Abstract
Objective:
To examine predictors of stroke recurrence in patients with a high vs a low likelihood of having an incidental patent foramen ovale (PFO) as defined by the Risk of Paradoxical Embolism (RoPE) score.
Methods:
Patients in the RoPE database with cryptogenic stroke (CS) and PFO were classified as having a probable PFO-related stroke (RoPE score of >6, n = 647) and others (RoPE score of ≤6 points, n = 677). We tested 15 clinical, 5 radiologic, and 3 echocardiographic variables for associations with stroke recurrence using Cox survival models with component database as a stratification factor. An interaction with RoPE score was checked for the variables that were significant.
Results:
Follow-up was available for 92%, 79%, and 57% at 1, 2, and 3 years. Overall, a higher recurrence risk was associated with an index TIA. For all other predictors, effects were significantly different in the 2 RoPE score categories. For the low RoPE score group, but not the high RoPE score group, older age and antiplatelet (vs warfarin) treatment predicted recurrence. Conversely, echocardiographic features (septal hypermobility and a small shunt) and a prior (clinical) stroke/TIA were significant predictors in the high but not low RoPE score group.
Conclusion:
Predictors of recurrence differ when PFO relatedness is classified by the RoPE score, suggesting that patients with CS and PFO form a heterogeneous group with different stroke mechanisms. Echocardiographic features were only associated with recurrence in the high RoPE score group.
Randomized trials comparing endovascular patent foramen ovale (PFO) closure with antithrombotic therapy for stroke prevention in patients with cryptogenic stroke (CS) and PFO have been equivocal1–3 and identify the importance of careful patient selection. Closure may only benefit those with a high “PFO-attributable recurrence risk”—the joint probability that (1) the discovered PFO was pathogenic (i.e., causally related to the index event) and (2) the CS will recur. Identifying predictors of recurrence risk among those most likely to have had a PFO-related index event is of high clinical interest.
Previously, we identified variables that are associated with PFO prevalence in CS patients.4 Based on this and Bayes theorem, we developed the Risk of Paradoxical Embolism (RoPE) score, which estimates the probability that a PFO discovered in a CS patient is incidental or pathogenic. Patients with high RoPE scores (younger, no vascular risk factors, and a superficial infarct) are more likely to have pathogenic PFOs, while PFOs in patients with low RoPE scores (older with vascular risk factors) are probably incidental.
We examined factors associated with recurrence risk in patients with PFO and CS. We hypothesized that if RoPE scores segregate patients into those more and less likely to have PFO-related index events, then predictors of recurrence should differ between the groups. Further, PFO characteristics (e.g., shunt size and septal hypermobility) should be less influential in low RoPE score patients (PFO-unrelated strokes). Such a finding would indirectly validate the RoPE score and ultimately provide a basis for improving patient selection.
METHODS
The design of the RoPE Study including model derivation and validation, the details of the RoPE database, and neuroimaging and echocardiographic findings have been described previously.4–7 In brief, we created a database of 3,674 subjects with CS and known PFO status (all had transesophageal echocardiography [TEE] or transcranial Doppler) by combining existing cohort studies with protocol-driven follow-up. Index events were sudden-onset neurologic deficits presumed due to cerebral ischemia lasting longer than 24 hours or associated with anatomically relevant radiologic lesions (stroke) or lasting less than 24 hours with normal imaging (TIA). The operational definition of “cryptogenic” varied somewhat between databases but generally adhered to the TOAST criteria. To be included in the database, each cohort study was required to have a minimum of 85% follow-up for 1-year outcome and sufficient clinical, echocardiographic, and neuroradiologic data to permit multivariate modeling. Our analyses are retrospective but come from the RoPE database, which was formed by combining existing cohort studies, all of which included prospectively collected data.
