Abstract
Objective:
To evaluate the efficacy of rituximab (R) when added to high-dose methotrexate (HD-MTX) in patients with newly diagnosed immunocompetent primary CNS lymphomas (PCNSLs).
Methods:
Immunocompetent adults with newly diagnosed PCNSL treated at The Johns Hopkins Hospital between 1995 and 2012 were investigated. From 1995 to 2008, patients received HD-MTX monotherapy (8 g/m2 initially every 2 weeks and after complete response [CR] monthly to complete 12 months of therapy). From 2008 to 2012, patients received the same HD-MTX with rituximab (375 mg/m2) with each HD-MTX treatment. CR rates and median overall and progression-free survival were analyzed for each patient cohort in this single-institution, retrospective study.
Results:
A total of 81 patients were identified: 54 received HD-MTX (median age 66 years) while 27 received HD-MTX/R (median age 65 years). CR rates were 36% in the HD-MTX cohort and 73% in the HD-MTX/R cohort (p = 0.0145). Median progression-free survival was 4.5 months in the HD-MTX cohort and 26.7 months in the HD-MTX/R cohort (p = 0.003). Median overall survival was 16.3 months in the HD-MTX cohort and has not yet been reached in the HD-MTX/R cohort (p = 0.01).
Conclusions:
The addition of rituximab to HD-MTX appears to improve CR rates as well as overall and progression-free survival in patients with newly diagnosed PCNSL. Comparisons of long-term survival in the 2 cohorts await further maturation of the data.
Classification of evidence:
This study provides Class III evidence that in immunocompetent patients with PCNSL, HD-MTX plus rituximab compared with HD-MTX alone improves CR and overall survival rates.
Primary CNS lymphomas (PCNSLs) account for 2% to 3% of primary brain cancers. Although these tumors are rare, there is the potential for cure and therefore efforts have been made to identify the optimal treatment strategy for PCNSLs.1–11 High-dose methotrexate (HD-MTX) is the backbone of most modern chemotherapy regimens. Various MTX-based regimens (with or without radiation therapy) have been assessed with overall similar results of relatively high response rates, but long-term control rates have been limited. Until 2008, patients with newly diagnosed PCNSL at Johns Hopkins were treated with HD-MTX as outlined in the New Approaches to Brain Tumor Therapy (NABTT) Study.1
Given that the vast majority of PCNSLs are CD20-expressing B-cell lymphomas and that rituximab, a CD20-targeted monoclonal antibody, has demonstrated significant improvement in overall survival (OS) in virtually all systemic B-cell lymphomas, it is hypothesized that rituximab may improve the response rate and long-term control of PCNSLs. Despite concerns that this large monoclonal antibody would not be able to cross the blood-brain barrier, preliminary data suggest that rituximab may have activity in PCNSLs.11–13 Based on these observations, rituximab (given with every cycle of HD-MTX) was added to the institutional standard protocol for patients with newly diagnosed PCNSL at Johns Hopkins.
This retrospective review was undertaken to assess whether the addition of rituximab to the HD-MTX regimen described by the NABTT CNS Consortium improves complete response (CR) rates, progression-free survival (PFS), or OS in patients with newly diagnosed PCNSL.
METHODS
Study objectives.
The primary objective of this institutional review board–approved, single-institution, retrospective study was to determine whether the addition of rituximab to HD-MTX (HD-MTX/R) improves the CR rate compared with HD-MTX alone in immunocompetent adult patients with newly diagnosed PCNSL (level of evidence: Class III).
Secondary objectives were to examine potential differences in OS and PFS in these 2 patient populations (level of evidence: Class III).
Patient population.
Immunocompetent patients with newly diagnosed and previously untreated PCNSL aged 18 years or older were identified using the Sidney Kimmel Comprehensive Cancer Center registry. HIV-positive patients or patients receiving immunosuppressive therapy at the time of diagnosis (with the exception of steroids) were excluded. All patients who received at least one treatment with HD-MTX (8 mg/m2 with dose adjustments based on estimated creatinine clearance) at The Johns Hopkins Hospital between 1995 and 2012 were included in the analysis.
Study measures.
In our institutional practice, MRI scans are obtained every 2 cycles of treatment and used as the primary means for assessing partial response or CR. The MRI protocol consisted of standard sagittal and axial T1-weighted, axial T2-weighted, fluid-attenuated inversion recovery, diffusion-weighted imaging, and sagittal and axial postcontrast T1-weighted images. For this study, all available imaging data were rereviewed centrally in a nonblinded manner by one radiologist (D.B.) using previously published PCNSL response criteria.14 Patients' responses were considered evaluable for CR if they had a baseline contrast MRI and if sufficient imaging data were available to determine when a CR was achieved or when the time of progression could be defined.
