Abstract
Intravascular stent therapy is considered a primary therapeutic option for most adults and adolescents with coarctation of the aorta. This review highlights the indications, technical considerations, procedural aspects, and limited long-term outcome data when using this intervention. Stent technology has continued to evolve with potential for further modifications since its inception in the early 1990s. The best therapeutic approach, e.g., stenting versus surgery, in the treatment of native coarctation continues to be debated due to the paucity of long-term clinical and imaging data in both groups.
Keywords: intravascular stent therapy, coarctation of the aorta, balloon angioplasty


Introduction
Coarctation of the aorta (COA) is a congenital cardiac anomaly accounting for 4% to 7% of all congenital heart disease (CHD).1,2 Most are diagnosed as neonates and infants and undergo corrective surgery as an accepted standard of practice. However, many cases are unrecognized until late childhood or adulthood.2 Transcatheter therapy in the form of balloon angioplasty was introduced in the 1980s as an alternative approach to treat recurrent coarctation following surgical therapy,3, 4 but both treatment strategies had major drawbacks, including recoarctation, residual hypertension, aortic wall injury causing dissection, and aneurysm formation.5, 6 To overcome some of these limitations, intravascular balloon-expandable stent therapy was introduced in the 1990s.7, 8 The bare metal stents, by providing a rigid endovascular prosthesis, maintained improved vessel diameter compared to balloon angioplasty of the coarctation segment. By providing a scaffold for the weakened aortic wall, many believe that stent placement would decrease the likelihood of aneurysm formation.9 Over the past decade, use of intravascular stent therapy for native and recurrent COA has become widely accepted for older children and adults.1, 9-15
Stent Technology and its Application for Treatment of COA
An intraluminal graft using stainless steel wire that allows dilatation of the stenotic lesion and provides an endoprosthesis to prevent recoil of the arterial wall was first developed and tested in dog arteries by Palmaz et al.16, 17 Dr. Mullins was the first to use intravascular stents (IS) in pulmonary arteries and systemic veins.18 This was soon followed by studies demonstrating its ability to be re-expanded in a swine model aorta,7 with eventual use of the stent to treat native and recurrent coarctation of the aorta.19-21 Since the mid-1990s, with its first use in an infant and followed by a small series in adults,19, 21, 22 IS therapy for COA has gained acceptance as a primary option for treating native and recurrent COA in adolescents and adults.10, 11, 15, 23, 24
Significant technological advances have been made in recent years to achieve the desirable characteristics of an ideal stent, including higher flexibility, lower profile, the ability to further dilate to adult size with somatic growth, minimal foreshortening with maximal expansion, high radial strength, non-sharp edges, and open cell strut configuration when a brachiocephalic vessel requires overlapping.2, 10, 23 Figure 1 shows the most commonly used stents for treatment of COA, and Table 1 and Figure 2 summarize the characteristics and radial strengths of stents currently available in the United States. Multiple single-center series and the multicenter retrospective and prospective report conducted by the Congenital Cardiovascular Interventional Study Consortium (CCISC) reported the acute, intermediate, and long-term data comparable or superior to both the surgical and transcatheter balloon angioplasty series.4, 25-28
Figure 1.
Images of the four most commonly used stents for treatment of coarctation of the aorta worldwide. CP: Cheatham-Platinum, which is available in the United States only under the COAST protocol.

Table 1.
Characteristics of stents currently available in the United States.
| Characteristics | Palmaz P188/308 | Palmaz 30/4010 | Genesis 19/29/39 | ITLD 16/26 | Mega 16/26/36 | Mega 16/26/36 |
| Nominal expansion sizes | 7-18 mm | 12-26 mm | 5-20 mm | 7-20 mm | 7-20 mm | 9-25 mm |
| Final wall thickness | 0.0055” | 0.0103” | 0.0095” | 0.0076” | 0.0093 | 0.0105 |
| Minimal crimp ID | 0.093” | N/T | 0.066” | N/A | N/A | N/A |
| Del sheath (5-8 mm) | 8 Fr | 10 Fr | 6 Fr | 8 Fr | 8 Fr | 9 Fr |
| Del sheath (9-12 mm) | 9 Fr | 10 Fr | 7 Fr | 9 Fr | 9 Fr | 10 Fr |
| Del sheath (14-16 mm) | 10 Fr | 11 Fr | 8 Fr | 10 Fr | 10 Fr | 11 Fr |
| Cell | Closed | Closed | Closed | Open | Open | Open |
| Pre-delivery flexibility | None | None | Yes | Yes | Yes | Yes |
Figure 2.
