transduction, LPS was added to the medium and the cells were incubated for an additional 24 h. The culture supernatant was removed and evaluated for IL-8 content. Alternatively, the cells were lysed and subjected to Western blot analysis by using rabbit anti-human Pyk2 Ab or β-Gal staining in the case of the control. In each well, at least 80% of the HUVEC appeared to be β-Gal-positive, confirming the high efficiency of our recombinant AAV-based approach (data not shown).
Cycloheximide treatment
HUVEC were either unstimulated or stimulated for 24 h with LPS (100 ng/ml) in the presence or absence of 10 μg/ml cycloheximide. Supernatants were harvested and the IL-8 production measured by ELISA.
IL-8 ELISA
After stimulation, the culture supernatants were collected, centrifuged, and processed for IL-8 quantification by commercially available ELISA kits (Endogen), per the manufacturer’s instructions.
Isolation of neutrophils
Human neutrophils were purified from normal donors by dextran sedimentation and Ficoll gradient centrifugation followed by hypotonic lysis of erythrocytes. The purity of the prepared neutrophils was >95% (as judged by the morphology of stained cytocentrifuged preparations) and the viability was >98% (as judged by the trypan blue dye exclusion method).
TEM assay of neutrophils
Briefly, ~100,000 HUVEC were added to fibronectin-coated 24-well Transculture chambers with a pore size of 3 μm (Costar; Corning) and grown for 3 days in 5% CO2 at 37°C. A total of 0.6 ml of medium from the untreated or LPS/Tyrphostin A9 or AAV/Pyk2MT-treated HUVEC was added to the lower compartment. In the upper compartment, 1 × 106 neutrophils in 0.1 ml of the EBM containing 0.5% FBS were added onto the HUVEC monolayer. Supernatants pretreated with 1000 U/ml polymyxin B and 20 μg/ml IL-8 Ab served as controls. The chambers were incubated for 4 h at 37°C in 5% CO2. The cells in the lower compartment were counted on a hemocytometer. The results are presented as mean ± SD of three separate experiments and are expressed as the increase in the number of cells migrating toward the lower compartment.
Western blotting and immunoprecipitation
Total cellular extracts from the LPS-treated cells were prepared by lysing the cells in radioimmunoprecipitation assay buffer (50 mM Tris-HCl (pH 7.4), 1% Nonidet P-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM PMSF, 10 μg/ml aprotinin, leupeptin, and pepstatin, 10 mM sodium vanadate, 10 mM sodium fluoride, and 10 mM sodium pyrophosphate). Proteins (50 μg) were size-fractionated by 8% SDS-PAGE and transferred onto nitrocellulose membranes. The membranes were blocked for 2–3 h with 5% nonfat milk and then incubated with the respective primary and secondary Abs for 2–3 h each. The membranes were washed three to four times for 15 min each with TBS and 0.05% Tween 20, and later developed by chemiluminescence (ECL System; GE Healthcare).
For the immunoprecipitation, equal amounts of protein from the stimulated time points were clarified by incubation with protein A-Sepharose CL-4B or GammaBind Sepharose beads (Amersham Biosciences) for 1 h at 4°C. The Sepharose beads were removed by brief centrifugation, and the supernatants were incubated with different primary Abs for 2 h at 4°C. Immunoprecipitation of the antigen-antibody complexes was performed by incubation at 4°C overnight with 50 μl of protein A-Sepharose or Gammabind Sepharose (50% suspension). Nonspecific interacting proteins were removed by washing the beads thrice with radioimmunoprecipitation RIPA buffer and once with PBS. Immunoprecipitated complexes were solubilized in 50 μl of 2X Laemmli buffer, and further analyzed by Western blotting, as described.
Immune complex kinase assay
HUVEC were lysed in modified RIPA buffer and equal amounts of cell lysates were immunoprecipitated with anti-Pyk2 Ab. The immunoprecipitates were incubated for 12 min at 23°C with 50 μl of Tris buffer containing 5 μCi [32P]ATP and 30 μg/ml poly(Glu-Tyr)4:1. The reaction was stopped by adding 2X SDS sample buffer and the 32P-labeled immune complexes were resolved on 7.5% SDS-PAGE. The gel was dried and the 32P-labeled proteins were made visible by autoradiography.
Cell surface staining and flow cytometry analysis
To detect TLR2 and TLR4 expression, HUVEC and THP-1 cells were stained with the monoclonal primary Abs, respectively, followed by staining with FITC- or PE-conjugated secondary Abs. Flow cytometry was conducted using a FACSCalibur cytometer and these data were analyzed by CellQuest software (BD Biosciences).
Small interfering RNA (siRNA)-mediated knockdown of Pyk2
RNA interference-mediated knockdown of Pyk2 was performed using SMARTpool Pyk2 duplex RNA oligonucleotides obtained from Dharmacon. A nontargeting siRNA (Qiagen) was used as the control. HUVEC were transfected with the siRNA using Lipofectamine 2000 reagent (Invitrogen Life Technologies), according to the manufacturer’s instructions. Pyk2 siRNA-mediated knockdown was estimated by detection of Pyk2 expression by Western blot analysis, 48 h after the initial transfection.
Statistical analysis
Reported data are mean ± SD of at least three independent experiments performed in duplicate or triplicate. The statistical significance was determined by the Student’s t test.
Results
LPS induces the tyrosine phosphorylation of Pyk2, but not FAK, in endothelial cells
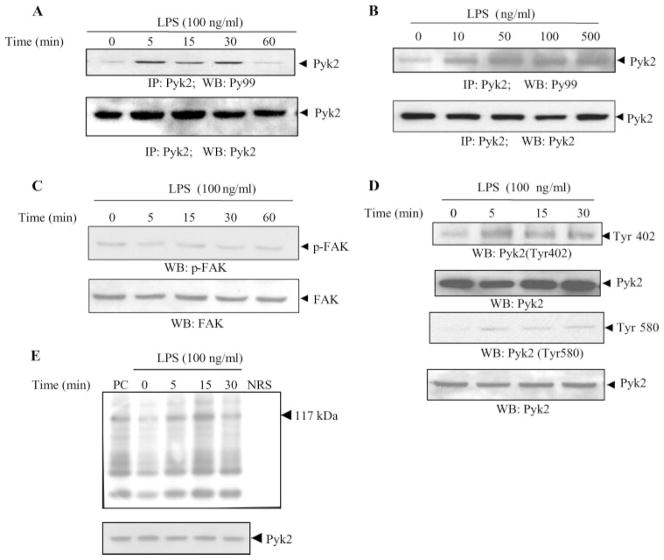
To investigate the signaling processes involved in LPS-induced cytokine production, we examined the ability of LPS to activate Pyk2. Pyk2 is a nonreceptor tyrosine kinase that is expressed abundantly in endothelial cells (22). Stimulation of endothelial cells with LPS (100 ng/ml) was accompanied by increased tyrosine phosphorylation of Pyk2, as demonstrated by immunoprecipitation experiments. We observed that LPS induces both a concentration and time-dependent tyrosine phosphorylation of Pyk2 (Fig. 1, A and B). The increase in Pyk2 phosphorylation was observed with as low as 10 ng/ml LPS and was most evident at 100 ng/ml LPS, 5–30 min after stimulation. Similar amounts of Pyk2 were immunoprecipitated in the samples, as demonstrated by blotting with anti-Pyk2 Ab (Fig. 1, A and B, bottom). In contrast, LPS did not induce an increase in FAK phosphorylation (Fig. 1C). We, therefore, focused the rest of our studies on Pyk2.
