Abstract
Objective:
To examine functional connectivity within the basal ganglia network (BGN) in a group of cognitively normal patients with early Parkinson disease (PD) on and off medication compared to age- and sex-matched healthy controls (HC), and to validate the findings in a separate cohort of participants with PD.
Methods:
Participants were scanned with resting-state fMRI (RS-fMRI) at 3T field strength. Resting-state networks were isolated using independent component analysis. A BGN template was derived from 80 elderly HC participants. BGN maps were compared between 19 patients with PD on and off medication in the discovery group and 19 age- and sex-matched controls to identify a threshold for optimal group separation. The threshold was applied to 13 patients with PD (including 5 drug-naive) in the validation group to establish reproducibility of findings.
Results:
Participants with PD showed reduced functional connectivity with the BGN in a wide range of areas. Administration of medication significantly improved connectivity. Average BGN connectivity differentiated participants with PD from controls with 100% sensitivity and 89.5% specificity. The connectivity threshold was tested on the validation cohort and achieved 85% accuracy.
Conclusions:
We demonstrate that resting functional connectivity, measured with MRI using an observer-independent method, is reproducibly reduced in the BGN in cognitively intact patients with PD, and increases upon administration of dopaminergic medication. Our results hold promise for RS-fMRI connectivity as a biomarker in early PD.
Classification of evidence:
This study provides Class III evidence that average connectivity in the BGN as measured by RS-fMRI distinguishes patients with PD from age- and sex-matched controls.
Functional changes in the basal ganglia (BG) lie at the heart of Parkinson disease (PD). fMRI employing motor tasks has identified abnormalities in BG and supplementary motor area.1,2 However, interpretation of those changes may be confounded by the fact that patients with PD have difficulty with performing motor tasks. Resting-state fMRI can overcome this problem by providing an index of activity across the whole brain while the participant is at rest. Independent component analysis (ICA) enables isolation of resting-state brain networks, where individual areas in a given network show tight functional connectivity.3 Recent studies demonstrated effectiveness of this technique in identifying changes in the default mode network4,5 and sensorimotor network6 in PD. Although ICA-derived BG network (BGN) has been reliably identified in other disease states,7–9 to our knowledge, no study so far has investigated it in PD.
In this case-control study, we aimed to investigate changes in the BGN in PD and test whether they can distinguish patients with PD from healthy controls. To that effect, we first developed a resting-state template using a large group of elderly controls, containing a BGN. Secondly, a discovery cohort was used to identify changes in the BGN and the effect of dopaminergic medication on its connectivity. In the last step, we employed a validation cohort to test reproducibility of the findings in a separate group of patients.
METHODS
Participants.
A breakdown of participant numbers at each stage of recruitment is presented in figure e-1 on the Neurology® Web site at Neurology.org. Thirty-two right-handed patients with early PD were recruited from the Oxford Parkinson's Disease Centre (OPDC) cohort. The OPDC recruits patients from the Thames Valley area with a diagnosis of idiopathic PD within the last 3 years according to UK PD Society Brain Bank criteria. Participants undergo assessment in designated research clinics covering medical interview, characterization of the motor and nonmotor features of PD (including the new Movement Disorder Society–Unified Parkinson's Disease Rating Scale [UPDRS]10), and cognitive assessment. Participants with PD in our study were divided into a discovery cohort and a validation cohort. The discovery cohort consisted of 19 medicated patients taking dopamine agonists, levodopa, MAO-B inhibitors, or a combination of those. However, perhaps not surprisingly given the short disease duration, there was no evidence for significant motor complications of therapy seen on the UPDRS-IV. Patients were scanned both off medication (after 12–17 hours of overnight withdrawal of dopaminergic medication, including levodopa, once daily and 3 times daily dopamine agonist preparations) and 60–90 minutes after taking their own medication in their best-defined “on” state. The validation cohort comprised 13 patients: 5 drug-naive and 8 medicated, all of whom were scanned off medication. The validation cohort tended to be older and had slightly lower Mini-Mental State Examination (MMSE) scores than the discovery cohort but these differences were not statistically significant, and there were no differences in sex composition, disease severity, disease duration, or levodopa equivalent daily dose11 (table 1). Additionally, the validation cohort tended to be older and had lower MMSE scores than the healthy control group, but the changes were statistically nonsignificant or marginally significant, respectively.
Table 1.