Radial strength characteristics of the most commonly used stents in the United States for treatment of coarctation of the aorta.
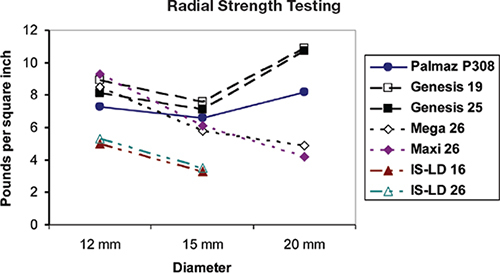
In the CCISC’s experience, the most widely used stents for treating COA in the United States are off-label use of the Genesis XD (Cordis Corp., Warren, NJ), the EV3 Maxi (EV3, Plymouth, MN), the Palmaz “10” series stent (Cordis, Warren, NJ), and the Cheatham-Platinum stent (NuMed Corp., Hopkington, NJ) per the ongoing COarctation of the Aorta Stent Trial (COAST). The ideal stent for treatment of COA should be dilatable from 12 mm to 22 mm, with limited foreshortening, adequate radial strength at maximal dilation, and resistance to fracture. The Palmaz XL stents, with the highest radial strength (Figure 2) and fracture resistant (the CCISC’s experience has noted no fractures in the XL series), have been limited for use in adults due to their high profile. The EV3 Mega and Max LD stent, with their open cell design, minimal foreshortening, and considerable flexibility, are the preferred stent for treatment of transverse aortic arch obstruction when the overlapping of head and neck vessels is likely to be required.2, 23
Indication and Catheterization Technique
The initial presentation of symptoms differs between infants and children/adults (“older patient”) presenting with native COA. The primary indication for intervening in the older patient is resting hypertension, an “accurate” upper- to lower-extremity systolic gradient of > 20 mm Hg, and/or the presence of symptoms (claudication/headaches). Children and young adults rarely have symptoms other than systolic hypertension, though the second most common symptom in this group is headaches. Diastolic hypertension and ventricular dysfunction are rarely observed in the older group. Obtaining an accurate noninvasive blood pressure measurement cannot be overemphasized. For the COAST trial, obtaining three consecutive blood pressure measurements in each extremity is required to increase the accuracy of the noninvasive measurements.2 Although echocardiographic imaging is extremely helpful in younger children and adolescents, further 3-dimensional (3D) magnetic resonance imaging (MRI) is usually required to plan the best course of action. Based on the experience of the CCISC, if the ratio of narrowed segment of the aorta (regardless if the narrowing is in the transverse aortic arch, isthmus, or coarctation site) to the level of the diaphragm is ≥ 0.60, intervention is rarely required.26
Catheterization Procedure
The procedure is most commonly performed under general anesthesia using the retrograde transfemoral approach. Administration of antibiotic prophylaxis and intravenous heparin at 75 to 100 mg/kg to maintain an activated clotting time of > 250 ms throughout the procedure is recommended. All patients should undergo a complete right- and left-heart catheterization. The coarctation segment is most commonly crossed using an angled catheter. Once peak-to-peak systolic pressures have been obtained across the coarctation segment, a detailed angiogram using either a pigtail or multitrack catheter is performed. The new Dyna computed tomography (CT)/rotational angiography imaging capabilities, when available, offer important additional imaging details of the aortic arch. Details of the entire transverse aortic arch, including brachiocephalic vessel location, isthmus, coarctation segment, and the aortic dimension at the level of the diaphragm, are essential. Final stent diameter is based on proximal arch diameter (transverse or distal arch), with the diameter not exceeding the size of aorta at the level of the