The clinical variables in the database are age (at index event), sex, race, hypertension, diabetes, coronary artery disease, hypercholesterolemia, smoking, a history of cerebral ischemia (TIA or stroke) prior to the index event, medications being taken at the time of the index event (antithrombotics, statins, hormone replacement therapy, oral contraceptives), and treatment medications after the index event (antiplatelets, anticoagulants). The echocardiographic variables used in this analysis are septal hypermobility, PFO shunting at rest, and physiologic shunt size. Details of how these data were measured in the component studies and merged into the RoPE database have been described previously.5 Septal hypermobility refers to interatrial septal excursion during the cardiac cycle of 10 mm or more from the midline. Shunting at rest refers to agitated saline contrast (bubbles) appearing in the left atrium within 3 cardiac cycles of right atrial opacification with normal respiration, i.e., not requiring Valsalva maneuver. Physiologic shunt size was defined by the maximal number of bubbles seen in the left atrium either at rest or after Valsalva maneuver. A large shunt was defined as greater than 10–15 bubbles in a single frame. The neuroradiologic variables were taken from imaging done at the time of the index event. They were infarct seen (yes/no), infarct location (superficial, deep), infarct size (large/small), multiple acute infarcts (yes/no), chronic (prior) stroke (yes/no). Details of the neuroimaging findings in RoPE have been described separately.6
Outcomes included stroke and TIA. Each outcome was re-adjudicated by the RoPE Outcome Adjudication Committee and categorized as stroke, TIA, nonstroke death, or no event.8 Cerebral ischemic events were assigned a pathophysiologic mechanism if possible. However, given the nature of the RoPE study, systematic workups were not performed to determine mechanism across studies. All outcomes, irrespective of the adjudicated mechanism of recurrence, were included for analysis. Sensitivity analysis was performed excluding nonstroke outcomes (i.e., TIA) and recurrent events of determined cause (i.e., ostensibly not PFO-related).
To support risk modeling, the database was divided into those with RoPE scores of 0–6 (n = 678, estimated PFO attributable fraction 40% [36%–43%]) and 7–10 (n = 646, estimated PFO attributable fraction 80% [77%–83%]). The cohort was divided into a high and low RoPE score group based on a threshold approximating the median score to maximize power (≤6 vs >6).