Demographic and clinical data of study participants
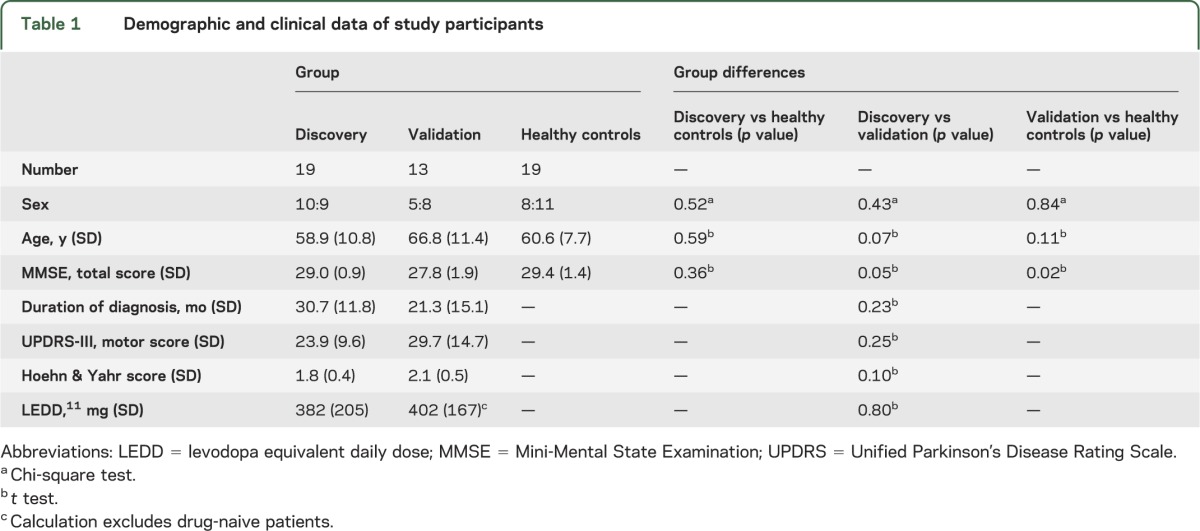
Nineteen right-handed healthy controls (HC), matched for age and sex to the discovery cohort, were recruited from the OPDC control group. Specifically, controls' age was matched to the average age of the scanned patients and the sex ratio was set at 1:1 at the start of the study. The HC participants were unrelated to patients with PD and were mostly spouses or friends of the participants with PD. Scanning procedures took place between November 2010 and February 2013. The size of the study groups was chosen based on average number of participants in similar published imaging studies.
Inclusion criteria for all participants in the study were right-handedness and ability to give informed consent. Exclusion criteria were standard contraindications to MRI scanning, dementia, other neurologic or psychiatric diseases, and more than one risk factor for cerebrovascular disease (hypertension, diabetes mellitus, hypercholesterolemia, cardiovascular disease). Specific exclusion criteria for the PD groups were physician-rated certainty of diagnosis <90%, more than mild tremor in “on” medication state (>2 on any tremor item of UPDRS-III), and presence of dyskinesia or dystonia to avoid motion artifacts during imaging. A specific exclusion criterion for controls was having first- or second-degree relatives with PD. Basic demographic characteristics of study participants are provided in table 1 and participant-specific details can be found in table e-1.
The “healthy” ICA template (described in detail below) was developed using 80 elderly healthy controls—19 from the OPDC cohort (described above) and 61 healthy control scans from 3 previously published studies from our institution12–14 that used the same scanner and acquisition protocol (see below). Inclusion criteria for those participants were right-handedness and MMSE ≥29. Exclusion criteria were significant brain atrophy and enlarged ventricles or intracranial pathology (stroke, tumor, cyst) on structural scans. Basic details of those participants can be found in table e-2.
Image acquisition.
All MRI data were acquired with a 3T Siemens (Erlangen, Germany) Trio MRI scanner with a 12-channel head coil. For each participant, T1-weighted images were obtained using a 3D magnetization-prepared rapid acquisition gradient echo sequence (192 axial slices, flip angle 8°, 1 × 1 × 1 mm3 voxel size, echo time [TE]/repetition time [TR]/inversion time = 4.7 ms/2,040 ms/900 ms).
Functional images were acquired using gradient echoplanar imaging (TR = 2,000 ms, TE = 28 ms, flip angle 89°, resolution 3 × 3 × 3.5 mm). Thirty-four axial slices were acquired per volume, covering both hemispheres with incomplete coverage of the cerebellum; 180 repetitions were acquired in 6 minutes. Participants were instructed to remain still and awake with eyes open.