diaphragm. Furthermore, the ratio of stent diameter to preintervention narrowest coarctation segment should be < 3.5.12 In taking a page from our adult colleagues, one cannot overemphasize the importance of achieving adequate vascular access and postprocedural hemostasis. During vascular access, we ensure that we enter the femoral artery at the level of the femoral head. Immediately upon entry with the arterial sheath, an angiogram is taken to ensure adequate vessel size and appropriate sheath entry site within the vessel. At that time we preclose with the Perclose A-T device (Abbott Vascular Device, Abbott Park, IL). A perfect example of how we avoided possible vessel injury is depicted in Figure 3 with a recent patient who required covered stent placement for both reobstruction and an aneurysm of her previously repaired coarctation segment. To provide a stable track to advance and deploy the balloon/stent segment, a Rosen wire (Cook Medical, Bloomington, IN) or exchange-length Amplatzer extra- or super-stiff (St. Jude Medical, St. Paul, MN) wire is positioned in the ascending aorta or right subclavian artery. The stent is hand-crimped on the balloon catheter, with the extremely low profile balloons requiring inflation to 0.6 atm to allow for adequate stent/balloon traction, thereby preventing stent slippage during advancement through the long sheath. From the CCISC consortium, the balloon-in-balloon is by far the preferred delivery balloon when initial stent dilation is ≥ 18 mm as it provides controlled stent expansion, the ability to adjust stent position following inner balloon expansion, and decreased stent foreshortening (Figure 4). For stents that are initially deployed on ≤ 16 mm balloon catheters, the Z-MED II™ balloon catheters (NuMed Inc., Hopkinton, NY) are the most widely used. For optimal stent positioning, we cover the proximal balloon with the delivery sheath and slowly expand the distal stent to its full size. We then pull the sheath off of the balloon catheter and deploy the remainder of the stent across the coarctation segment (Figure 5). For optimal stent delivery and to prevent stent malposition and migration, right ventricular pacing may be performed. Though this is rarely necessary for standard moderate-to-severe coarctations, this technique is essential for stent treatment of transverse aortic arch narrowing and patients with mild coarctation of the aorta. Following stent deployment, either a pigtail or multitrack catheter is used to obtain simultaneous pressure measurements across the stent. Multiple angiograms (or rotational angiography) is performed after stent placement. Stent implantation is considered successful if a gradient < 10 mm Hg and improvement in vessel caliber > 80% of the normal adjacent aortic arch is achieved. The majority of patients continue their antihypertensive medications and are restricted from contact sports for 1 month after the procedure. They are also placed on antiplatelet medication and are to follow endocarditis precautions for 6 months after stent placement.
Figure 3.
Importance of imaging the femoral artery before upsizing sheaths. (A) Note the very small distance between the origin of the SFA and the sheath entry site into the femoral artery (< 5 mm). (B) In the contralateral femoral arterial vessel in the same patient, the sheath entry is superior to the figure on the left (12 mm superior to the origin of the left SFA), thereby allowing the use of a Preclose device and less likely to cause SFA injury when upsizing the sheath for stent placement. SFA: superficial femoral artery.
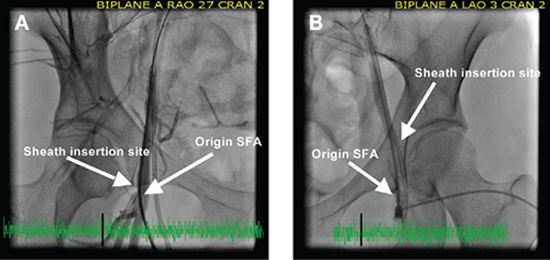
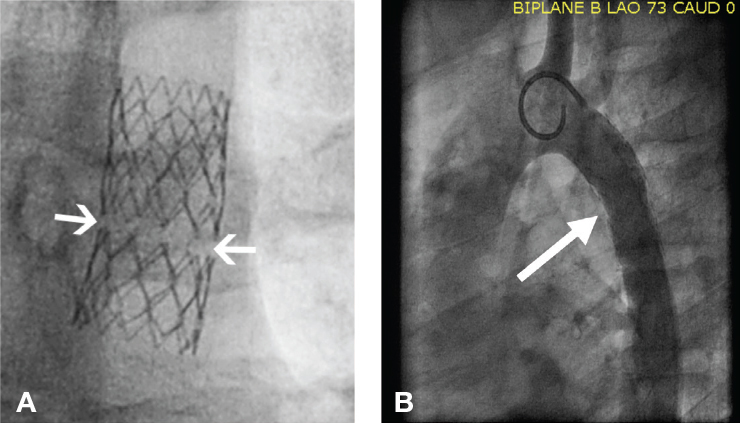
Figure 4.