All patients included in this study had survival information available from medical records and/or publically available vital statistics and could be evaluated for OS. Progression was defined as evidence of progression on imaging, based on clinical progression as documented in clinician notes, or death from disease progression. Patients without information to determine time of progression (such as patients who were lost to follow-up) were censored at the time last known to be alive and progression-free.
Performance status was determined retrospectively based on information available in patient charts. Because of the retrospective nature of this data collection, not all patients had a performance status documented at baseline. To be able to separately analyze data from patients who, based on their performance status, would have met common clinical trial enrollment criteria, patients were grouped into Eastern Cooperative Oncology Group (ECOG) scores 0–2 (typical criterion for enrollment in clinical trials) and ECOG scores ≥2 (poor performance status). A more detailed separation among the better performance status groups, ECOG scores 0, 1, and 2, was not possible based on available data in the patient charts.
Treatment regimen.
Cohort 1 (HD-MTX, 1995–2008) patients were treated according to the NABTT procotol.1 Cohort 2 (HD-MTX/R, 2008–2012) patients were treated with HD-MTX 8 g/m2 (day 1) and rituximab 375 mg/m2 (day 3) every 14 days until CR, progression or intolerable toxicities, or inability to receive HD-MTX. After CR, 2 more cycles were given every 14 days, followed by monthly treatments of HD-MTX/R for the total duration of 1 year. Each treatment cycle was standardized and required an inpatient admission. Patients received IV hydration and oral or IV sodium bicarbonate for urine alkalinization. Once alkalinization was achieved (urine pH ≥7.5), patients received premedication with an antiemetic and infusion of MTX at 8 g/m2 over 4 hours. The creatinine clearance was initially measured. Our practice changed in later years and from then on the calculated creatinine clearance was used.15 The dose was adjusted, if needed, based on the creatinine clearance or toxicity. The dose was reduced by the percentage reduction of the creatinine clearance below 100 (e.g., a creatinine clearance of 75 mL/min resulted in a dose reduction of 25%). During and after infusion of MTX, IV hydration and urine alkalinization were continued. MTX levels were obtained at 24 and 48 hours postinfusion and until the MTX level was ≤0.20 µM. Rituximab 375 mg/m2 was administered on day 3 of treatment. The initial infusion was done over 60 minutes for the first cycle, and it was reduced to a 30-minute infusion during subsequent doses if no infusion reaction was observed.
Statistical analysis.
Patient baseline characteristics were summarized using descriptive statistics. The proportion of patients with CR was assumed to follow an independent binomial distribution. Chi-square test statistics were used for proportional comparison. OS time was calculated from the time initial treatment started until death from any cause, or censored if the subject was alive at the time of last follow-up. PFS time was calculated from the time initial treatment started until the date the disease progressed, or censored if the patient had no progressive disease at the time of last follow-up. Survival probability was estimated using the Kaplan-Meier method.16 The confidence interval (CI) of median survival time was constructed by the method of Brookmeyer and Crowley.17 Differences in survival or PFS between treatments or between tumor response groups were evaluated with the log-rank test. All p values are reported as 2-sided, and all analyses were conducted using SAS software (version 9.2; SAS Institute, Cary, NC).
Standard protocol approvals, registrations, and patient consents.
This retrospective study was approved by the institutional review board at Johns Hopkins University. Consent from patients was not required and was waived.