Image processing.
Analysis of the resting-state scans was performed using MELODIC v3.03, part of the FSL software package.15 The images were motion-corrected and unwarped using a fieldmap.
It has recently been shown that head motion during a resting-state scan may affect functional connectivity.16,17 We therefore took particular care to account for any effects of motion in our analyses and used a previously described ICA-based denoising approach.18 Subsequently, standard processing steps19,20 were used to register individual images to the Montreal Neurological Institute space (details in supplementary material).
Connectivity analysis.
In order to create an unbiased template of resting-state networks typical for healthy elderly participants, a group ICA implemented in MELODIC was performed on all 80 controls (19 from OPDC cohort and 61 from 3 previous studies). The BGN was identified as one of 50 components in the set (details in e-Methods). Differences in BG resting functional connectivity across cohorts were then analyzed using the dual-regression method21 (details in e-Methods).
At the discovery stage, spatial BGN connectivity maps of 19 PD-“off” participants were compared to 19 age- and sex-matched HC participants using unpaired t test implemented in the FSL tool, Randomise (v2.1). Randomise provides exact false-positive control using a permutation testing implemented in threshold-free cluster enhancement (TFCE),22 which enhances sensitivity to spatially limited effects. Significant clusters (at p < 0.05 after family-wise error correction) were then used as a mask to identify medication effects in a paired t test comparison of 19 PD-“off” and 19 PD-“on” participants. The significance was set at p < 0.05, false discovery rate corrected within the above mask. PD-“on” patients were also compared to HC participants with an unpaired t test, using TFCE in Randomise.
In order to characterize connectivity changes between PD-“off” and HC in more detail, a post hoc analysis was performed on parameter estimates (PE) values. Specifically, PE were averaged participant-wise within a binary mask containing only significant clusters from the PD-“off” vs HC analysis, which produced a single value for each participant representative of individual BGN connectivity. A receiver operating characteristic (ROC) curve was generated to determine an optimal threshold for separating the 2 groups based on average connectivity. Averaged PE were also investigated for correlations with disease severity (UPDRS-III, Hoehn & Yahr), disease duration, and age using Pearson r.
A validation stage was included in the study design to address potential bias related to small study size. Average connectivity was extracted from 13 patients with PD from the validation cohort, using a mask of significant clusters from the discovery stage. A threshold identified in the ROC analysis was applied to the validation cohort and its accuracy in this group was determined.
Standard protocol approvals, registrations, and patient consents.
The experiments were undertaken with the understanding and written consent of each participant, with the approval from the local ethics committee, and in compliance with national legislation and the Declaration of Helsinki.
Primary research question.
Can the average connectivity in the ICA-derived BGN, as measured by resting-state functional MRI, distinguish between patients with PD and matched healthy controls? This study provides Class III evidence that average resting-state functional connectivity in the BGN distinguishes patients with PD from age-matched normal controls with 100% sensitivity and 89.5% specificity (area under the curve 0.972, 95% confidence interval [CI] 0.921–1.0). Additionally, the connectivity threshold tested on the validation cohort achieved 85% accuracy.
RESULTS
ICA consistently identified a BGN including the putamen and caudate bilaterally as well as anterior parts of the thalamus (figure 1). This network explained 30%–60% of the variance of voxels within the BG (figure e-2).
Figure 1. Basal ganglia network.
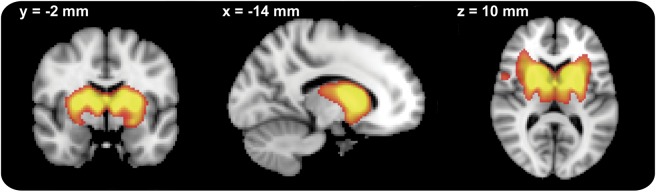
The image is thresholded at z > 4.
Discovery cohort.
At the discovery stage, voxel-wise comparison of 19 PD-“off” patients to 19 age- and sex-matched controls demonstrated reduced BGN connectivity in the PD-“off” group in 16,273 voxels spread across 9 clusters (figure 2): putamen and caudate bilaterally, midbrain, superior temporal gyrus bilaterally, dorsolateral prefrontal cortex bilaterally, medial prefrontal cortex, and precuneus.
Figure 2. Group comparison between patients with Parkinson disease “off” medication and the healthy control group in the discovery group.