Patient characteristics were summarized. One- and 2-year stroke/TIA recurrence rates were estimated using Kaplan-Meier curves. To support Cox proportional hazard (PH) regression modeling, we estimated missing data points using single imputation based on the averaged results of 10 multiply imputed databases. Separate Cox PH regression models were developed on each of these strata using all the variables shown in table 1. Variables that were significant at the p ≤ 0.05 threshold in either model were included as candidates together with their interaction with RoPE strata in a model derived from the entire cohort. Variables were retained in the model if their interaction term was significant at the p ≤ 0.1 threshold or their main effect was significant at the p ≤ 0.05 threshold.
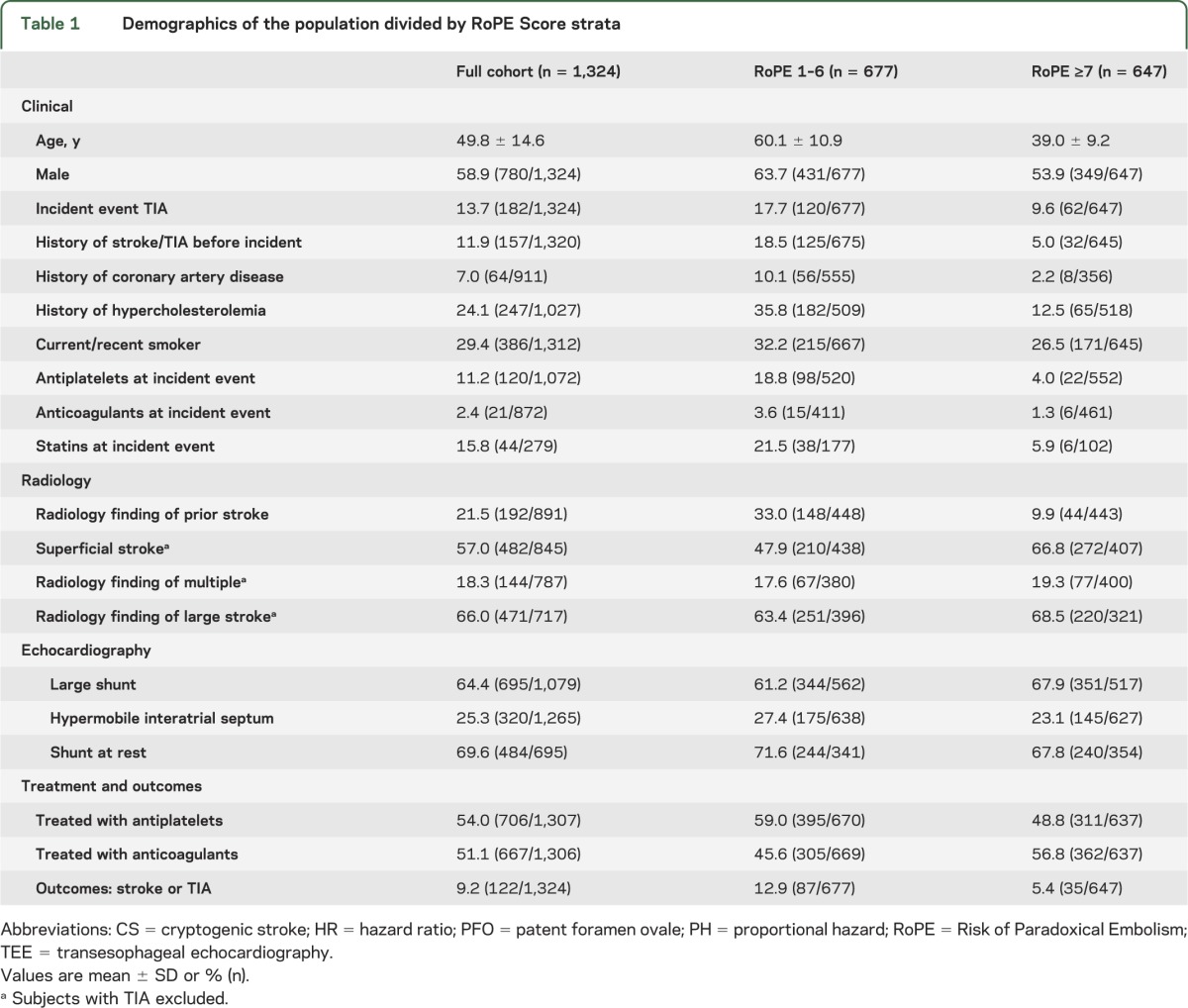
Table 1.
Demographics of the population divided by RoPE Score strata
Because some patients in the RoPE database were eventually treated with mechanical closure, we examined the potential influence of informative censoring on our results, by (1) examining patient characteristics of those closed compared to other study patients; (2) examining the impact of excluding these patients on the results of the multivariable model; and (3) performing multiple sensitivity analyses assigning stroke outcomes to the patients who were censored due to closure, including incident rates considerably higher than clinically anticipated.
Standard protocol approvals, registrations, and patient consent.
This study was approved by the Tufts Medical Center internal review board.
RESULTS
Demographics of the population are presented in table 1. Of the 3,674 subjects in the RoPE database, there are 1,543 with PFO and adequate study-level subject follow-up. We excluded 219 subjects because the PFO was not evaluated by TEE (n = 120), or because subjects did not have adequate follow-up or outcome data (n = 59), or consent for follow-up was not obtained (n = 40). The remaining 1,324 subjects had a median follow-up of 2.2 years (interquartile range 1.0–3.6). More than 1 year follow-up was available for 93%. Of the 133 total outcomes, 11 were excluded as no event after the adjudication process, leaving a total of 122 outcomes (76 stroke and 46 TIA) for this analysis. A mechanism was identified for the recurrent event in 5 subjects (3 stroke and 2 TIA) and was either cryptogenic or lacked sufficient information for adjudication in the remaining 117. All patients in the database were under the care of study neurologists and were treated with medical therapies, i.e., antithrombotic medication and risk factor modification according to local practice.