FIGURE 1.
LPS induces tyrosine phosphorylation and enzymatic activity of Pyk2 in endothelial cells. HUVEC were stimulated with LPS (100 ng/ml) for the indicated periods of time (A) or with various concentrations of LPS for 15 min (B). The lysates were then immunoprecipitated with Abs to Pyk2. The immunoprecipitates were analyzed by Western blotting with Abs to phosphotyrosine (Py99). The same blot was then probed with anti-Pyk2 Ab. C and D, Lysates obtained from HUVEC stimulated with LPS (100 ng/ml) for various periods of time were also analyzed by Western blotting with Abs to phospho-FAK (p-FAK) (C) or with Abs to phospho-Pyk2 (Tyr 402) and phospho-Pyk2 (Tyr 580) (D). E, The kinase activity in the immunoprecipitates was assessed as the ability of Pyk2 to phosphorylate the synthetic substrate poly(Glu-Tyr)4:1. A typical autoradiograph is shown (117 kDa). (top). NRS, Normal rabbit serum; PC, positive control. The total abundance of Pyk2 protein was analyzed by Western blot analysis of the lysates, as indicated (bottom). Data show one representative experiment of three independent experiments performed.
Pyk2 has a structure similar to FAK as it contains an autophosphorylation site (Tyr402), sites involved in kinase activation (Tyr579/580), and a site homologous to the Grb2-binding site (Tyr881) in FAK (11). Endothelial cells treated with LPS were analyzed for the phosphorylation and activation of Pyk2 using Abs specific for these sites of tyrosine phosphorylation. We observed that LPS enhanced the phosphorylation of residues 402 and 580 (Fig. 1D), indicating an important function for these residues in LPS-induced Pyk2 activation. However, LPS did not markedly induce phosphorylation of residue 881 (data not shown).
LPS induces Pyk2 tyrosine kinase activity
To assess whether increased Pyk2 tyrosine phosphorylation leads to the induction of Pyk2 enzyme activity, Pyk2 immunoprecipitates from control or LPS-stimulated HUVEC were assayed for Pyk2 activity based on its ability to phosphorylate a synthetic substrate poly(Glu-Tyr)4:1 in an in vitro kinase assay (Fig. 1E). As a positive control, Pyk2 activity was stimulated with 100 ng/ml vascular endothelial growth factor for 2 min. LPS significantly increased the activation of Pyk2 and its tyrosine kinase activity peaked at 15 min.
Inhibition of Pyk2 activation attenuates LPS-induced IL-8 production
Because Pyk2 is rapidly being recognized as a tyrosine kinase of central importance in diverse cell types, we hypothesized that Pyk2 could be involved in the LPS signaling that mediates acute inflammatory responses in endothelium. Thus, we tested the possible role of Pyk2 in LPS-induced cytokine production. Tyrphostin A9 (AG17) is the most selective of tyrosine kinase inhibitors that block the Pyk2 signaling pathway (23), thus providing an effective tool for investigating the role of this tyrosine kinase in cellular signaling. Our initial studies testing the possible involvement of Pyk2 in LPS-induced cytokine expression using Ab arrays demonstrated that certain cytokines that are known to be up-regulated on stimulation with LPS, such as GROα, IL-1ra, IL-6, IL-8, IP-10, and migration inhibitory factor, were inhibited by the specific Pyk2 inhibitor, Tyrphostin A9 (data not shown). Because IL-8 is the prototypic CXC chemokine (9) and it contributes most significantly to the inflammatory process in endothelial cells (7), we further investigated the molecular mechanisms of Pyk2 in LPS-induced IL-8 production.
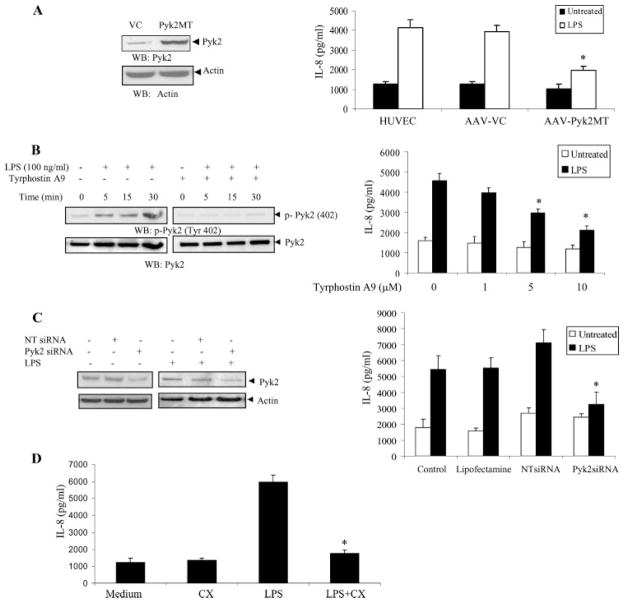
We used three approaches to study the role of Pyk2 in regulating IL-8 production: 1) expression of a dominant negative form of Pyk2 that removes a key lysine involved in ATP binding, 2) use of a specific chemical inhibitor, and 3) knockdown of Pyk2 protein expression using siRNA.