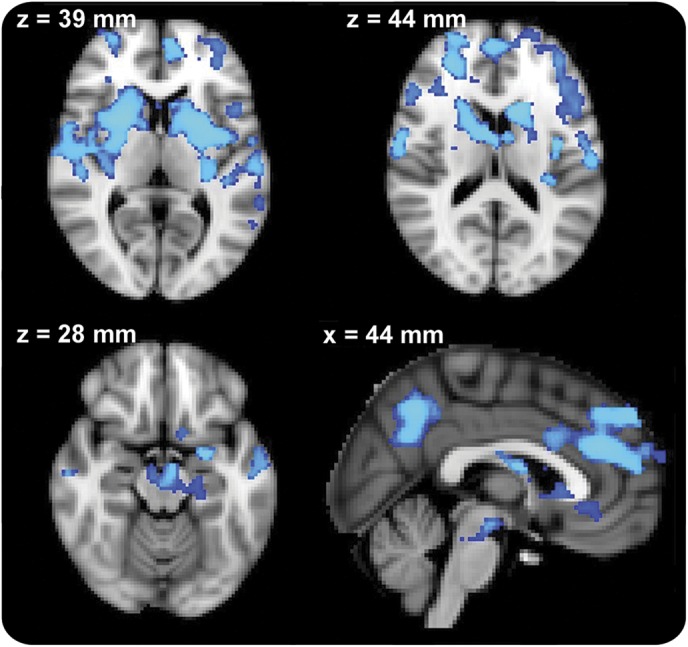
Significant clusters are located in the putamina bilaterally, medial frontal area, bilateral prefrontal areas, and precuneus. Images are displayed in radiologic convention (right is left). Slice location is displayed in Montreal Neurological Institute coordinates. Clusters are thresholded at p < 0.05 after correction for family-wise error.
Voxel-wise comparison of those same 19 patients with PD in “off” and “on” medication states revealed significantly increased connectivity in the “on” state affecting mostly the BG (figure 3). PD-“on” patients did not show any significant difference in connectivity when compared to the HC group.
Figure 3. Medication effect in the discovery group.
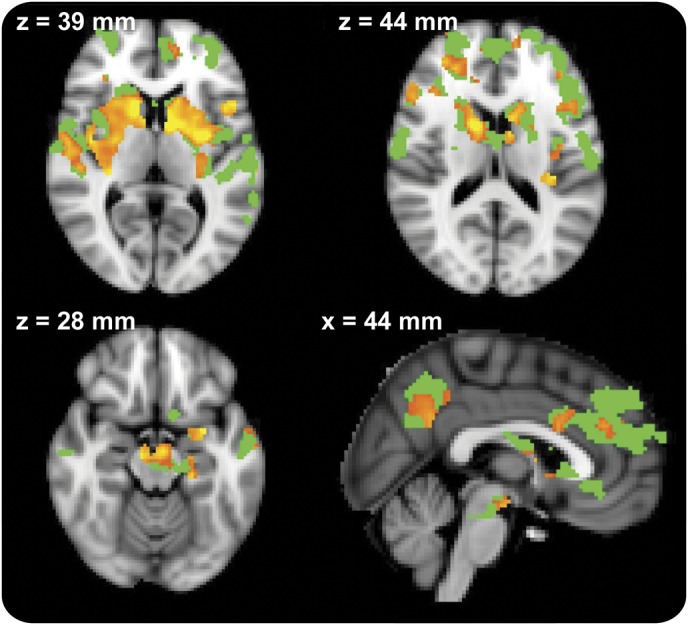
Clusters with increased connectivity after medication (red−yellow) are shown on the background of the mask of significantly different clusters from the Parkinson disease−“off” vs healthy controls comparison (green). Images are displayed in radiologic convention (right is left). Slice location is displayed in Montreal Neurological Institute coordinates. Clusters are thresholded at p < 0.05 after false discovery rate correction.
Average PE from the significant BGN clusters in PD-“off”/HC comparison were 1.04 (95% CI 0.42–1.65) in the discovery cohort and 7.59 (95% CI: 5.61–9.56) in the control group. In ROC analysis of averaged PE from the significant BGN clusters in PD-“off”/HC comparison, the area under the curve was 0.972 (95% CI 0.921–1.0). The cutoff value of 3.88 differentiated the PD-“off” from HC group with 100% sensitivity and 89.5% specificity (figure 4).
Figure 4. Average parameter estimates from the significantly different basal ganglia network clusters.