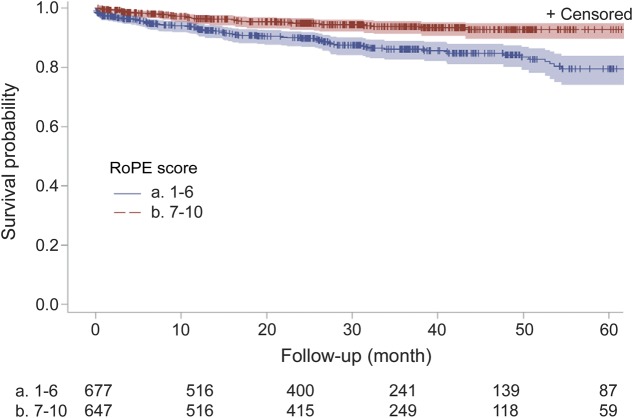
The stroke/TIA recurrence rates differed by RoPE strata. For those with low RoPE scores, the 1-year and 2-year rates were 7% and 10%, respectively, and only 4% and 5% for those with high RoPE scores (figure; KM plot; p < 0.0001, log-rank test). Of the 5 recurrent events adjudicated as having a known cause, 4 were in the low RoPE score group.
Figure. Kaplan-Meier plot of recurrent stroke-free survival by Risk of Paradoxical Embolism (RoPE) stratum.
Subjects presenting with TIA rather than stroke had a higher risk for recurrence (hazard ratio [HR] 1.69, 1.05–2.74), but there was no interaction with RoPE strata (table 2).
Table 2.
Adjusted hazard ratios from multivariable model of recurrent stroke/TIA
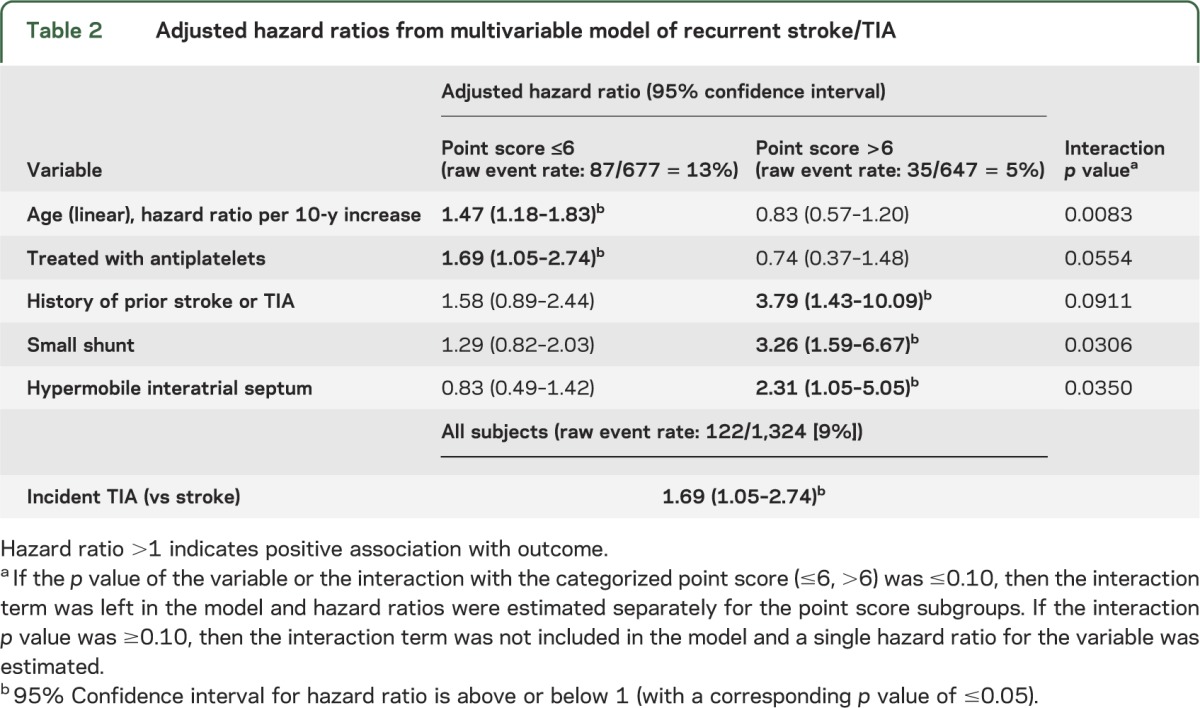
Other variables were associated with recurrence but only in 1 RoPE stratum or another (interaction p value ≤0.10). In those with low RoPE scores, there was an increased risk of recurrence (1) with older age (HR 1.47, 1.18–1.83) and (2) in those who were treated with antiplatelet drugs after their index event (HR 1.69, 1.05–2.74). These variables were not influential in the high RoPE score stratum. There were 3 variables that were only associated with recurrence in the high RoPE score group: a history of stroke or TIA (HR 3.79, 1.43–10.09), a hypermobile interatrial septum (HR 2.31, 1.05–5.05), and a small shunt (HR 3.26, 1.59–6.67) (table 2). Shunt at rest was not associated with recurrence in either group. A sensitivity analysis done after excluding the French study (in which the association between a hypermobile interatrial septum and recurrence risk was initially reported) did not change the direction of the associations with the echocardiographic variables. The characteristics of the 187 patients who were closed differed from those who continued with medical therapy by being younger, less likely to smoke, less likely to have CAD, and more likely to have been on anticoagulants at the time of their incident event (table e-1 on the Neurology® Web site at Neurology.org). However, sensitivity analyses excluding these patients (table e-2) or randomly assigning to them stroke outcomes at the time of closure at rates considerably higher than those otherwise anticipated showed that our main results were robust to the full range of plausible outcome rates in these patients (appendix e-1).
DISCUSSION