Use of the balloon-in-balloon catheter for placement of a covered stent across a coarctation aneurysm. The inner balloon is approximately one-half the size of the larger outer diameter balloon. In this particular patient, (A) the inner balloon is inflated to 10 mm, allowing for final stent positioning, before the outer balloon (B) is inflated to 20 mm.
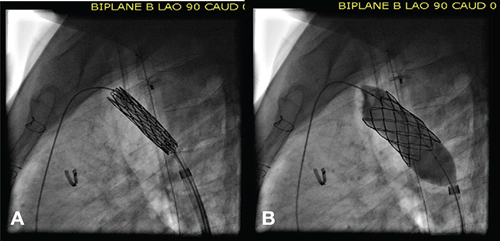
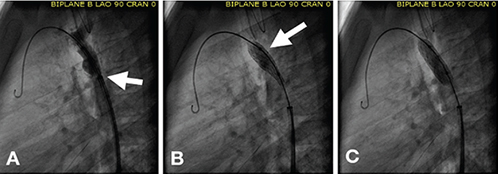
Figure 5.
Deployment technique for stents mounted on a single (non-BIB) balloon catheter. (A) The sheath is still covering the proximal balloon/stent catheter. (B) The catheter is gradually inflated, dilating the distal balloon/stent apparatus to its full size. (C) Final inflation of the balloon/stent apparatus is done with the sheath fully withdrawn off of the balloon catheter. This technique allows for controlled and precise stent positioning across the coarctation site. BIB: balloon-in-balloon.
Stenting Complex Aortic Arch and Aneurysm
Precatheterization 3D MRI or CT assessment of the hypoplastic aortic arch is essential to delineate arch anatomy, level and degree of obstruction, and its relation to the head and neck vessels. The baseline hemodynamic and angiographic evaluation are similar to simple COA. The stiff wire is positioned most often in the ascending aorta to provide a natural aortic arch curve. Stent placement across the aortic arch would most likely overlap partially or completely with the head and neck vessels. In the CCISC’s experience of more than 90 patients undergoing stent treatment of transverse aortic arch obstruction, the majority have used a single stent to completely overlap the major branches rather than placing a stent proximal and distal to the arch vessels. In the CCISC Registry, the open-cell design stents (ev3 Mega™ and Max™ LD, ev3 Endovascular, Plymouth, MN) are the preferred stent in the treatment of transverse arch obstruction, as their cells can be separately dilated so as to not impinge on the lumen of the brachiocephalic vessel. These stents, due to their open-cell design, have better conformity to the aortic arch contour than their closed-cell counterparts.
The treatment of aortic aneurysm with associated COA involves the use of a covered stent. In the United States, the use of a covered stent requires FDA approval under compassionate use unless the center is participating in the COAST II trial. The aneurysm can be located proximal or distal to the coarctation site and at times is in close proximity to the left subclavian artery (LSA). Covering the LSA could lead to left-arm ischemia, claudication, or possible vertebralbasilar infarct if the vertebral artery arises off the LSA and there is inadequate flow through the circle of Willis circulation on the contralateral side. Alternatively placing a surgical jump graft from the carotid to the LSA prior to placement of a covered stent may be preferable to extensive arch surgery.23 In addition, prior placement of a vascular plug in the LSA to prevent communication from the carotid to the blind aneurysmal pouch may be necessary to prevent thrombus formation and extension to the LSA following covered stent placement within the aortic arch. Figure 6 (A and B) illustrates a large aneurysm at the base of the left subclavian artery and its treatment using a covered stent. In the absence of covered stents, centers not participating in the COAST trial have the option of using a self-fabricated polytetrafluoroethylene material, suturing it to the stent edges, and crimping it on the balloon catheter.29
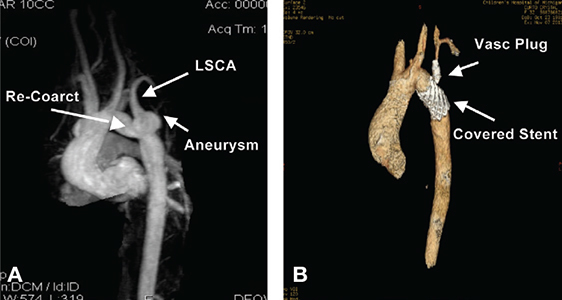
Figure 6.