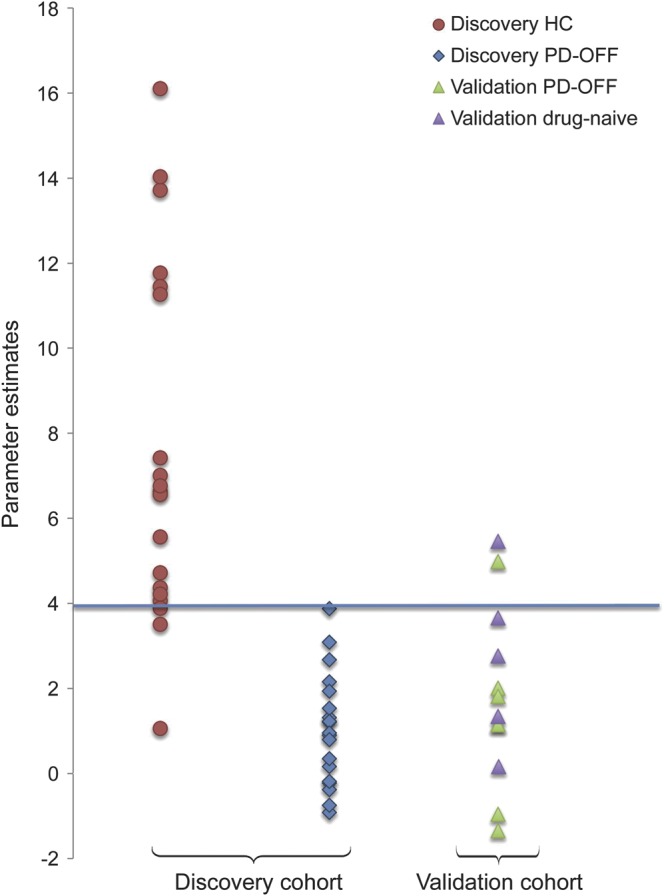
In the discovery cohort, Parkinson disease (PD)−“off” patients are shown as blue diamonds and controls as red circles. The validation cohort consists of PD-“off” patients, as green triangles, and PD drug-naive patients, as violet triangles. The blue line indicates a threshold with 100% sensitivity and 89.5% specificity for differentiating PD-“off” from healthy controls (HC) in the discovery cohort. Fitting this threshold to the validation cohort provides 85% accuracy for classifying patients with PD.
We did not find any significant correlations between averaged BGN connectivity and UPDRS-III, Hoehn & Yahr, disease duration, or age (either in the PD or the control groups).
Validation cohort.
In the 13 patients with PD from the validation cohort, average parameter estimates from the significant BGN clusters were 1.72 (95% CI 0.52–2.93). Applying the threshold of average connectivity in the significant BGN clusters derived from the discovery group correctly identified participants with PD in 85% of cases (11 cases of 13) (figure 4). One drug-naive participant and one medicated patient demonstrated average connectivity values above the threshold.
DISCUSSION
This study demonstrates widely reduced functional connectivity in the BGN in cognitively intact patients with PD. The difference in connectivity robustly separates the PD group off medication from controls. Administration of dopaminergic medication improves deficient connectivity in this network. Moreover, the abnormal connectivity is validated on a separate group of drug-naive and medicated patients.
Changes in the BGN in the PD-“off” vs HC analysis were focused in the putamen bilaterally (figure 2). Decreased functional connectivity in the putamen, understood as reduced coherence of the blood oxygenation level–dependent signal, are in line with findings of a previous study reporting reduced regional homogeneity23 and reduced degree of seed-based connectivity24 in a few voxels in the putamen. Another group examined seed-based functional connectivity of the caudate and anterior and posterior putamen with the rest of the brain.25 They did not find reduced connectivity within the seeds themselves but did report a difference in the right insula, immediately next to our focus of reduced connectivity in right putamen. A recent study26 using a similar technique of seed-based BG connectivity showed generally reduced connectivity with the cerebellum and particularly brainstem—a finding supported by our study. However, comparison of seed-based studies with ICA-based analyses needs to be done with caution since correlations in the former are based on a simple averaging of time series from the ROI of choice while in the latter only variance specific to the component of interest is taken into account.27
The physiologic significance of reduced connectivity of the BG may be clarified by reference to the traditional task-related fMRI and PET studies. Abnormal activations have been reported in the BG in a wide range of paradigms: simple hand movements,2,28,29 working memory and planning,30 set shifting and feedback,31,32 and temporal processing.33,34 Of note, only 3 out of the studies cited above28,30,33 showed increased activations in the BG in patients with PD, with the majority reporting reduced activity. It is not clear yet how the task fMRI findings relate to the resting-state changes. We hypothesize, however, that reduced functional connectivity in the BG, as shown in our resting-state experiment, may lead to reduced recruitment of this network seen in task fMRI.