Our analysis shows that patients with CS and PFO have different risk factors for short-term recurrence when segregated by RoPE score strata. Those with low RoPE scores (indicating that the index event was less likely to be PFO-related) have recurrent strokes in association with a conventional vascular risk factor (i.e., age) with no clear influence from the echocardiographic characteristics of their probable incidental PFOs. On the other hand, the echocardiographic characteristics of the PFOs in those with high RoPE scores are strongly associated with recurrence. This suggests different stroke mechanisms for these different strata; the importance of echocardiographic factors seen in the high RoPE score patients provides confirmatory evidence for the PFO-relatedness of the initial stroke in these patients.
A limitation in using the RoPE score alone to select patients for percutaneous closure is that those patients with high RoPE scores were found to be at much lower risk for stroke recurrence compared to those with low RoPE scores.4 Thus, while low RoPE score patients might be expected to have limited benefit from closure because a large proportion of the index events are due to mechanisms unrelated to the PFO and unaddressed by closure, high RoPE score patients may have limited benefit because they are unlikely to have recurrent strokes when treated medically.
In CS patients, a prior clinical history of stroke is less common (∼10%) than is a chronic stroke seen at the time of index event imaging (∼25%).6 Both are associated with recurrence but the clinical history appears especially influential in the high RoPE score group. This suggests that high RoPE score patients who present with recurrent stroke are at a particularly high risk of another event. In both RoPE strata, however, TIA again shows itself to be an unstable cerebrovascular condition with a higher risk of recurrence than in those with a definitive stroke.9
The observation that echocardiographic characteristics are only influential in the high RoPE score group is intuitive and was anticipated. A hypermobile (“aneurysmal”) interatrial septum has been implicated as a risk factor for recurrent stroke since the 2001 article by Mas et al.8 We confirm that finding, although we note that those data formed part of the RoPE dataset.
Our result that there is a strong relationship between smaller shunt size and recurrent stroke is counterintuitive and was unanticipated. The finding could be argued to be a consequence of an unreliable variable—interobserver agreement for grading shunt size was only moderate.8 Furthermore, physiologic shunt size can vary from day to day and is dependent on hydration status, technical factors during the injection of the agitated saline, and patient cooperation, especially with Valsalva.10 However, lack of reliability or consistency in shunt grading should produce a bias toward the null, and thus is unlikely to explain the discovery of a strong association, albeit in an unexpected direction.