(A & B) 3-dimensional reconstructive images of pre- and postcovered stent treatment of both recoarctation and aneurysm at the base of the LSCA. Figure B shows successful elimination of both the coarctation segment and aneurysm with a Cheatham-Platinum covered stent. Placement of a vascular plug was performed in the proximal LSCA following distal left common carotid to distal LSCA jump graft placement performed the previous day. LSCA: left subclavian artery.
Outcome and Adverse Events
Apart from numerous single institutional series, recent multicenter reports from the CCISC registry have shown excellent acute, intermediate, and long-term success. In a total of 565 procedures with a median age of 15 years (mean 18), Forbes et al. demonstrated procedural success in 97.9% (defined as gradient < 20 mm Hg and COA to DAO ratio of > 0.8).11 There was significant improvement in pre-versus post-COA dimensions, systolic gradient, and COA to DAO dimensions. A recent report by Holzer et al.13 in a cohort of 302 patients showed acute procedural success in 96%, intermediate success in 86% (41% patients completed follow-up), and long-term success in 77% of patients. The procedural success was defined as UL/LL systolic gradient of < 20 mm Hg, lack of significant recurrent obstruction, and freedom from unplanned reintervention. At long-term follow-up, continued hypertension was noted in 23% (systolic blood pressure > 95%) and 32% were on antihypertensive medications. With regard to IS therapy and outcome on complex aortic arch obstruction, Holzer and colleagues have reported the largest series to date on 40 patients with aortic arch stent placement. Acute procedural success was noted in 90% and the neck vessels were crossed in 75% without adverse events.23
Aortic wall complications, including dissection/rupture, may lead to circulatory collapse and death. Fortunately its incidence is rare at 1.6% as shown by Forbes et al. in their cohort of 565 procedures.11 Pre-stent angioplasty, balloon coarctation ratio > 3.5, abdominal COA. and age > 40 yrs were all significantly related to encountering aortic wall complications.11, 30, 31 Immediate availability of a covered stent or surgical backup is essential to deal with aortic wall emergencies, and anticipatory preparedness prior to IS in the catheterization lab is warranted. The true incidence of aortic aneurysm remains elusive, with studies reporting in the range of 5% to 9%.13, 15, 26 Forbes et al. reported a balloon to coarctation ratio > 3.5 and pre-stent angioplasty as risk factors for encountering aneurysms at intermediate follow-up.26 Most aneurysms are small and require conservative management, though there have been situations where progression of the aneurysm size has occurred, requiring covered stent placement years later. Recurrent stenosis requiring reintervention is reported to be 2.7% to 4% in the two large CCISC studies.13, 26 Severe intimal stenosis, stent fracture, stent recoil, and adjacent somatic growth are the potential factors requiring reintervention with angioplasty and or second stent placement. All stents have been observed to fracture, with the current rates ranging from 4.5% to 12% at intermediate follow-up.12, 32 The closed-cell stents note individual cell fractures, though the Genesis XD stents typically fracture along the sigma hinge point into two pieces (Figure 7).12 To date, there have been no reported distal fragment embolization of any stent used at the coarctation site. In the CCISC experience, 30% of patients with known stent fracture required repeat reintervention due to obstruction.24 Other adverse events such as stent malposition and vascular injury at access sites accounted for < 5% of cases and cerebrovascular accidents were rare at < 1%.13
Figure 7.
(A) Genesis XD stent fracture noted 2 years after placement within a coarctation site. Note the circumferential fracture along the sigma hinges, which is typical for the Genesis XD stent. (B) In spite of the circumferential stent fracture, no reobstruction is observed within the Genesis XD stent. Arrows mark the location of the stent fracture.