As a first approach, we constructed AAV vectors expressing a kinase-inactive Pyk2 mutant (Pyk2K457A) and then HUVEC were transduced with this dominant negative Pyk2. In this mutant, mutation of Lys457 to alanine in the tyrosine kinase domain of Pyk2 creates an inactive Pyk2 that inhibits wild-type Pyk2 activity. We observed that endothelial cells transduced with the catalytically inactive Pyk2 mutant (AAV-Pyk2MT) exhibited significantly attenuated LPS-induced IL-8 production as compared with the vector control (β-Gal) transduced cells (Fig. 2A, right). The inhibitory effect was present over a range of LPS stimulations (10–100 ng/ml) (data not shown) with maximal inhibition occurring at 100 ng/ml LPS. It should be noted that a basal production of IL-8 was always present in the supernatant. Overexpression of Pyk2MT in HUVEC was confirmed by Western blot analysis of the cell lysates (Fig. 2A, left). This result provides direct evidence for the role of Pyk2 in LPS-induced IL-8 production.
FIGURE 2.
Inhibition of Pyk2 activation blocks LPS-induced IL-8 production in endothelial cells. A, HUVEC were transduced with recombinant AAV-expressing Pyk2 mutant (Pyk2MT) or AAV β-Gal vector control (VC). Overexpression of the mutant was demonstrated by Western blot analysis with anti-Pyk2 Abs 48 h after transduction (top left). Anti-actin Ab was used as an internal control (bottom left). Transduced cells were tested for their ability to produce IL-8 upon LPS stimulation (100 ng/ml) using a commercial ELISA kit (Endogen) (right).*, p < 0.05 vs the AAV vector control (AAV-VC). Data represent the mean ± SD of three independent experiments. B, HUVEC were pretreated with vehicle or Tyrphostin A9 (5 μM) for 1 h at 37°C. The cells were then stimulated with LPS for various time periods in EBM with 0.5% FBS. The cells were lysed and analyzed by Western blotting with anti-phospho-Pyk2 (Tyr 402) Abs (left). The same blots were probed with anti-Pyk2 Abs. HUVEC preincubated with vehicle or various concentrations of Tyrphostin A9 for 1 h were cultured without or with LPS (100 ng/ml) and the concentration of IL-8 in the culture supernatants was determined 24 h after stimulation (right).*, p < 0.05 compared with the vehicle control. Data represent the mean ± SD of three independent experiments. C, HUVEC were transfected with Pyk2-specific siRNA and nontargeting siRNA (NT siRNA) using Lipofectamine in the absence or presence of LPS (100 ng/ml). The knock down of Pyk2 expression was analyzed by Western blotting with anti-Pyk2 Abs (left). Anti-actin Ab was used as an internal control. The supernatants from the siRNA transfected cells were analyzed for IL-8 expression after stimulation with LPS (100 ng/ml) (right).*, p < 0.05 compared with the nontargeting siRNA control. D, HUVEC were treated with LPS (100 ng/ml) in the absence or presence of 10 μg/ml cyclohexamide (CX). After 24 h, the supernatants were collected and analyzed for IL-8 expression.*, p < 0.05 compared with the LPS control. Data represent the mean ± SD of three independent experiments.
We also demonstrated that Tyrphostin A9, a specific inhibitor of Pyk2, inhibited LPS-induced Pyk2 phosphorylation in a time-dependent manner (Fig. 2B, left). Furthermore, the inhibitor blocked LPS-induced IL-8 expression in the endothelial cells in a concentration-dependent manner (Fig. 2B, right).
As a third approach, we decreased Pyk2 expression in HUVEC using siRNA Pyk2. Our optimization studies indicated that the expression of Pyk2 protein was specifically decreased by transfection with the siRNA Pyk2 duplex (100 nM), but not by transfection with a nontargeting siRNA (Fig. 2C, left). At this siRNA concentration (100 nM), there was minimal loss of cell viability (<10%) (data not shown). There was no difference in the Pyk2 knock down in the absence or presence of LPS (100 ng/ml). The Pyk2 siRNA-treated cells showed reduced IL-8 expression compared with cells treated with nontargeting siRNA control (Fig. 2C, right). Taken together, these data suggest that Pyk2-dependent activation is an important part of the LPS-IL-8 signaling pathway.
Cycloheximide treatment at 10 μg/ml completely blocked LPS-induced IL-8 production, confirming that the up-regulation of IL-8 expression was due to de novo synthesis (Fig. 2D). To exclude a possible toxic effect of cyclohexamide, cells that had been cultured in its presence were washed and restimulated with LPS in the absence of cyclohexamide, and IL-8 production was measured after 24 h. HUVEC were still able to respond to LPS (data not shown).
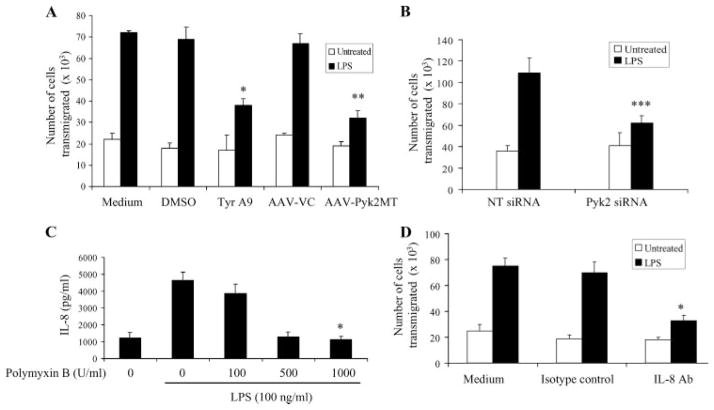
Inhibition of Pyk2 activation reduces the LPS-induced TEM of neutrophils
IL-8 has been shown to be a potent mediator of the extravasation and accumulation of neutrophils at the sites of injury by their adhesion to and migration through the endothelial lining (24). Hence, the TEM of neutrophils toward the supernatants of LPS-stimulated endothelial cells transduced with AAV-Pyk2MT or pretreated with Tyrphostin A9 was assessed to determine whether the IL-8 produced was functionally active. As shown, transduction of LPS-stimulated endothelial cells with Pyk2MT and pretreatment with Tyrphostin A9 significantly inhibited neutrophil migration across a HUVEC monolayer compared with the supernatants of cells treated with LPS only (Fig. 3A). To confirm that the carryover of Tyrphostin A9 did not influence IL-8-mediated neutrophil migration, rIL-8 (100 ng/ml) was added to the supernatants with or without Tyrphostin A9. There was no difference in IL-8-mediated transmigration of neutrophils toward these supernatants with or without Tyrphostin A9 (data not shown). In addition, we also observed that supernatants from the Pyk2-specific siRNA-treated cells showed attenuated neutrophil transmigration, in comparison to the supernatants of cells treated with nontargeting siRNA (Fig. 3B). The TEM of neutrophils toward any residual LPS present in the supernatant was eliminated by pretreatment with polymyxin B. Polymyxin B was used at a concentration of 1000 U because almost complete inhibition of LPS-induced IL-8 expression was seen at this concentration (Fig. 3C).