Conventional wisdom has it that a larger shunt supports the initial diagnosis of PFO-relatedness and also identifies those at higher risk of recurrence. Indeed, others have found that PFOs discovered in CS patients are more frequently large than those discovered in patients with stroke of known cause.11 This finding, however, does not necessarily imply that patients with larger shunts therefore are at a higher risk of recurrence. Indeed, in the PICSS trial, for example, large shunt was a risk factor for stroke at the index event, but not for stroke recurrence. Our finding that small shunts are associated with an increased risk of recurrence in those without conventional vascular risk factors highlights the importance of considering that mechanisms other than paradoxical embolism (e.g., in situ thrombus) may also play a role in the association of PFO with CS, as others have also noted.12
Our findings also highlight the need for clarity regarding the definition of “high-risk” PFO patients. The literature on this issue is confusing. It is common that authors conflate the factors associated with the confidence of a PFO-associated diagnosis (e.g., younger age, CS, absence of vascular risk factors, peripherally located brain infarcts) with the risk for recurrence and some have made treatment recommendations on the basis of this. One such article relied on 11 references to support the features that the authors identified as “associated with recurrent paradoxical events.”13 Of those 11 references, 6 were without recurrence data entirely, 4 were not CS populations, and 1 was a CS population with no recurrent events.
This analysis is limited by the potential variability of protocols and definitions within the different studies that comprise the RoPE database. Efforts were made to harmonize the variables but some misclassification is inevitable. There still is no standardized approach for describing PFO anatomical and physiologic characteristics by transesophageal echocardiography, the absence of which hampers studies like ours. There are other variables that may be related to recurrence risk but that were not uniformly available in the component datasets and so were excluded for this analysis. Variables, such as hypercoagulable states, Valsalva-like activities immediately prior to symptom onset, and exogenous hormone therapy, could be tested in subgroups in future analyses. These database limitations also apply to the RoPE score itself, which does not include all potentially important variables for distinguishing patients with pathogenic vs incidental PFOs. Nevertheless, the study shows that this score appears to identify subgroups of patients with greatly different risks of recurrence and different determinants of recurrence. This is consistent with our hypothesis that the score disaggregates CS patients into groups likely to have different mechanisms.
Other limitations include the fact that our statistical power was decreased by excluding 601 subjects from the RoPE database for reasons mentioned above. Power was also reduced by the relatively short mean follow-up in the entire dataset, although some subjects were followed for as long as 15 years. Predictors of stroke over longer periods of time than is available in the RoPE database may not be the same as those identified here. However, later follow-up will, by definition, be in older patients in whom competing mechanisms of stroke become more common. Therefore, PFO-related variables may become less influential. Finally, we were unable to subclassify most of the recurrent events, thus limiting our ability to determine PFO or other relatedness.
Among patients with CS and PFO, stroke recurrence rates are higher in the stratum least likely to have a PFO-attributable CS and lower in the stratum most likely to have a PFO-attributable CS. Recurrence predictors differ by RoPE strata, suggesting different mechanisms in these groups. Echocardiographic characteristics predict recurrence risk only in those with high, but not low, RoPE scores. In those with RoPE scores of 7 or greater, hypermobile interatrial septa and smaller shunts are predictive of stroke recurrence, suggesting that paradoxical embolism is responsible for only some of the PFO-associated strokes. These data have implications for patient selection that are contrary to recommendations made by consensus rather than by reliable models of stroke recurrence.14 However, in order to use the information in this analysis for better patient selection, our predictive models should be optimized into a single score that will stratify patients by “PFO-attributable recurrence risk”—a combination of (1) predictors of attributable fraction and (2) predictors of recurrence risk. This should then be tested on the extant randomized clinical trial data, as we plan to do in subsequent studies.7
Supplementary Material
GLOSSARY
- CS
cryptogenic stroke
- HR
hazard ratio
- PFO
patent foramen ovale
- PH
proportional hazard
- RoPE
Risk of Paradoxical Embolism
- TEE
transesophageal echocardiography
Footnotes
Editorial, page 204
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
D.M.K.: designed and conceptualized the study, analyzed and interpreted data in the study, drafted the manuscript, revised, reviewed, and approved the manuscript. D.E.T.: designed and conceptualized the study, analyzed and interpreted data in the study, contributed study data, and revised, reviewed, and approved the manuscript. R.R.: analyzed and interpreted data in the study, supervised database management, revised, reviewed, and approved the manuscript. M.S.V.E., P.M., C.W.: contributed study data, analyzed and interpreted data in the study, revised, reviewed, and approved the manuscript. J.-L.M., J.S., S.H., E.D., M.R.D., C.J., H.P.M., M.-L.M., K.N., F.P.: contributed study data, revised, reviewed, and approved the manuscript. J.G., J.S.L.: revised, reviewed, and approved the manuscript.