Reduced connectivity was also seen in the dorsolateral and medial prefrontal cortex as well as the precuneus. The connectivity between striatum and prefrontal areas has been linked to executive function,35 which is known to be compromised early in PD.36 It could then be hypothesized that connectivity deficit in this area may be linked to PD-specific executive deficit. Similarly, the precuneus as the main hub of the DMN network has been related to cognitive function and other nodes of the DMN have recently been shown to display reduced functional connectivity in relation to cognitive impairment in PD.4
Administration of dopaminergic medication clearly improved connectivity as shown in the PD-“off”/PD-“on” comparison, leading to disappearance of any significant differences between PD and HC. This finding suggests that reduced connectivity in the BGN is a functional rather than structural phenomenon and is related to PD-specific dopamine-dependent processes. Another possible explanation could be that reduced connectivity is somehow a reflection of tremor activity during scanning, which may be ameliorated by medication. This is, however, highly unlikely as 7 out of 19 patients did not have any resting tremor (table e-1) and yet they demonstrated the same changes as the rest of the group. Moreover, lack of significant differences in motion between the groups speaks against a strong influence of head movements on the results.
A recent study6 also showed increase in connectivity in patients with PD after levodopa. The authors investigated the sensorimotor network but the fact that connectivity off and on medication changed in the same direction as in our study suggests a common underlying pathologic mechanism in both networks.
BGN connectivity did not correlate with any clinical indices of severity in our study. This may indicate that BGN connectivity, similar to SN hyperechogenicity as identified by transcranial sonography,37 is a trait marker of disease, reflecting a constitutional fault of the network and not the resulting clinical symptoms. Alternatively, the damage in the BGN may be profound enough to produce a floor effect where gradation of severity is lost.38
Reduced connectivity in the BGN separated the PD-“off” from the HC group with high sensitivity and specificity (100% and 89.5%, respectively). A particularly strong finding from this study is a confirmation of this result in the validation cohort, 85% of which showed results below the derived threshold. The fact that 4 out of 5 drug-naive patients also showed reduced BGN connectivity increases robustness of the finding and speaks against it being a simple effect of short-term medication withdrawal.
Development of the elderly healthy control ICA template enabled us to isolate resting-state networks that were unbiased by the pathologic changes in PD. The approach has been successfully used previously.39,40 Moreover, the template can be reused to test reproducibility of original findings on a separate group, as done in our study, and may serve to identify pathologic changes in at-risk groups in the future.
A major limitation of our study is small sample size of the discovery cohort. We addressed that weakness by adding a validation cohort to assess reproducibility of the results; however, testing larger independent samples with longitudinal follow-up is necessary. Moreover, we excluded participants with tremor, which limits the generalizability of our findings. However, a previous study employing seed-based connectivity in PD did not find any difference in BG connectivity between tremor-dominant and nontremor patients.25
Functional connectivity at rest, analyzed with an observer-independent method, showed clear abnormalities in patients with PD in areas relevant to disease pathophysiology. This finding was successfully cross-validated on a separate group of drug-naive and medicated patients. Additionally, connectivity changes dramatically improved after dopaminergic medication. Application of this technique to a larger baseline cohort and longitudinal scanning will help critically evaluate the role of resting-state functional MRI as a potential imaging biomarker for early PD.
Supplementary Material
ACKNOWLEDGMENT
The authors thank all the patients and healthy control participants.
GLOSSARY
- BG
basal ganglia
- BGN
basal ganglia network
- CI
confidence interval
- HC
healthy controls
- ICA
independent component analysis
- MMSE
Mini-Mental State Examination
- OPDC
Oxford Parkinson's Disease Centre
- PD
Parkinson disease
- PE
parameter estimates
- ROC
receiver operating characteristic
- TE
echo time
- TFCE
threshold-free cluster enhancement
- TR
repetition time
- UPDRS
Unified Parkinson's Disease Rating Scale
Footnotes
Editorial, page 202
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. Szewczyk-Krolikowski: literature review, experimental design, data acquisition, image data analysis, result interpretation, statistical analysis, manuscript drafting and revision. Dr. Menke: experimental design, data acquisition, and manuscript revision. Dr. Rolinski: data acquisition and manuscript revision. Dr. Duff: data analysis and manuscript revision. Dr. Salimi-Khorshidi: data analysis and manuscript revision. Dr. Filippini: image data analysis and manuscript revision, provision of additional imaging datasets. Dr. Zamboni: provision of additional imaging data sets. Dr. Hu: experimental design, result interpretation, and manuscript revision. Dr. Mackay: experimental design, result interpretation, and manuscript revision.