FIGURE 3.
Inhibition of the LPS-induced production of IL-8 decreased the TEM of human neutrophils. Supernatants from the Tyrphostin A9-treated (Tyr A9) and Pyk2 mutant-overexpressing (AAV-Pyk2MT) (A) or Pyk2-specific siRNA-treated (Pyk2 siRNA) and nontargeting siRNA (NT siRNA) (B) HUVEC cells were stimulated with LPS (100 ng/ml) and tested for their ability to induce neutrophil migration using a TEM assay.*, p < 0.05 vs the vehicle control. **, p < 0.05 compared with the AAV vector control (AAV-VC). ***, p < 0.05 vs the nontargeting siRNA control. C, HUVEC pretreated with various concentrations of polymyxin B were stimulated with 100 ng/ml LPS. The IL-8 content in the supernatants was measured after 24 h of stimulation. *, p < 0.05 vs the LPS control. D, Supernatants from untreated or LPS-treated HUVEC pretreated with IL-8 blocking Ab or an isotype control were also tested for their ability to induce neutrophil migration across the endothelium using the TEM assay. *, p < 0.05 vs the isotype control. Data represent mean ± SD of three independent experiments.
To specifically test the role of IL-8 in migration, 20 μg/ml human IL-8-specific goat Ab was added to the supernatants for 1 h before the assay was initiated. The results demonstrated that anti-IL-8 Ab blocked the neutrophil migration across the HUVEC monolayer, which was induced by the LPS-treated supernatants (Fig. 3D). These results confirmed that the secreted IL-8 in the supernatants was functionally active and hence indicated a physiological function of Pyk2 activity in regulating IL-8 production.
LPS-induced Pyk2 activation leading to IL-8 expression is mediated by TLR4
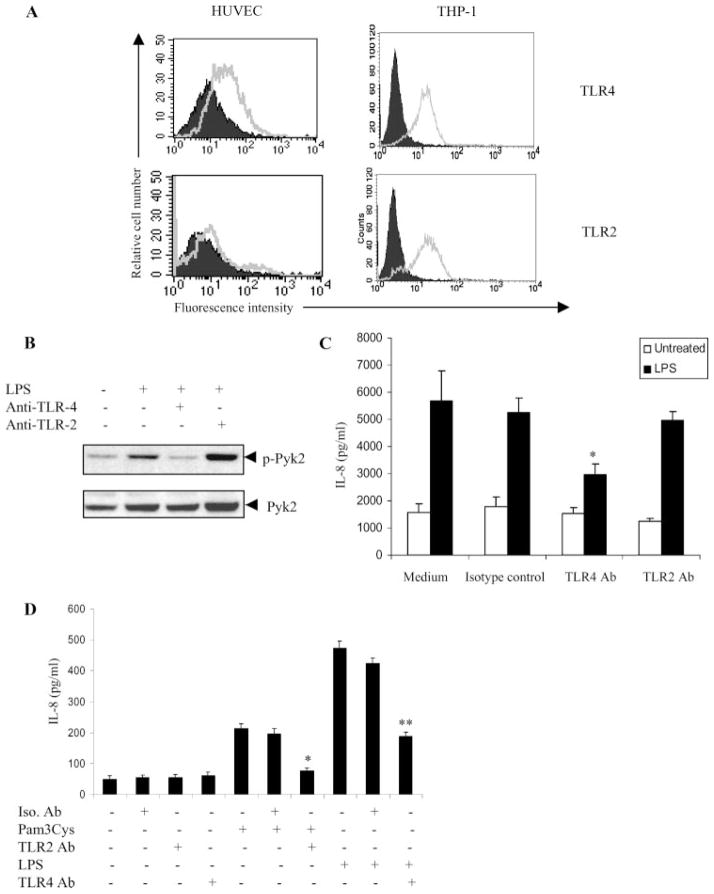
Previous studies have indicated that TLR4, and to a certain extent TLR2, are the main receptors for LPS in endothelial cells (25–27). To investigate the involvement of TLR2 and TLR4 in LPS-responsive HUVEC, we first analyzed the expression pattern of TLRs in HUVEC by FACS analysis. TLR4 was detected at moderate levels on the surface of HUVEC (Fig. 4A, top left). However, TLR2 expression was minimal (Fig. 4A, bottom left). THP-1, a monocytic cell line was used as a positive control for expression of TLR2 and TLR4 (Fig. 4A, right).
FIGURE 4.
The LPS-Pyk2-IL-8 pathway is TLR4-dependent. A, HUVEC and THP-1 were stained using Abs against TLR4 (left) or TLR2 (right) and analyzed by flow cytometry. Cells stained with control IgG represent the Ab control (AbC). Ab control (filled histogram) and TLR expression (open histogram) are indicated. B, HUVEC were pretreated with anti-TLR4 or anti-TLR2 blocking Ab (10 μg/ml) or isotype control for 1 h at 37°C. The cells were then stimulated with LPS (100 ng/ml) for 15 min in EBM with 0.5% FBS. The cells were lysed and analyzed by Western blotting with anti-phospho-Pyk2 (Tyr 402) Abs (top). The same blots were probed with anti-Pyk2 Abs (bottom). C, HUVEC preincubated with anti-TLR4 Ab, anti-TLR2 Ab, or isotype control for 1 h were cultured with or without LPS (100 ng/ml). The concentration of IL-8 in the culture supernatants was determined 24 h after stimulation. *, p < 0.05 compared with the vehicle control. Data represent the mean ± SD of three independent experiments. D, Vitamin D3-differentiated THP-1 cells preincubated with isotype control (Iso. Ab) or anti-TLR2 Ab for 1 h were cultured with or without Pam3Cys (10 pg/ml). Similarly, THP-1 cells, preincubated with isotype control (Iso. Ab) or anti-TLR4 Ab for 1 h were cultured with or without LPS (100 ng/ml). The concentration of IL-8 in the culture supernatants was determined 24 h after stimulation. *, p < 0.05 compared with the isotype control. **, p < 0.05 compared with the isotype control.