STUDY FUNDING
Partially funded by grants UL1 RR025752 and R01 NS062153 from the NIH.
DISCLOSURE
D. Thaler is a consultant to AGA Medical Corporation and has consulted for WL Gore Associates. R. Ruthazer, C. Weimar, J. Mas, J. Serena, E. Di Angelantonio, F. Papetti, S. Homma, H. Mattle, K. Nedeltchev, M. Mono, C. Jaigobin, P. Michel, M. Elkind, M. Di Tullio, J. Lutz, and J. Griffith report no disclosures relevant to the manuscript. D. Kent has consulted for WL Gore Associates. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Carroll JD, Saver JL, Thaler DE, et al. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med 2013;368:1092–1100 [DOI] [PubMed] [Google Scholar]
- 2.Meier B, Kalesan B, Mattle HP, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med 2013;368:1083–1091 [DOI] [PubMed] [Google Scholar]
- 3.Furlan AJ, Reisman M, Massaro J, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med 2012;366:991–999 [DOI] [PubMed] [Google Scholar]
- 4.Kent DM, Ruthazer R, Weimar C, et al. An index to identify stroke-related vs incidental patent foramen ovale in cryptogenic stroke. Neurology 2013;81:619–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thaler DE, Di Angelantonio E, Di Tullio MR, et al. The Risk of Paradoxical Embolism (RoPE) Study: initial description of the completed database. Int J Stroke 2013;8:612–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thaler DE, Ruthazer R, Di Angelantonio E, et al. Neuroimaging findings in cryptogenic stroke patients with and without patent foramen ovale. Stroke 2013;44:675–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kent DM, Thaler DE. The Risk of Paradoxical Embolism (RoPE) Study: developing risk models for application to ongoing randomized trials of percutaneous patent foramen ovale closure for cryptogenic stroke. Trials 2011;12:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mas JL, Arquizan C, Lamy C, et al. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med 2001;345:1740–1746 [DOI] [PubMed] [Google Scholar]
- 9.Johnston SC, Leira EC, Hansen MD, Adams HP., Jr Early recovery after cerebral ischemia risk of subsequent neurological deterioration. Ann Neurol 2003;54:439–444 [DOI] [PubMed] [Google Scholar]
- 10.Johansson MC, Eriksson P, Guron CW, Dellborg M. Pitfalls in diagnosing PFO: characteristics of false-negative contrast injections during transesophageal echocardiography in patients with patent foramen ovales. J Am Soc Echocardiogr 2010;23:1136–1142 [DOI] [PubMed] [Google Scholar]
- 11.Homma S, Sacco RL, Di Tullio MR, Sciacca RR, Mohr JP. Effect of medical treatment in stroke patients with patent foramen ovale: patent foramen ovale in Cryptogenic Stroke Study. Circulation 2002;105:2625–2631 [DOI] [PubMed] [Google Scholar]
- 12.Messe SR, Silverman IE, Kizer JR, et al. Practice parameter: recurrent stroke with patent foramen ovale and atrial septal aneurysm: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2004;62:1042–1050 [DOI] [PubMed] [Google Scholar]
- 13.Wu LA, Malouf JF, Dearani JA, et al. Patent foramen ovale in cryptogenic stroke: current understanding and management options. Arch Intern Med 2004;164:950–956 [DOI] [PubMed] [Google Scholar]
- 14.Pristipino C, Anzola GP, Ballerini L, et al. Management of patients with patent foramen ovale and cryptogenic stroke: a collaborative, multidisciplinary, position paper. Catheter Cardiovasc Interv 2013;82:E38–E51 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.