Introduction
The angular vestibulo-ocular reflex (aVOR) maintains images on the fovea of the retina during head movement. When the aVOR is impaired, images slip off the fovea of the retina resulting in gaze instability during head motion. In superior canal dehiscence syndrome (SCDS), surgical plugging and/or resurfacing of the superior canal (SC) is the primary treatment when symptoms become debilitating. Common symptoms associated with SCDS include autophony (abnormal loudness perception of one’s own voice), dizziness in response to loud sounds and/or pressure changes, disequilibrium, conductive hyperacusis, pulsatile tinnitus and conductive hearing loss (1;2). While surgical treatment of SCDS improves autophony, disequilibrium, hearing, and dizziness in response to sound and/or pressure (1–4), surgical plugging is expected to result in reduced aVOR in the plane of the affected SC, which has been confirmed with scleral search coils during head impulse testing (5;6). While reduced aVOR function temporarily impairs balance (7), the long-term effect of SC plugging on gaze stabilization is unknown.
The dynamic visual acuity (DVA) test is a clinical assessment of gaze stability and the functional aVOR. DVA is the difference in visual acuity between head stationary (static) and moving (dynamic) conditions (8). During DVA testing, subjects view an optotype, (e.g. the letter “E”), which becomes progressively smaller. While visual acuity is minimally degraded during head motion in healthy subjects, this decrement becomes significant in vestibular hypofunction (8–11).
DVA is measured actively or passively. During active DVA, subjects move their own head (typically horizontally, as if saying “no”), which is a predictable rotation. During passive DVA, the examiner moves the patient’s head unpredictably. Canal plane DVA using transient, unpredictable head thrusts (htDVA) measures gaze stability separately in the plane of each semicircular canal (5). Improvement in active and passive htDVA has been documented in individuals with unilateral or bilateral vestibular loss who participate in gaze stability exercises, with little change in control groups given placebo exercises (9;10;12). Improvement in passive DVA is attributed to an increase in the number of preprogrammed compensatory saccades, while improvement in active DVA also was due to improved VOR gain (12). Horizontal, active DVA has not been reported in the SCDS population. However, in a small number of SCDS cases (n = 6) abnormal passive canal plane htDVA has been documented in the plane of the plugged SC (5). Although balance function normalizes over time following SCDS surgical repair (7), it is unknown if gaze stability, measured by active or passive canal plane htDVA also normalizes over time. The purpose of the present investigation was to: 1) characterize normal, horizontal, active DVA and passive canal plane htDVA across ages to establish control data, and 2) to determine if horizontal active DVA and passive canal plane htDVA are significantly different in individuals with SCDS before, immediately after surgical repair (acute phase, within 10 days), and greater than 6 weeks after surgical repair (non-acute phase). We hypothesized that horizontal active DVA and passive htDVA in the plane of the affected SC would be abnormal in the acute stage following surgical repair. We further hypothesized that active DVA would normalize over time but that htDVA in the plane of the plugged SC would remain permanently abnormal.
Materials and Methods
Study Population
Thirty-two patients with SCDS (mean age 48 years, range 23 – 61) and 51 normal control subjects (~ 10 subjects per decade) participated in the study. By case history, normal control subjects denied hearing loss or history of balance, dizziness, or neurologic complaints. Normal control subjects were separated into age categories:
Age 20 – 29 years (mean 23 ± 3.1, n = 10)
Age 30 – 39 years (mean 35 ± 3.1, n = 11)
Age 40 – 49 years (mean 45 ± 3.8, n = 10)
Age 50 – 59 years (mean 55 ± 3.1, n = 10)
Age 60+ years (mean 66 ± 5.4, n = 10, range: 61 – 78)
SCDS was diagnosed on case history, visualization of a dehiscent SC on high resolution CT with multi-planar reconstructions of the SC, and clinical assessments, including cervical and ocular VEMPs, bedside assessments consisting of head impulses in each canal plane, examination for Tullio phenomenon and Hennebert sign, and air- and bone-conduction audiometry. Minimal criteria for SCDS diagnosis were (1) visualization of SCD on CT and (2) at least one physiologic finding consistent with a third mobile window in the affected ear (i.e., nystagmus in the plane of the affected SC elicited by loud tones or external auditory canal pressure, abnormal VEMP testing, or negative bone conduction thresholds). All subjects gave informed consent for testing approved by the Institutional Review Board at the Johns Hopkins University School of Medicine.