To further determine the functional role of TLR4 and TLR2 in LPS-induced Pyk2 activation and IL-8 expression, the ability of anti-TLR4- and anti-TLR2-neutralizing Abs to block these LPS-induced effects was examined. At concentrations of 10 μg/ml, anti-TLR4 Ab significantly inhibited both the Pyk2 activation (Fig. 4B) and IL-8 production (Fig. 4C) induced by LPS. However, anti-TLR2 Ab did not have any effect. The functional ability of the TLR2 and TLR4 blocking Ab was confirmed by inhibition of Pam3Cys-induced IL-8 production and LPS-induced IL-8 production, respectively, in vitamin D3-differentiated THP-1 cells (Fig. 4D). In THP-1 cells, Pam3Cys, an analog of bacterial lipoprotein has been shown to induce IL-8 production via TLR2 (28), whereas LPS has been shown to induce IL-8 production through TLR4. (29). Taken together, these findings suggest that LPS requires cell surface TLR4 to induce Pyk2 activation leading to IL-8 production in endothelial cells.
Pyk2 regulates IL-8 production through the p38 MAPK pathway
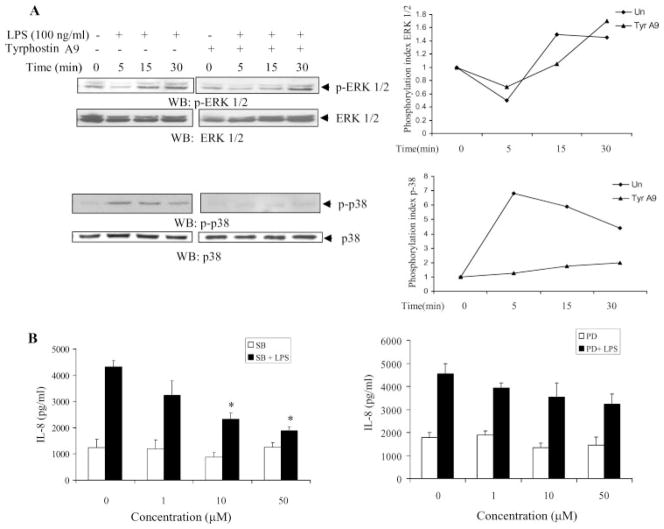
Pyk2 has been shown to act as an essential intermediate providing a link between extracellular stimuli and MAPK signaling pathways in various cell lines (30). Of the signaling pathways activated by LPS in endothelial cells, the p44/42 MAPK and p38 MAPK pathways have been shown to be directly involved in the production of cytokines (31–34). Hence, we determined whether inhibition of Pyk2 with Tyrphostin A9 blocked MAPK activation. Treatment of HUVEC with LPS resulted in the phosphorylation of both ERK and p38 MAPK, as reported previously (31–34). However, pre-treatment with Tyrphostin A9 significantly inhibited the LPS-induced p38 phosphorylation (Fig. 5A, bottom left), but did not significantly influence ERK phosphorylation (Fig. 5A, top left), suggesting that p38 MAPK may be an important downstream molecule of Pyk2 in LPS-induced IL-8 production. The phosphorylation indices of ERK and p38 MAPK are shown in Fig. 5A (right). The involvement of the p38 MAPK pathway was confirmed by the dose-dependent blocking of LPS-induced IL-8 with a specific inhibitor of p38 MAPK, SB203580 (Fig. 5B, left). Though the use of a specific ERK kinase inhibitor, PD98059, reduced the LPS-induced expression of IL-8 slightly, the difference was not statistically significant (Fig. 5B, right).
FIGURE 5.
Pyk2 regulates LPS-induced IL-8 expression through the p38 MAPK pathway. A, HUVEC was pretreated with vehicle or Tyrphostin A9 (5 μM) for 1 h at 37°C. The cells were then stimulated with LPS for various periods of time in EBM with 0.5% FBS. The cells were lysed and analyzed by Western blotting with anti-phospho-ERK1/2 (p-ERK1/2) Abs (upper left blots) or anti-phospho-p38 (p-38) Abs (lower left blots). The same blots were probed with anti-ERK1/2 or anti-p38 Abs, respectively. Data show one representative experiment of three independent experiments. For quantitative analysis of protein phosphorylation, the ratio of phosphorylation vs total protein in each lane was obtained by densitometry. The phosphorylation index of ERK and p38 was determined by calculating the value of this ratio in each lane and presenting the ratio as the fold increase over the control value (unstimulated sample; 0), which was designated as 1 (right). B, HUVEC pretreated with vehicle, the p38 MAPK inhibitor (SB203580, SB) (left) or the ERK kinase inhibitor (PD98059, PD) (right) for 1 h were stimulated with 100 ng/ml LPS and then the concentration of IL-8 in the supernatants was measured 24 h after stimulation. *, p < 0.05 compared with the vehicle control. Data represent mean ± SD of three independent experiments.
Discussion
Pyk2 is rapidly becoming recognized as a tyrosine kinase of central importance in diverse cell types such as neuronal cells, hematopoietic cells, liver epithelial cells, and vascular smooth muscle cells (11, 12, 14, 35). However, little is known about Pyk2 regulation in vascular endothelial cells. In this study, we present data indicating that activation of Pyk2 plays a key role in modulating LPS-induced IL-8 production in endothelial cells. We have demonstrated Pyk2 activation by an increase in both tyrosine phosphorylation and enzyme activity of Pyk2. Though previous studies on endothelial cells have shown that Pyk2 is tyrosine-phosphorylated in response to stimulation with G protein-coupled receptor of agonists, vascular endothelial growth factor, and the cytokine IL-1α (13, 36), this study is the first to our knowledge to demonstrate the activation of Pyk2 in response to LPS in endothelial cells.
The proline-rich kinase-2 Pyk2 is a cytoplasmic tyrosine kinase showing considerable sequence homology and structural similarity to FAK, including consensus motifs in the catalytic domain. Despite the high sequence homology, structural similarities, and common downstream effector molecules between FAK and Pyk2, recent studies provide evidence of their different subcellular localization and regulatory mechanisms (37, 38). In the present study, LPS did not induce the tyrosine phosphorylation of FAK, and therefore the activities of Pyk2 and FAK are likely to be regulated by different upstream molecules in LPS-stimulated endothelial cells.