Comparison between Stent/Balloon Angioplasty versus Surgery
Carr published a comparison of balloon angioplasty and stent (n = 633) versus surgical patients (n = 213) after reviewing publications from 1995-2005. Stent therapy had lower morbidity compared to surgery (9% vs 11%, range 1%-20% vs. 1%-25%). The restenosis rates, however, were higher in the stent group at 11% (0%-25%) versus 2% (0%-9%) in the surgical group. Postprocedure freedom from hypertension was similar (61% vs. 65%). The long-term protocol for hypertension and aortic wall imaging data were lacking significantly in the surgical group.25 Hager et al. published the longest surgical follow-up data (27 yrs) in a cohort of 404 patients. He noted that 57% of patients had persistent hypertension and only 13% were due to restenosis. The risk factors for long-term hypertension were surgical repair with prosthetic material, male sex, residual gradient, and older age at follow-up. Despite good anatomical results, this report highlights the prevalence of long-term hypertension with various contributing factors other than arch reobstruction.33 The CCISC registry was created to specifically address some of these factors, and recent data published by Forbes et al. comparing all three modalities in a cohort of 350 patients (stent n = 217, angioplasty n = 67, surgery n = 72) showed acute significant improvement in systolic blood pressure and upper- to lower-extremity gradient. Stent patients had fewer acute complications than surgical and balloon angioplasty patients (2.3%, 8.1%, and 9.8%, P < 0.001). At intermediate-term follow-up, there were no significant differences in resting hypertension, the COA:DAO were significantly higher (0.98 surgical, 0.80 stent, 0.79 balloon angioplasty), and aortic wall injury was 12.6% in surgical, 7.1% in stent, and 43.6% in balloon angioplasty patients.26 Improved follow-up in all three groups is necessary before a definitive statement can be made among these treatment modalities regarding long-term outcomes.
Conclusion
Significant improvement in both outcomes and avoidance of acute/intermediate complications have been observed in IS treatment of coarctation of the aorta over the past 10 years. At this time, acute and intermediate follow-up results favor IS treatment of coarctation in older children and adults, though long-term results remain speculative. Balloon angioplasty or surgical treatment of smaller children (< 25 kg), especially when end-to-end anastomosis can be performed, continues to be the procedure of choice in this group. Accurate comparisons between the two treatment strategies are limited by the paucity of long-term imaging and clinical follow-up data. Further follow-up is required to determine which treatment a particular patient (and coarctation anatomy) should undergo to obtain the best results.
Funding Statement
Funding/Support: The authors have no funding disclosures.
Footnotes
Conflict of Interest Disclosure: The authors have completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
Contributor Information
Thomas J. Forbes, Children’s Hospital of Michigan, Detroit, Michigan
Srinath T. Gowda, Children’s Hospital of Michigan, Detroit, Michigan
References
- 1.Golden AB, Hellenbrand WE. Coarctation of the aorta: stenting in children and adults. Catheter Cardiovasc Interv. 2007 Feb 1;69(2):289–99.. doi: 10.1002/ccd.21009. [DOI] [PubMed] [Google Scholar]
- 2.Ringel RE, Gauvreau K, Moses H, Jenkins KJ. Coarctation of the Aorta Stent Trial (COAST): study design and rationale. Am Heart J. 2012 Jul;164(1):7–13.. doi: 10.1016/j.ahj.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Lock JE, Bass JL, Amplatz K, Fuhrman BP, Castaneda-Zuniga W. Balloon dilation angioplasty of aortic coarctations in infants and children. Circulation. 1983 Jul;68(1):109–16.. doi: 10.1161/01.cir.68.1.109. [DOI] [PubMed] [Google Scholar]