Surgical Technique
Surgical techniques for plugging the dehiscent SC have been described previously (2;7). Of the 32 patients diagnosed with SCDS, 27 elected to have the affected SC surgically plugged. The remaining 5 patients are represented in the “pre-surgery” group although they did not choose to pursue surgery. During the surgical procedure, image guidance was used to confirm the location of the dehiscent SC. Size of the dehiscence was measured by a small scale, in millimeters, laid next to the dehiscence. With the use of magnification, dehiscence length and width were measured to calculate area. The SC was then plugged with fascia and bone pâté, filling the perilymphatic space and thus compressing the membranous canal without disruption. Since 2009, following plugging of the canal, a layer of Hydroset Bone Cement (Stryker Corporation) several millimeters thick was additionally placed over the repair and allowed to set until palpably firm. Finally, a fascia graft was overlaid and sealed in place with fibrin glue.
Dynamic Visual Acuity
the DVA test protocol has been described previously (5;8;13). In brief, subjects were seated 2 meters from a high resolution 18.1-viewable-inch monitor with a refresh rate of 85 Hz. Subjects who normally wore glasses or contact lenses were instructed to wear them during all testing. Static visual acuity (SVA), active DVA, and passive htDVA were measured. For all testing, subjects viewed five optotypes per acuity level, with optotype size decrementing in steps equivalent to a visual acuity change of 0.1 LogMAR (log10X, where X = the minimum angle resolved, in arcmin, with 1 arcmin = 1/60°) (14). The optotype, the letter ‘E’, randomly rotated each trial by 0, 90, 180, or 270°. Visual acuity testing took approximately 30 minutes to complete.
Static Acuity
SVA was determined first with the subject’s head comfortably fixed in space. SVA was the LogMAR score where the subject failed to correctly identify five optotypes at the same acuity level or reached the LogMAR value of 0.000 (equivalent to 20/20 on the Snellen chart).
Active DVA
For active DVA, a single-axis Watson rate sensor (Micromedical Technologies, Inc., Chatham, IL, USA) was positioned on the subject’s head so the sensor’s axis of maximum sensitivity approximately aligned with the horizontal canal. Subjects were instructed to actively shake their head (“no”). Two trials were completed. During one trial, the optotype flashed in response to rightward head movements and in the other trial the optotype flashed in response to leftward head movements. During each trial, the optotype “E” was displayed on the monitor only when the rate sensor detected head velocity between 120–180 degrees/s for more than 40 ms. The optotype flashed for no longer than 85 ms, during which the head would have rotated 9 – 13.5 degrees. The subject was allowed to view each optotype a maximum of three times, at which point the computer no longer displayed the letter and the subject was required to guess the orientation (i.e., a forced choice of one of four possible orientations). Once the subject indicated a response, the next trial started. Testing was terminated once the subject incorrectly identified all five optotype presentations at one acuity level (e.g., 20/40) or reached the LogMAR value of 0.000. The difference was calucated between the SVA LogMAR score and the active DVA LogMAR score.
Passive Canal Plane htDVA
For htDVA, the rate sensor was positioned on the subject’s head so that the sensor’s axis of maximum sensitivity approximately aligned with the canal being tested (i.e., horizontal, superior, or posterior canal). For example, when testing the left superior and right posterior canal pair, the sensor was placed at 45° right (subject’s perspective) off the mid-sagittal line bisecting the skull. For htDVA, the examiner stood behind the participant and performed head impulses in the plane of each canal while the participant identified the direction of the optotype. One practice trial for horizontal htDVA was completed before starting. Scoring for htDVA was identical to that described above for active DVA. For consistency, all htDVA stimuli were primarily delivered by either MCS or KLJ.
Statistical Analysis
Statistical analyses were completed using SPSS (version 18, Chicago, IL). Pearson product-moment correlation was used to calculate the relationship between visual acuity outcomes and age. Analysis of Variance (ANOVA) was used to compare means between groups. Post-hoc pairwise comparisons were performed when significant. An alpha <0.05 was used to determine statistical significance.
Results
Normal Control Group
Fifty-one normal control subjects (mean: 44.78 years, range 19 – 78) completed SVA, active DVA and htDVA (Table 1). A significant relationship was found between age and all visual acuity outcomes with the exception of htDVA in the right SC (Table 1), consistent with the decline of both static and dynamic visual acuity with age. A within-groups ANOVA across all normal subjects failed to reveal significant mean differences between right versus left active DVA [F (1, 50) = 0.829, p = 0.367]; therefore, mean active DVA is reported. There were also no significant mean differences between individual canal htDVA [F (5, 250) = 1.103, p = 0.359] across all normal subjects.
Table 1.