Several lines of recent evidence suggest that Pyk2 tyrosine phosphorylation could be closely linked to inflammatory processes in multiple cell types (12, 13, 20). In endothelial cells, the LPS-induced production of inflammatory cytokines has been reported to contribute significantly to the inflammatory process (7). Preliminary cytokine array studies in our laboratory indicated IL-8 to be among the most prominent chemokines attenuated in LPS-stimulated endothelial cells treated with Tyrphostin A9 (data not shown). Hence, we further investigated the molecular mechanism by which Pyk2 regulates LPS-induced IL-8 production using AAV-expressing a Pyk2 kinase-inactive mutant, Tyrphostin A9, and Pyk2-specific siRNA. Using all three approaches, we were able to demonstrate decreased LPS-induced IL-8 production, in comparison to the controls. Overexpression of a catalytically inactive form of Pyk2, Pyk2-K457A in pulmonary endothelial cells has been demonstrated to result in defects in cell adhesion, spreading and migration (39, 40). Although Pyk2-specific siRNA blocked IL-8 expression compared with the nontargeted siRNA control, the basal levels of IL-8 in the controls treated only with siRNA were marginally higher than the levels in untreated control. The higher basal levels of IL-8 produced on treatment with only siRNA are in agreement with a recent study that shows that siRNA may nonspecifically trigger the production of IL-8 (41). The physiological relevance of these experiments was confirmed by the inhibition of human neutrophil transmigration toward the supernatants of the LPS-treated HUVEC that were transduced with AAV-Pyk2MT, pretreated with Tyrphostin A9 or transfected with Pyk2-specific siRNA, in comparison to the LPS-treated controls, suggesting that Pyk2 modulates neutrophil infiltration across the endothelial cells via regulation of IL-8 production.
Increasing evidence suggests that central to the recognition of LPS expression in endothelial cells is a family of transmembrane proteins known as TLRs that have leucine-rich repeats in their extracellular domains (42, 43). Most effector cells of the innate immune system, such as monocytes and endothelial cells express TLR2 and TLR4 (26). Our studies on the expression of these two receptors in HUVEC confirmed a previous report (26) that indicated that HUVEC predominantly express TLR4 and weakly express TLR2. Using anti-TLR4 blocking Abs, we were able to demonstrate the inhibition of both LPS-induced Pyk2 activation and IL-8 production. From this demonstration, it seems reasonable to suggest that TLR4 is an important part of the Pyk2 signaling pathway, acting to transmit LPS-dependent signals that lead to IL-8 production.
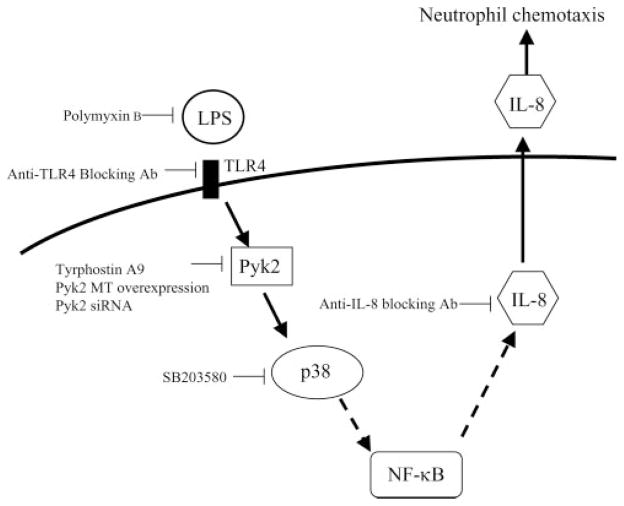
Pyk2 has been shown to function as an essential intermediate providing a link between extracellular stimuli and signaling pathways involving MAPKs (30, 44). The central role of p38 MAPK-dependent signaling for LPS-induced endothelial cell activation has been highlighted in several recent studies (32, 33, 45). We were able to demonstrate that p38 MAPK is an important part of the signaling cascade downstream of Pyk2 in LPS-IL-8 pathway in endothelial cells. Based on our data, we therefore propose a model that suggests that, as a result of the interaction of LPS with TLR4 on the cell surface, a signaling route is initiated via Pyk2 and the p38 MAPK pathway leading to the production of IL-8 (Fig. 6), possibly through NF-κB activation. Activation of NF-κB, downstream to p38 MAPK, is known to be an essential prerequisite for LPS-induced IL-8 expression in endothelial cells (33, 46).
FIGURE 6.
Proposed scheme of Pyk2 regulation of the signaling pathway leading to IL-8 expression in endothelial cells upon LPS stimulation.
In summary, we show that LPS induces Pyk2 activation in endothelial cells. The specific inhibition of Pyk2 activity was paralleled by a strong reduction in both LPS-induced IL-8 production and neutrophil chemotaxis, pinpointing a key role of Pyk2 in the LPS-induced production of the inflammatory cytokine. Because LPS-induced IL-8 production is known to be involved in the pathogenesis of sepsis and several inflammatory diseases, these results indicate that Pyk2 may represent a novel target for the development of innovative therapeutic strategies against inflammatory conditions.
Acknowledgments
We thank Janet Delahanty for editing the manuscript.
Footnotes
This work is supported in part by Grants AI49140 and CA109527 from the National Institutes of Health (to R.K.G.).