- 4.Shaddy RE, Boucek MM, Sturtevant JE, Ruttenberg HD, Jaffe RB, Tani LY, et al. Comparison of angioplasty and surgery for unoperated coarctation of the aorta. Circulation. 1993 Mar;87(3):793–9.. doi: 10.1161/01.cir.87.3.793. [DOI] [PubMed] [Google Scholar]
- 5.Hager A, Schreiber C, Nutzl S, Hess J. Mortality and restenosis rate of surgical coarctation repair in infancy: a study of 191 patients. Cardiology. 2009;112(f1):36–41.. doi: 10.1159/000137697. [DOI] [PubMed] [Google Scholar]
- 6.Rao PS, Galal O, Smith PA, Wilson AD. Five- to nine-year follow-up results of balloon angioplasty of native aortic coarctation in infants and children. J Am Coll Cardiol. 1996 Feb;27(2):462–70.. doi: 10.1016/0735-1097(95)00479-3. [DOI] [PubMed] [Google Scholar]
- 7.Morrow WR, Palmaz JC, Tio FO, Ehler WJ, VanDellen AF, Mullins CE. Re-expansion of balloon-expandable stents after growth. J Am Coll Cardiol. 1993 Dec;22(7):2007–13.. doi: 10.1016/0735-1097(93)90791-x. [DOI] [PubMed] [Google Scholar]
- 8.O’Laughlin MP, Perry SB, Lock JE, Mullins CE. Use of endovascular stents in congenital heart disease. Circulation. 1991 Jun;83(6):1923–39.. doi: 10.1161/01.cir.83.6.1923. [DOI] [PubMed] [Google Scholar]
- 9.Weber HS, Cyran SE. Endovascular stenting for native coarctation of the aorta is an effective alternative to surgical intervention in older children. Congenit Heart Dis. 2008 Jan-Feb;3(1):54–9.. doi: 10.1111/j.1747-0803.2007.00148.x. [DOI] [PubMed] [Google Scholar]
- 10.Ebeid MR, Prieto LR, Latson LA. Use of balloon-expandable stents for coarctation of the aorta: initial results and intermediate-term follow-up. J Am Coll Cardiol. 1997 Dec;30(7):1847–52.. doi: 10.1016/s0735-1097(97)00408-7. [DOI] [PubMed] [Google Scholar]
- 11.Forbes TJ, Garekar S, Amin Z, Zahn E, Nykanen D, Moore P, et al. Procedural results and acute complications in stenting native and recurrent coarctation of the aorta in patients over 4 years of age: a multi-institutional study. Catheter Cardiovasc Interv. 2007 Aug 1;70(2):276–85.. doi: 10.1002/ccd.21164. [DOI] [PubMed] [Google Scholar]
- 12.Forbes TJ, Moore P, Pedra CA, Zahn EM, Nykanen D, Amin Z, et al. Intermediate follow-up following intravascular stenting for treatment of coarctation of the aorta. Catheter Cardiovasc Interv. 2007 Oct 1;70(4):569–77.. doi: 10.1002/ccd.21191. [DOI] [PubMed] [Google Scholar]
- 13.Holzer R, Qureshi S, Ghasemi A, Vincent J, Sievert H, Gruenstein D, et al. Stenting of aortic coarctation: acute, intermediate, and long-term results of a prospective multi-institutional registry--Congenital Cardiovascular Interventional Study Consortium (CCISC). Catheter Cardiovasc Interv. 2010 Oct 1;76(4):553–63.. doi: 10.1002/ccd.22587. [DOI] [PubMed] [Google Scholar]
- 14.Mohan UR, Danon S, Levi D, Connolly D, Moore JW. Stent implantation for coarctation of the aorta in children < 30 kg. JACC Cardiovasc Interv. 2009 Sep;2(9):877–83.. doi: 10.1016/j.jcin.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Qureshi AM, McElhinney DB, Lock JE, Landzberg MJ, Lang P, Marshall AC. Acute and intermediate outcomes and evaluation of injury to the aortic wall, as based on 15 years experience of implanting stents to treat aortic coarctation. Cardiol Young. 2007 Jun;17(3):307–18.. doi: 10.1017/S1047951107000339. [DOI] [PubMed] [Google Scholar]
- 16.Palmaz JC, Sibbitt RR, Reuter SR, Garcia F, Tio FO. Expandable intrahepatic portacaval shunt stents: early experience in the dog. AJR Am J Roentgenol. 1985 Oct;145(4):821–5.. doi: 10.2214/ajr.145.4.821. [DOI] [PubMed] [Google Scholar]
- 17.Palmaz JC, Sibbitt RR, Tio FO, Reuter SR, Peters JE, Garcia F. Expandable intraluminal vascular graft: a feasibility study. Surgery. 1986 Feb;99(2):199–205.. [PubMed] [Google Scholar]
- 18.Mullins CE, O’Laughlin MP, Vick GW, 3rd, Mayer DC, Myers TJ, Kearney RA, et al. Implantation of balloon-expandable intravascular grafts by catheterization in pulmonary arteries and systemic veins. Circulation. 1988 Jan;77(1):188–99.. doi: 10.1161/01.cir.77.1.188. [DOI] [PubMed] [Google Scholar]