STUDY FUNDING
The research is funded by the Monument Trust Discovery Award from Parkinson's UK to the Oxford Parkinson Disease Centre. The authors are supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre based at Oxford University Hospitals NHS Trust and University of Oxford and the Dementias and Neurodegenerative Diseases Research Network (DeNDRoN).
DISCLOSURE
K. Szewczyk-Krolikowski has received research support from Parkinson's UK. R. Menke has received research support from Parkinson's UK. M. Rolinski has received research support from the NIHR Oxford Biomedical Centre. E. Duff, G. Salimi-Khorshidi, N. Filippini, and G. Zamboni report no disclosures relevant to the manuscript. M. Hu has received research support from Parkinson's UK. C. Mackay has received research support from Parkinson's UK and the NIHR Oxford Biomedical Research Centre. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Prodoehl J, Spraker M, Corcos D, Comella C, Vaillancourt D. Blood oxygenation level-dependent activation in basal ganglia nuclei relates to specific symptoms in de novo Parkinson's disease. Mov Disord 2010;25:2035–2043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rowe J, Stephan KE, Friston K, Frackowiak R, Lees A, Passingham R. Attention to action in Parkinson's disease: impaired effective connectivity among frontal cortical regions. Brain 2002;125:276–289 [DOI] [PubMed] [Google Scholar]
- 3.Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philosophical Trans R Soc B Biol Sci 2005;360:1001–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tessitore A, Esposito F, Vitale C, et al. Default-mode network connectivity in cognitively unimpaired patients with Parkinson disease. Neurology 2012;79:2226–2232 [DOI] [PubMed] [Google Scholar]
- 5.Krajcovicova L, Mikl M, Marecek R, Rektorova I. The default mode network integrity in patients with Parkinson's disease is levodopa equivalent dose-dependent. J Neural Transm 2012;119:443–454 [DOI] [PubMed] [Google Scholar]
- 6.Esposito F, Tessitore A, Giordano A, et al. Rhythm-specific modulation of the sensorimotor network in drug-naive patients with Parkinson's disease by levodopa. Brain 2013;136:710–725 [DOI] [PubMed] [Google Scholar]
- 7.Robinson S, Basso G, Soldati N, et al. A resting state network in the motor control circuit of the basal ganglia. BMC Neurosci 2009;10:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laird AR, Fox PM, Eickhoff SB, et al. Behavioral interpretations of intrinsic connectivity networks. J Cogn Neurosci 2011;23:4022–4037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo C, Li Q, Xia Y, et al. Resting state basal ganglia network in idiopathic generalized epilepsy. Hum Brain Mapp 2012;33:1279–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goetz CG, Fahn S, Martinez-Martin P, et al. Movement Disorder Society–sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): process, format, and clinimetric testing plan. Mov Disord 2007;22:41–47 [DOI] [PubMed] [Google Scholar]
- 11.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. 2010;25:2649–2653 [DOI] [PubMed] [Google Scholar]
- 12.Zamboni G, Wilcock GK, Douaud G, Drazich E. Resting functional connectivity reveals residual functional activity in Alzheimer's disease. Biol Psychiatry 2013;74:375–383 [DOI] [PubMed] [Google Scholar]
- 13.Filippini N, Nickerson LD, Beckmann CF, et al. Age-related adaptations of brain function during a memory task are also present at rest. Neuroimage 2012;59:3821–3828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filippini N, Ebmeier KP, MacIntosh BJ, et al. Differential effects of the APOE genotype on brain function across the lifespan. Neuroimage 2011;54:602–610 [DOI] [PubMed] [Google Scholar]
- 15.Woolrich MW, Jbabdi S, Patenaude B, et al. Bayesian analysis of neuroimaging data in FSL. Neuroimage 2009;45(suppl 1):S173–S186 [DOI] [PubMed] [Google Scholar]
- 16.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 2012;59:2142–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Dijk KRA, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage 2012;59:431–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith SM, Miller KL, Moeller S, et al. Temporally-independent functional modes of spontaneous brain activity. Proc Natl Acad Sci USA 2012;109:3131–3136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002;17:825–841 [DOI] [PubMed] [Google Scholar]