RESULTS
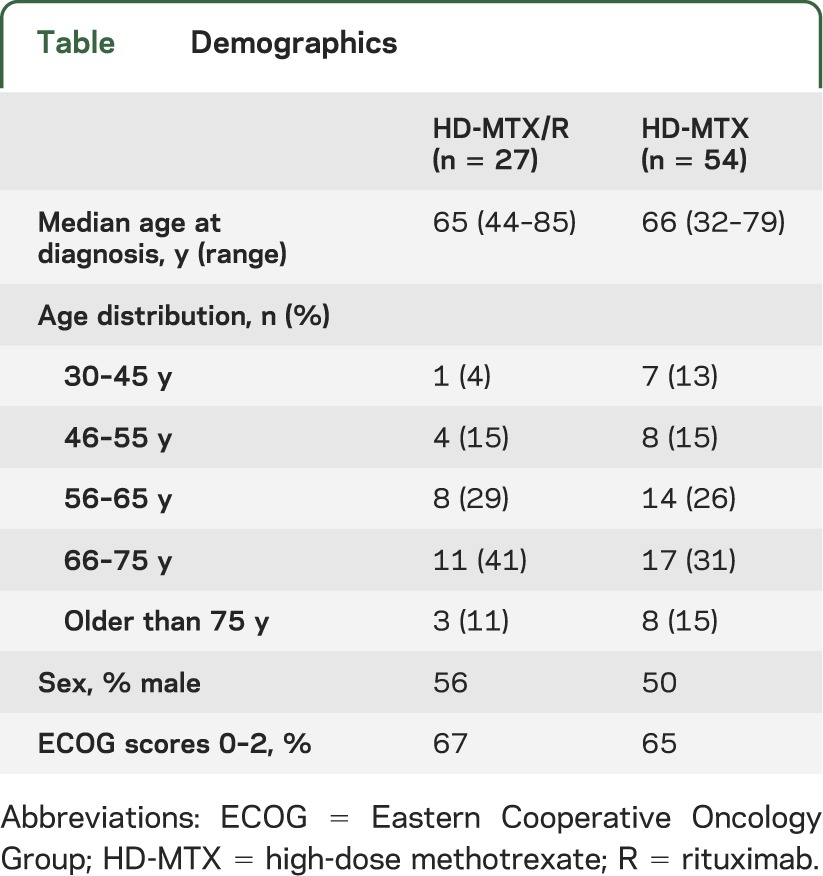
A total of 81 patients with newly diagnosed PCNSL who received at least one dose of HD-MTX (8 g/m2) at Johns Hopkins between 1995 and 2012 were identified for this retrospective analysis. Fifty-four patients received treatment with MTX monotherapy (HD-MTX) and 27 patients received HD-MTX/R. Of these, there were sufficient data to evaluate the responses of 50 patients (93%) in the HD-MTX cohort and 24 patients (89%) in the HD-MTX/R cohort. The median follow-up time was 16.2 months (range, 0.6–176.2 months) in the HD-MTX group and 18.1 months (0.9–70.3 months) in the combination therapy group. The 7 patients who could not be evaluated for response had insufficient imaging data or available medical records (e.g., patients who initiated their treatment at The Johns Hopkins Hospital and who continued it elsewhere). These patients could, however, be included in the survival analysis. The 2 cohorts showed a similar distribution of age, performance status, and sex (table).
Table.
Demographics
CR was identified in 36% of patients in the HD-MTX monotherapy cohort and in 73% of patients who received HD-MTX/R (p = 0.0145). Overall complete and partial responses were 60% and 89%, respectively. The median number of cycles to CR was 5 (range, 2–15) in the HD-MTX monotherapy cohort and 5 (range, 2–21) in the combination cohort.
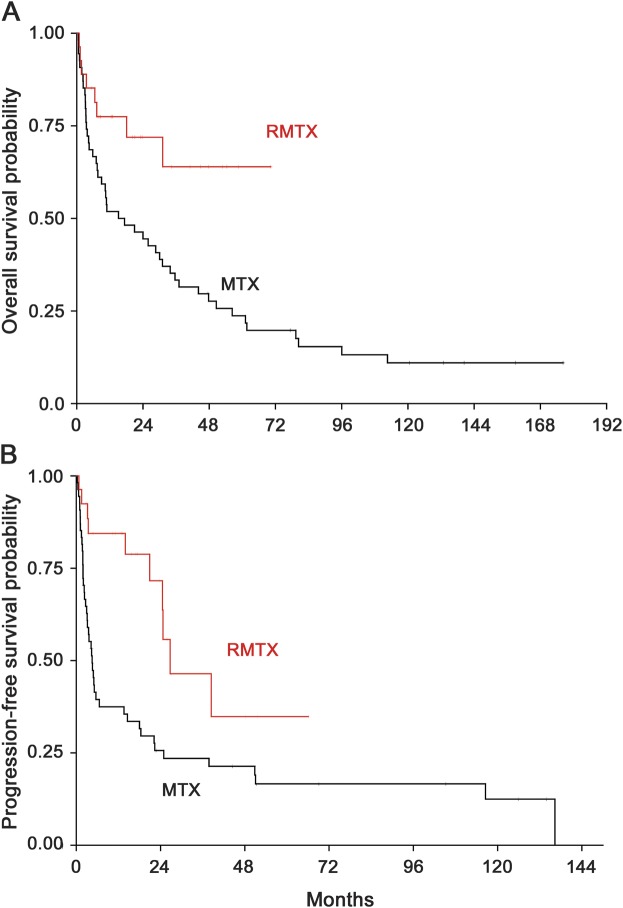
Median OS (all 81 patients were included in the analysis) was 16.3 months (95% CI: 7.4–31.1 months) in the HD-MTX monotherapy cohort and it has not yet been reached in the HD-MTX/R cohort (p = 0.01; figure 1A). Median PFS was 4.5 months (95% CI: 2.9–13.6 months) in the HD-MTX monotherapy cohort compared with 26.7 months in the combination therapy cohort (95% CI: 20.9 months to not reached) (p = 0.003; figure 1B).