- 19.Bulbul ZR, Bruckheimer E, Love JC, Fahey JT, Hellenbrand WE. Implantation of balloon-expandable stents for coarctation of the aorta: implantation data and short-term results. Cathet Cardiovasc Diagn. 1996 Sep;39(1):36–42.. doi: 10.1002/(SICI)1097-0304(199609)39:1<36::AID-CCD7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Suarez de Lezo J, Pan M, Romero M, Medina A, Sequra J, Lafuente M, et al. Immediate and follow-up findings after stent treatment for severe coarctation of aorta. Am J Cardiol. 1999 Feb 1;83(3):400–6.. doi: 10.1016/s0002-9149(98)00877-7. [DOI] [PubMed] [Google Scholar]
- 21.Suarez de Lezo J, Pan M, Romero M, Medina A, Sequra J, Pavlovic D, et al. Balloon-expandable stent repair of severe coarctation of aorta. Am Heart J. 1995 May;129(5):1002–8.. doi: 10.1016/0002-8703(95)90123-x. [DOI] [PubMed] [Google Scholar]
- 22.Redington AN, Hayes AM, Ho SY. Transcatheter stent implantation to treat aortic coarctation in infancy. Br Heart J. 1993 Jan;69(1):80–2.. doi: 10.1136/hrt.69.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holzer RJ, Chisolm JL, Hill SL, Cheatham JP. Stenting complex aortic arch obstructions. Catheter Cardiovasc Interv. 2008 Feb 15;71(3):375–82.. doi: 10.1002/ccd.21357. [DOI] [PubMed] [Google Scholar]
- 24.Suarez de Lezo J, Pan M, Romero M, Sequra J, Pavlovic D, Ojeda S, et al. Percutaneous interventions on severe coarctation of the aorta: a 21-year experience. Pediatr Cardiol. 2005 Mar-Apr;26(2):176–89.. doi: 10.1007/s00246-004-0961-5. [DOI] [PubMed] [Google Scholar]
- 25.Carr JA. The results of catheter-based therapy compared with surgical repair of adult aortic coarctation. J Am Coll Cardiol. 2006 Mar 21;47(6):1101–7.. doi: 10.1016/j.jacc.2005.10.063. [DOI] [PubMed] [Google Scholar]
- 26.Forbes TJ, Kim DW, Du W, Turner DR, Holzer R, Amin Z, et al. Comparison of surgical, stent, and balloon angioplasty treatment of native coarctation of the aorta: an observational study by the CCISC (Congenital Cardiovascular Interventional Study Consortium). J Am Coll Cardiol. 2011 Dec 13;58(25):2664–74.. doi: 10.1016/j.jacc.2011.08.053. [DOI] [PubMed] [Google Scholar]
- 27.Padua LM, Garcia LC, Rubira CJ, de Oliveira Carvalho PE. Stent placement versus surgery for coarctation of the thoracic aorta. Cochrane Database Syst Rev. 2012 May 16;5 doi: 10.1002/14651858.CD008204.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Egan M, Holzer RJ. Comparing balloon angioplasty stenting and surgery in the treatment of aortic coarctation. Expert Rev Cardiovasc Ther. 2009 Nov;7(11):1401–12.. doi: 10.1586/erc.09.111. [DOI] [PubMed] [Google Scholar]
- 29.Holzer R, Concilio K, Hijazi ZM. Self-fabricated covered stent to exclude an aortic aneurysm after balloon angioplasty for post-surgical recoarctation. J Invasive Cardiol. 2005;17:177–9.. [PubMed] [Google Scholar]
- 30.Fletcher SE, Cheatham JP, Froeming S. Aortic aneurysm following primary balloon angioplasty and secondary endovascular stent placement in the treatment of native coarctation of the aorta. Cathet Cardiovasc Diagn. 1998;44:40–4.. doi: 10.1002/(sici)1097-0304(199805)44:1<40::aid-ccd10>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 31.Tzifa A, Ewert P, Brzezinska-Rajszys G, et al. Covered Cheatham-platinum stents for aortic coarctation: early and intermediate-term results. J Am Coll Cardiol. 2006 Apr 4;47(7):1457–63.. doi: 10.1016/j.jacc.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 32. Forbes, Thomas (Children’s Hospital of Michigan, Detroit, Michigan). Conversation with: Richard Ringel (National PI COAST Trial, Johns Hopkins School of Medicine, Baltimore, MD). date. [Google Scholar]
- 33.Hager A, Kanz S, Kaemmerer H, Schreiber C, Hess J. Coarctation Long-term Assessment (COALA): significance of arterial hypertension in a cohort of 404 patients up to 27 years after surgical repair of isolated coarctation of the aorta even in the absence of restenosis and prosthetic material. J Thorac Cardiovasc Surg. 2007 Sep;134(3):738–45.. doi: 10.1016/j.jtcvs.2007.04.027. [DOI] [PubMed] [Google Scholar]