- 20.Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage 2009;48:63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Filippini N, MacIntosh BJ, Hough MG, et al. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci USA 2009;106:7209–7214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 2009;44:83–98 [DOI] [PubMed] [Google Scholar]
- 23.Wu T, Long X, Zang Y, et al. Regional homogeneity changes in patients with Parkinson's disease. Hum Brain Mapp 2009;30:1502–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu T, Wang J, Wang C, et al. Basal ganglia circuits changes in Parkinson's disease patients. Neurosci Lett 2012;524:55–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helmich RC, Derikx LC, Bakker M, Scheeringa R, Bloem BR, Toni I. Spatial remapping of cortico-striatal connectivity in Parkinson's disease. Cereb Cortex 2010;20:1175–1186 [DOI] [PubMed] [Google Scholar]
- 26.Hacker CD, Perlmutter JS, Criswell SR, Ances BM, Snyder AZ. Resting state functional connectivity of the striatum in Parkinson's disease. Brain 2012;135:3699–3711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joel SE, Caffo BS, van Zijl PCM, Pekar JJ. On the relationship between seed-based and ICA-based measures of functional connectivity. Magn Reson Med 2011;66:644–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cerasa A, Hagberg GE, Peppe A, et al. Functional changes in the activity of cerebellum and frontostriatal regions during externally and internally timed movement in Parkinson's disease. Brain Res Bull 2006;71:259–269 [DOI] [PubMed] [Google Scholar]
- 29.Wu T, Wang L, Hallett M, Li K, Chan P. Neural correlates of bimanual anti-phase and in-phase movements in Parkinson's disease. Brain 2010;133:2394–2409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Owen AM, Doyon J, Dagher A, Sadikot A, Evans AC. Abnormal basal ganglia outflow in Parkinson's disease identified with PET: implications for higher cortical functions. Brain 1998;121:949–965 [DOI] [PubMed] [Google Scholar]
- 31.Monchi O, Petrides M, Mejia-Constain B, Strafella AP. Cortical activity in Parkinson's disease during executive processing depends on striatal involvement. Brain 2007;130:233–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monchi O, Petrides M, Doyon J, Postuma RB, Worsley K, Dagher A. Neural bases of set-shifting deficits in Parkinson's disease. J Neurosci 2004;24:702–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jahanshahi M, Jones CRG, Zijlmans J, et al. Dopaminergic modulation of striato-frontal connectivity during motor timing in Parkinson's disease. Brain 2010;133:727–745 [DOI] [PubMed] [Google Scholar]
- 34.Harrington DL, Castillo GN, Greenberg PA, et al. Neurobehavioral mechanisms of temporal processing deficits in Parkinson's disease. Plos One 2011;6:e17461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gordon EM, Devaney JM, Bean S, Vaidya CJ. Resting-state striato-frontal functional connectivity is sensitive to DAT1 genotype and predicts executive function. Cereb Cortex Epub Aug 22, 2013 [DOI] [PMC free article] [PubMed]
- 36.Dirnberger G, Jahanshahi M. Executive dysfunction in Parkinson's disease: a review. J Neuropsychol 2013;7:193–224 [DOI] [PubMed] [Google Scholar]
- 37.Brooks DJ, Pavese N. Imaging biomarkers in Parkinson's disease. Prog Neurobiol 2011;95:614–628 [DOI] [PubMed] [Google Scholar]
- 38.Martin WRW, Wieler M, Stoessl AJ, Schulzer M. Dihydrotetrabenazine positron emission tomography imaging in early, untreated Parkinson's disease. Ann Neurol 2008;63:388–394 [DOI] [PubMed] [Google Scholar]
- 39.Cole DM, Beckmann CF, Oei NYL, Both S, van Gerven JMA, Rombouts SARB. Differential and distributed effects of dopamine neuromodulations on resting-state network connectivity. Neuroimage 2013;78:59–67 [DOI] [PubMed] [Google Scholar]
- 40.Cole DM, Oei NYL, Soeter RP, et al. Dopamine-dependent architecture of cortico-subcortical network connectivity. Cereb Cortex 2012;23:1509–1516 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