Figure 1. Overall and progression-free survival in patients with PCNSL treated with HD-MTX with or without rituximab.
Overall (A) and progression-free (B) survival of patients with newly diagnosed PCNSL treated with HD-MTX/R vs HD-MTX between 1995 and 2012. The shorter follow-up of the combination arm is explained by the later introduction of rituximab, which was added to the HD-MTX regimen starting in 2008. HD-MTX = high-dose methotrexate; PCNSL = primary CNS lymphoma; R = rituximab.
To compare our results with data from previously published studies, we also performed subgroup data analysis of patients with an ECOG performance status of ≤2 (because it had been used as an eligibility criterion in prior clinical trials). Including only these better performance status patients, the median OS for patients treated with HD-MTX alone was 28.6 months (95% CI: 7.4–50.6 months), and it has not yet been reached in the combination therapy group. Median PFS in these patients was 5.2 months (95% CI: 3–22.2 months) in the monotherapy cohort and 26.7 months in the combination cohort (p = 0.016).
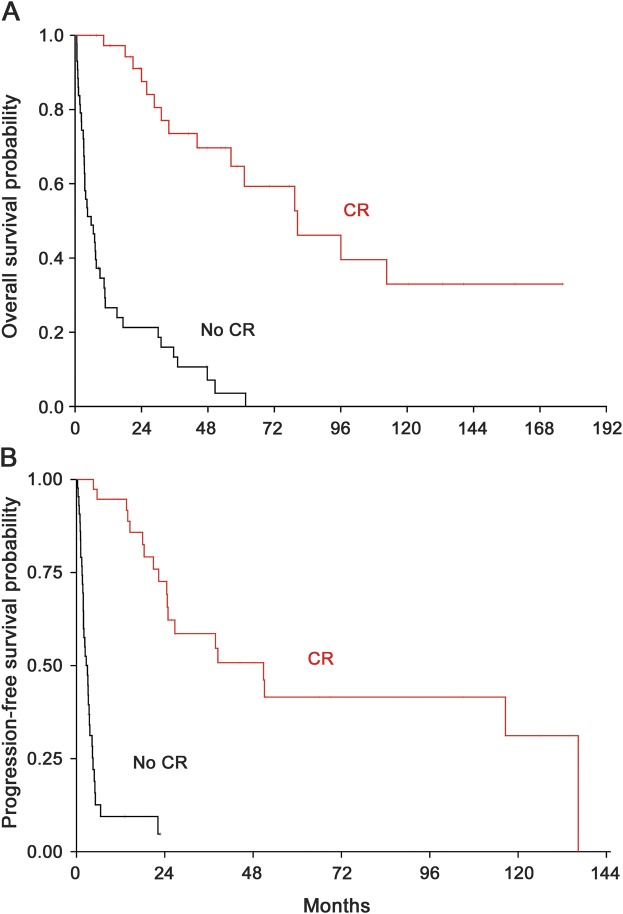
We then assessed median OS and PFS in all patients who had achieved a CR (both groups combined) compared with those who did not achieve a CR. In patients who did achieve a CR, median OS was 80.4 months vs only 5.8 months (95% CI: 3.3–9 months) in patients who did not achieve a CR (p < 0.0001; figure 2A). Median PFS was 50.9 months (95% CI: 24.5–136.4 months) vs 3 months (95% CI: 1.9–3.5 months), respectively (p < 0.0001; figure 2B). This illustrates that virtually the only patients able to achieve long PFS and OS are those who achieved a CR (figure 2).
Figure 2. Survival of patients with CR vs others.
Overall (A) and progression-free (B) survival of patients treated with HD-MTX or HD-MTX/R (both groups combined) who did achieve a CR vs those who did not. CR = complete response; HD-MTX = high-dose methotrexate; R = rituximab.