Abbreviations used in this paper: FAK, focal adhesion kinase; siRNA, small interfering RNA; AAV, adeno-associated virus; EBM, endothelial basal medium; TEM, transendothelial migration; β-Gal, β-galactosidase.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Hack CE, Zeerleder S. The endothelium in sepsis: source of and a target for inflammation. Crit Care Med. 2001;29:S21–S27. doi: 10.1097/00003246-200107001-00011. [DOI] [PubMed] [Google Scholar]
- 2.Trepels T, Zeiher AM, Fichtlscherer S. The endothelium and inflammation. Endothelium. 2006;13:423–429. doi: 10.1080/10623320601061862. [DOI] [PubMed] [Google Scholar]
- 3.Berman RS, Frew JD, Martin W. Endotoxin-induced arterial endothelial barrier dysfunction assessed by an in vitro model. Br J Pharmacol. 1993;110:1282–1284. doi: 10.1111/j.1476-5381.1993.tb13956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bannerman DD, Goldblum SE. Endotoxin induces endothelial barrier dysfunction through protein tyrosine phosphorylation. Am J Physiol. 1997;273:L217–L226. doi: 10.1152/ajplung.1997.273.1.L217. [DOI] [PubMed] [Google Scholar]
- 5.Raetz CR. Biochemistry of endotoxins. Annu Rev Biochem. 1990;59:129–170. doi: 10.1146/annurev.bi.59.070190.001021. [DOI] [PubMed] [Google Scholar]
- 6.Munshi N, Fernandis AZ, Cherla RP, Park IW, Ganju RK. Lipopolysaccharide-induced apoptosis of endothelial cells and its inhibition by vascular endothelial growth factor. J Immunol. 2002;168:5860–5866. doi: 10.4049/jimmunol.168.11.5860. [DOI] [PubMed] [Google Scholar]
- 7.Bierhaus A, Chen J, Liliensiek B, Nawroth PP. LPS and cytokine-activated endothelium. Semin Thromb Hemostasis. 2000;26:571–587. doi: 10.1055/s-2000-13214. [DOI] [PubMed] [Google Scholar]
- 8.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 9.Smart SJ, Casale TB. Interleukin-8-induced transcellular neutrophil migration is facilitated by endothelial and pulmonary epithelial cells. Am J Respir Cell Mol Biol. 1993;9:489– 495. doi: 10.1165/ajrcmb/9.5.489. [DOI] [PubMed] [Google Scholar]
- 10.Smith WB, Gamble JR, Clark-Lewis I, Vadas MA. Interleukin-8 induces neutrophil transendothelial migration. Immunology. 1991;72:65–72. [PMC free article] [PubMed] [Google Scholar]
- 11.Avraham H, Park SY, Schinkmann K, Avraham S. RAFTK/Pyk2-mediated cellular signalling. Cell Signal. 2000;12:123–133. doi: 10.1016/s0898-6568(99)00076-5. [DOI] [PubMed] [Google Scholar]
- 12.Astier A, Avraham H, Manie SN, Groopman J, Canty T, Avraham S, Freedman AS. The related adhesion focal tyrosine kinase is tyrosine-phosphorylated after β1-integrin stimulation in B cells and binds to p130cas. J Biol Chem. 1997;272:228–232. doi: 10.1074/jbc.272.1.228. [DOI] [PubMed] [Google Scholar]
- 13.Keogh RJ, Houliston RA, Wheeler-Jones CP. Human endothelial Pyk2 is expressed in two isoforms and associates with paxillin and p130Cas. Biochem Biophys Res Commun. 2002;290:1470–1477. doi: 10.1006/bbrc.2002.6350. [DOI] [PubMed] [Google Scholar]
- 14.Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio JM, Plowman GD, Rudy B, Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca2+-induced regulation of ion channel and MAP kinase functions. Nature. 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 15.Qian D, Lev S, van Oers NS, Dikic I, Schlessinger J, Weiss A. Tyrosine phosphorylation of Pyk2 is selectively regulated by Fyn during TCR signaling. J Exp Med. 1997;185:1253–1259. doi: 10.1084/jem.185.7.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okazaki H, Zhang J, Hamawy MM, Siraganian RP. Activation of protein-tyrosine kinase Pyk2 is downstream of Syk in FcεRI signaling. J Biol Chem. 1997;272:32443–32447. doi: 10.1074/jbc.272.51.32443. [DOI] [PubMed] [Google Scholar]
- 17.Miyazaki T, Takaoka A, Nogueira L, Dikic I, Fujii H, Tsujino S, Mitani Y, Maeda M, Schlessinger J, Taniguchi T. Pyk2 is a downstream mediator of the IL-2 receptor-coupled Jak signaling pathway. Genes Dev. 1998;12:770–775. doi: 10.1101/gad.12.6.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tokiwa G, Dikic I, Lev S, Schlessinger J. Activation of Pyk2 by stress signals and coupling with JNK signaling pathway. Science. 1996;273:792–794. doi: 10.1126/science.273.5276.792. [DOI] [PubMed] [Google Scholar]
- 19.Di Cioccio V, Strippoli R, Bizzarri C, Troiani G, Cervellera MN, Gloaguen I, Colagrande A, Cattozzo EM, Pagliei S, Santoni A, et al. Key role of proline-rich tyrosine kinase 2 in interleukin-8 (CXCL8/IL-8)-mediated human neutrophil chemotaxis. Immunology. 2004;111:407–415. doi: 10.1111/j.1365-2567.2004.01822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katagiri T, Takahashi T, Sasaki T, Nakamura S, Hattori S. Protein-tyrosine kinase Pyk2 is involved in interleukin-2 production by Jurkat T cells via its tyrosine 402. J Biol Chem. 2000;275:19645–19652. doi: 10.1074/jbc.M909828199. [DOI] [PubMed] [Google Scholar]
- 21.Madry H, Cucchiarini M, Terwilliger EF, Trippel SB. Recombinant adeno-associated virus vectors efficiently and persistently transduce chondrocytes in normal and osteoarthritic human articular cartilage. Hum Gene Ther. 2003;14:393–402. doi: 10.1089/104303403321208998. [DOI] [PubMed] [Google Scholar]
- 22.Tang H, Hao Q, Fitzgerald T, Sasaki T, Landon EJ, Inagami T. Pyk2/CAKβ tyrosine kinase activity-mediated angiogenesis of pulmonary vascular endothelial cells. J Biol Chem. 2002;277:5441–5447. doi: 10.1074/jbc.M110673200. [DOI] [PubMed] [Google Scholar]
- 23.Fuortes M, Melchior M, Han H, Lyon GJ, Nathan C. Role of the tyrosine kinase pyk2 in the integrin-dependent activation of human neutrophils by TNF. J Clin Invest. 1999;104:327–335. doi: 10.1172/JCI6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qi J, Goralnick S, Kreutzer DL. Fibrin regulation of interleukin-8 gene expression in human vascular endothelial cells. Blood. 1997;90:3595–3602. [PubMed] [Google Scholar]
- 25.Andreakos E, Sacre SM, Smith C, Lundberg A, Kiriakidis S, Stonehouse T, Monaco C, Feldmann M, Foxwell BM. Distinct pathways of LPS-induced NF-κB activation and cytokine production in human myeloid and non-myeloid cells defined by selective utilization of MyD88 and Mal/TIRAP. Blood. 2004;103:2229–2237. doi: 10.1182/blood-2003-04-1356. [DOI] [PubMed] [Google Scholar]