None of the 27 patients who received rituximab in addition to MTX during their initial therapy discontinued the rituximab treatment, indicating that the addition of rituximab was feasible and well tolerated. Toxicity data that may have been related to rituximab was not collected prospectively.
DISCUSSION
Two distinct treatment regimens for patients with newly diagnosed PCNSL were administered at The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins between 1995 and 2012: HD-MTX with or without rituximab. This retrospective comparison of outcomes demonstrates significantly improved CR rates and improved PFS in the MTX/R group compared with the MTX monotherapy group. Although there is limited follow-up in the patients receiving MTX and rituximab, it appears that there is also likely to be an improvement in OS in this population when compared with HD-MTX alone. It is important to note that the prognostic factors of age and performance status in these 2 cohorts were similar (table).
Our study has intrinsic limitations given that it is a retrospective, single-institution analysis. These include suboptimal matching of the 2 patient cohorts because patients were not prospectively assigned to the different treatment arms using prognostic factors for randomization and the fact that the radiology review was done in a nonblinded manner. In addition, rituximab was added more recently and long-term survival data cannot yet be compared. It is of note that CR rate and survival in the HD-MTX monotherapy group of this study are lower than that reported in the literature.1 The difference is likely explained by selection bias of patients in published prospective studies compared with our analysis. Whereas fixed eligibility criteria were used in prospective studies, our retrospective analysis included all patients who had received at least one cycle of treatment (HD-MTX or HD-MTX/R with intention to treat) irrespective of their baseline performance status, which is a known prognostic factor in PCNSL.5,18 In other words, our study included patients who would not have been eligible for participation in prospective trials that are using common eligibility criteria.
Despite these limitations, our data indicate that the addition of rituximab to MTX appears to be beneficial to patients. The risk of adding rituximab to MTX-containing regimens is minimal, because side effects of rituximab are rather mild, and infusion-related reactions can be managed clinically.11,19
Our findings are in agreement with other published studies that have attempted to demonstrate improved outcomes when rituximab is added to another regimen. Birnbaum et al.13 reported a CR rate of 100% in 17 patients treated with their standard regimen of MTX/ifosfamide plus rituximab vs 68.4% in 19 patients treated with MTX/ifosfamide alone. A phase II study of HD-MTX plus rituximab, administered biweekly for 4 to 6 cycles for induction and after response up to 4 cycles every 4 weeks for maintenance, showed a CR of 60% in 26 of 40 patients and a favorable toxicity profile.11
No data exist regarding the optimal duration of rituximab therapy. Our retrospective data suggest that patients on the HD-MTX/R arm have disease progression later than patients on the HD-MTX monotherapy arm. This raises the question of whether continued rituximab beyond the 12 months of treatment used routinely at Johns Hopkins may lead to further improvement in PFS and possibly OS. Another question that has not been answered is how much, if any, blood-brain barrier disruption is required for the rituximab to reach the CD20-positive lymphoma cells. Clinical research in PCNSL is challenging because these are rare neoplasms and OS in patients treated with HD-MTX–based regimens is comparatively long. In addition, there are significant differences in MTX-based regimens that are used among different institutions.20
Our findings suggest that there is a significant improvement in patient outcomes when rituximab is added to HD-MTX–based regimens in patients with PCNSLs. These findings should be confirmed in a prospective, randomized, controlled trial. However, in absence of higher level evidence, the addition of rituximab to HD-MTX–based regimens is reasonable to consider given its documented survival benefit in virtually all other lymphomas, its tolerability, and growing evidence of antitumoral activity in PCNSLs.
GLOSSARY
- CI
confidence interval
- CR
complete response
- ECOG
Eastern Cooperative Oncology Group
- HD-MTX
high-dose methotrexate
- NABTT
New Approaches to Brain Tumor Therapy
- OS
overall survival
- PCNSL
primary CNS lymphoma
- PFS
progression-free survival
- R
rituximab
AUTHOR CONTRIBUTIONS
Matthias Holdhoff, MD, PhD: drafting/revising, concept/design, analysis of data. Prakash Ambady, MD: drafting/revising, analysis of data. Ahmed Abdelaziz, MD, and Guneet Sarai, MD: analysis of data. David Bonekamp, MD, PhD, and Jaishri O. Blakeley, MD: drafting/revising, analysis of data. Stuart A. Grossman, MD, and Xiaobu Ye, MD, MS: drafting/revising, concept/design, analysis of data. All authors have approved the final version of the manuscript.