- 26.Faure E, Equils O, Sieling PA, Thomas L, Zhang FX, Kirschning CJ, Polentarutti N, Muzio M, Arditi M. Bacterial lipopolysaccharide activates NF-κB through toll-like receptor 4 (TLR-4) in cultured human dermal endothelial cells: differential expression of TLR-4 and TLR-2 in endothelial cells. J Biol Chem. 2000;275:11058–11063. doi: 10.1074/jbc.275.15.11058. [DOI] [PubMed] [Google Scholar]
- 27.Yang RB, Mark MR, Gray A, Huang A, Xie MH, Zhang M, Goddard A, Wood WI, Gurney AL, Godowski PJ. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature. 1998;395:284–288. doi: 10.1038/26239. [DOI] [PubMed] [Google Scholar]
- 28.Uehara A, Yang S, Fujimoto Y, Fukase K, Kusumoto S, Shibata K, Sugawara S, Takada H. Muramyldipeptide and diaminopimelic acid-containing desmuramylpeptides in combination with chemically synthesized Toll-like receptor agonists synergistically induced production of interleukin-8 in a NOD2- and NOD1-dependent manner, respectively, in human monocytic cells in culture. Cell Microbiol. 2005;7:53–61. doi: 10.1111/j.1462-5822.2004.00433.x. [DOI] [PubMed] [Google Scholar]
- 29.Yang S, Tamai R, Akashi S, Takeuchi O, Akira S, Sugawara S, Takada H. Synergistic effect of muramyldipeptide with lipopolysaccharide or lipoteichoic acid to induce inflammatory cytokines in human monocytic cells in culture. Infect Immun. 2001;69:2045–2053. doi: 10.1128/IAI.69.4.2045-2053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dikic I, Tokiwa G, Lev S, Courtneidge SA, Schlessinger J. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature. 1996;383:547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- 31.Schumann RR, Pfeil D, Lamping N, Kirschning C, Scherzinger G, Schlag P, Karawajew L, Herrmann F. Lipopolysaccharide induces the rapid tyrosine phosphorylation of the mitogen-activated protein kinases erk-1 and p38 in cultured human vascular endothelial cells requiring the presence of soluble CD14. Blood. 1996;87:2805–2814. [PubMed] [Google Scholar]
- 32.Hashimoto S, Gon Y, Matsumoto K, Maruoka S, Takeshita I, Hayashi S, Asai Y, Jibiki I, Machino T, Horie T. Selective inhibitor of p38 mitogen-activated protein kinase inhibits lipopolysaccharide-induced interleukin-8 expression in human pulmonary vascular endothelial cells. J Pharmacol Exp Ther. 2000;293:370–375. [PubMed] [Google Scholar]
- 33.Hippenstiel S, Soeth S, Kellas B, Fuhrmann O, Seybold J, Krull M, Eichel-Streiber C, Goebeler M, Ludwig S, Suttorp N. Rho proteins and the p38-MAPK pathway are important mediators for LPS-induced interleukin-8 expression in human endothelial cells. Blood. 2000;95:3044–3051. [PubMed] [Google Scholar]
- 34.Rocic P, Govindarajan G, Sabri A, Lucchesi PA. A role for PYK2 in regulation of ERK1/2 MAP kinases and PI 3-kinase by ANG II in vascular smooth muscle. Am J Physiol. 2001;280:C90–C99. doi: 10.1152/ajpcell.2001.280.1.C90. [DOI] [PubMed] [Google Scholar]
- 35.Gismondi A, Bisogno L, Mainiero F, Palmieri G, Piccoli M, Frati L, Santoni A. Proline-rich tyrosine kinase-2 activation by β1 integrin fibronectin receptor cross-linking and association with paxillin in human natural killer cells. J Immunol. 1997;159:4729– 4736. [PubMed] [Google Scholar]
- 36.Anfosso F, Bardin N, Vivier E, Sabatier F, Sampol J, Dignat-George F. Outside-in signaling pathway linked to CD146 engagement in human endothelial cells. J Biol Chem. 2001;276:1564–1569. doi: 10.1074/jbc.M007065200. [DOI] [PubMed] [Google Scholar]
- 37.Zheng C, Xing Z, Bian ZC, Guo C, Akbay A, Warner L, Guan JL. Differential regulation of Pyk2 and focal adhesion kinase (FAK): the C-terminal domain of FAK confers response to cell adhesion. J Biol Chem. 1998;273:2384–2389. doi: 10.1074/jbc.273.4.2384. [DOI] [PubMed] [Google Scholar]
- 38.Schaller MD, Sasaki T. Differential signaling by the focal adhesion kinase and cell adhesion kinase β. J Biol Chem. 1997;272:25319–25325. doi: 10.1074/jbc.272.40.25319. [DOI] [PubMed] [Google Scholar]
- 39.Basile JR, Afkhami T, Gutkind JS. Semaphorin 4D/plexin-B1 induces endothelial cell migration through the activation of PYK2, Src, and the phosphatidylinositol 3-kinase-Akt pathway. Mol Cell Biol. 2005;25:6889– 6898. doi: 10.1128/MCB.25.16.6889-6898.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuwabara K, Nakaoka T, Sato K, Nishishita T, Sasaki T, Yamashita N. Differential regulation of cell migration and proliferation through proline-rich tyrosine kinase 2 in endothelial cells. Endocrinology. 2004;145:3324–3330. doi: 10.1210/en.2003-1433. [DOI] [PubMed] [Google Scholar]
- 41.Pauls E, Senserrich J, Bofill M, Clotet B, Este JA. Induction of interleukins IL-6 and IL-8 by siRNA. Clin Exp Immunol. 2007;147:189–196. doi: 10.1111/j.1365-2249.2006.03263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF. A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci USA. 1998;95:588–593. doi: 10.1073/pnas.95.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RA. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–1318. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 44.Della Rocca GJ, van Biesen T, Daaka Y, Luttrell DK, Luttrell LM, Lefkowitz RJ. Ras-dependent mitogen-activated protein kinase activation by G protein-coupled receptors: convergence of Gi- and Gq-mediated pathways on calcium/calmodulin, Pyk2, and Src kinase. J Biol Chem. 1997;272:19125–19132. doi: 10.1074/jbc.272.31.19125. [DOI] [PubMed] [Google Scholar]
- 45.Kotlyarov A, Neininger A, Schubert C, Eckert R, Birchmeier C, Volk HD, Gaestel M. MAPKAP kinase 2 is essential for LPS-induced TNF-α biosynthesis. Nat Cell Biol. 1999;1:94–97. doi: 10.1038/10061. [DOI] [PubMed] [Google Scholar]
- 46.Munoz C, Pascual-Salcedo D, Castellanos MC, Alfranca A, Aragones J, Vara A, Redondo JM, de Landazuri MO. Pyrrolidine dithiocarbamate inhibits the production of interleukin-6, interleukin-8, and granulocyte-macrophage colony-stimulating factor by human endothelial cells in response to inflammatory mediators: modulation of NF-κB and AP-1 transcription factors activity. Blood. 1996;88:3482–3490. [PubMed] [Google Scholar]