STUDY FUNDING
Supported by U01 CA137443 (Adult Brain Tumor Consortium core grant) and P30CA006973 (Sidney Kimmel Comprehensive Cancer Center core grant).
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Batchelor T, Carson K, O'Neill A, et al. Treatment of primary CNS lymphoma with methotrexate and deferred radiotherapy: a report of NABTT 96-07. J Clin Oncol 2003;21:1044–1049 [DOI] [PubMed] [Google Scholar]
- 2.Thiel E, Korfel A, Martus P, et al. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): a phase 3, randomised, non-inferiority trial. Lancet Oncol 2010;11:1036–1047 [DOI] [PubMed] [Google Scholar]
- 3.Zhu JJ, Gerstner ER, Engler DA, et al. High-dose methotrexate for elderly patients with primary CNS lymphoma. Neuro Oncol 2009;11:211–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah GD, Yahalom J, Correa DD, et al. Combined immunochemotherapy with reduced whole-brain radiotherapy for newly diagnosed primary CNS lymphoma. J Clin Oncol 2007;25:4730–4735 [DOI] [PubMed] [Google Scholar]
- 5.Abrey LE, Ben-Porat L, Panageas KS, et al. Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol 2006;24:5711–5715 [DOI] [PubMed] [Google Scholar]
- 6.Gavrilovic IT, Hormigo A, Yahalom J, DeAngelis LM, Abrey LE. Long-term follow-up of high-dose methotrexate-based therapy with and without whole brain irradiation for newly diagnosed primary CNS lymphoma. J Clin Oncol 2006;24:4570–4574 [DOI] [PubMed] [Google Scholar]
- 7.Abrey LE, Moskowitz CH, Mason WP, et al. Intensive methotrexate and cytarabine followed by high-dose chemotherapy with autologous stem-cell rescue in patients with newly diagnosed primary CNS lymphoma: an intent-to-treat analysis. J Clin Oncol 2003;21:4151–4156 [DOI] [PubMed] [Google Scholar]
- 8.DeAngelis LM, Seiferheld W, Schold SC, Fisher B, Schultz CJ; Radiation Therapy Oncology Group Study 93-10. Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: Radiation Therapy Oncology Group Study 93-10. J Clin Oncol 2002;20:4643–4648 [DOI] [PubMed] [Google Scholar]
- 9.Kim YR, Kim SH, Chang JH, et al. Early response to high-dose methotrexate, vincristine, and procarbazine chemotherapy-adapted strategy for primary CNS lymphoma: no consolidation therapy for patients achieving early complete response. Ann Hematol 2013;93:211–219 [DOI] [PubMed] [Google Scholar]
- 10.Gerstner ER, Carson KA, Grossman SA, Batchelor TT. Long-term outcome in PCNSL patients treated with high-dose methotrexate and deferred radiation. Neurology 2008;70:401–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chamberlain MC, Johnston SK. High-dose methotrexate and rituximab with deferred radiotherapy for newly diagnosed primary B-cell CNS lymphoma. Neuro Oncol 2010;12:736–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Batchelor TT, Grossman SA, Mikkelsen T, Ye X, Desideri S, Lesser GJ. Rituximab monotherapy for patients with recurrent primary CNS lymphoma. Neurology 2011;76:929–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birnbaum T, Stadler EA, von Baumgarten L, Straube A. Rituximab significantly improves complete response rate in patients with primary CNS lymphoma. J Neurooncol 2012;109:285–291 [DOI] [PubMed] [Google Scholar]
- 14.Abrey LE, Batchelor TT, Ferreri AJ, et al. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol 2005;23:5034–5043 [DOI] [PubMed] [Google Scholar]
- 15.Gerber DE, Grossman SA, Batchelor T, Ye X. Calculated versus measured creatinine clearance for dosing methotrexate in the treatment of primary central nervous system lymphoma. Cancer Chemother Pharmacol 2007;59:817–823 [DOI] [PubMed] [Google Scholar]
- 16.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–481 [Google Scholar]
- 17.Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics 1982;38:29–41 [Google Scholar]
- 18.Ferreri AJ, Blay JY, Reni M, et al. Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience. J Clin Oncol 2003;21:266–272 [DOI] [PubMed] [Google Scholar]
- 19.Kimby E. Tolerability and safety of rituximab (MabThera). Cancer Treat Rev 2005;31:456–473 [DOI] [PubMed] [Google Scholar]
- 20.Swinnen LJ. Primary central nervous system lymphoma: recent progress, many remaining questions. Curr Opin Oncol 2009;21:393–396 [DOI] [PubMed] [Google Scholar]