Visual acuity measures (in logMAR, see text) for normal control subjects across decades of age. Each cell contains mean and 1 SD (in parentheses) values. Conditions are: Static acuity (head stationary), Active DVA (participant actively rotating head horizontally in a sinusoidal fashion), and passive head thrusts exciting the named semicircular canal. htDVA ALL refers to mean DVA LogMAR score across all 6 directions of canal plane DVA testing.
| Age Group | Static Acuity | Active DVA | Right Horizontal | Left Horizontal | Right Superior | Left Superior | Right Posterior | Left Posterior | htDVA ALL |
|---|---|---|---|---|---|---|---|---|---|
| 20 – 29 | 0.005 (0.012) | 0.033 (0.027) | 0.043 (0.035) | 0.045 (0.035) | 0.047 (0.039) | 0.052 (0.034) | 0.052 (0.063) | 0.057 (0.026) | 0.049 (0.039) |
| 30 – 39 | 0.003 (0.035) | 0.034 (0.048) | 0.061 (0.079) | 0.051 (0.07) | 0.092 (0.066) | 0.094 (0.061) | 0.074 (0.078) | 0.065 (0.047) | 0.073 (0.067) |
| 40 – 49 | 0.002 (0.006) | 0.025 (0.027) | 0.048 (0.041) | 0.022 (0.021) | 0.048 (0.045) | 0.043 (0.03) | 0.05 (0.044) | 0.051 (0.053) | 0.044 (0.04) |
| 50 – 59 | 0.02 (0.023) | 0.054 (0.033) | 0.087 (0.063) | 0.072 (0.056) | 0.089 (0.084) | 0.078 (0.053) | 0.085 (0.049) | 0.076 (0.082) | 0.081 (0.063) |
| 60 + | 0.053 (0.061) | 0.113 (0.087) | 0.12 (0.114) | 0.138 (0.097) | 0.11 (0.086) | 0.128 (0.104) | 0.164 (0.115) | 0.121 (0.056) | 0.13 (0.095) |
| All | 0.016 (0.037) | 0.052 (0.058) | 0.072 (0.075) | 0.065 (0.071) | 0.077 (0.069) | 0.079 (0.067) | 0.085 (0.083) | 0.074 (0.059) | 0.075 (0.071) |
| r (p-value) | 0.4* (0.004) | 0.431* (0.002) | 0.331* (0.018) | 0.425* (0.002) | 0.268 (0.057) | 0.302* (0.031) | 0.377* (0.006) | 0.349* (0.012) | 0.34* (< 0.001) |
Bottom row: Relationship (r) between visual acuity and age, where *denotes significance at alpha < 0.05.
SCDS Group – Demographics
Of the 32 patients with SCDS, visual acuity testing was completed at varying time periods: pre-SCDS surgical repair (n = 16), post-SCDS surgery acute (n = 12) and post-SCDS surgery non-acute (n = 21). Not all patients could be tested at each time period, mainly because many patients traveled from distances for surgery and could not make all follow-up appointments. Of the patients, 9 were tested both pre-SCDS surgery and post-SCDS surgery non-acute, 1 was tested both pre-SCDS surgery and post-SCDS surgery acute, and 9 were tested both post-SCDS surgery acute and post-SCDS surgery non-acute. Because not all patients completed testing during all time periods, a within-group ANOVA could not be completed; therefore, subject groups were trimmed to eliminate overlap in participation groups and a between-group ANOVA was used to compare visual acuity performance between SCDS and normal control subjects. Due to the relationship between age and visual acuity performance in normal control subjects, patients in the SCDS group (age range 32 – 61) were compared to an age-matched subset of the normal control subjects by decade. Subject groups were:
Pre-SCDS surgical repair (n = 11, mean: 47 years, range 33 – 60)
Post-SCDS surgery acute (n = 10, mean: 48 years, range 32 – 61)
Post-SCDS surgery non-acute (n = 11, mean: 48 years, range 33 – 56)
Age-matched, normal controls (n = 33, mean: 46 years, range 32 – 61)
Of the 32 patients diagnosed with SCDS, 7 were diagnosed with left SCDS, 12 with right SCDS, and 13 with bilateral SCDS. Of the 13 bilateral SCDS patients, the more symptomatic ear underwent surgical repair. Results from tests of this ear were used for all groups.
SCDS Group – Visual Acuity Outcomes
There was no mean difference in SVA between any of the groups [1-way ANOVA F (3, 61) = 0.603, p = 0.616)]. For the SCDS group, active DVA towards the affected SCDS-side was compared to mean active DVA in the normal control group and there was no mean difference between groups [1-way ANOVA F (3, 61) = 2.222, p = 0.095)].
A mixed groups factorial ANOVA was completed to investigate mean differences in canal plane htDVA following surgical repair of SCDS, with group (Pre-SCDS surgical repair, Post-SCDS surgery acute, Post-SCDS surgery non-acute, and Age-matched, normal controls) as the between-subjects factor and htDVA for each semicircular canal (6 canals) as the within-subjects factor. For those with SCDS, htDVA towards the affected SCDS-side was compared to the right side of normal control subjects, and the contralateral, unaffected (or less symptomatic)-SCDS side was compared to the left side of normal control subjects (Table 2). Figure 1 shows mean visual acuity parameters (SVA, active DVA, and htDVA) across groups. There was a main effect of canal plane [F (5, 300) = 12.96, p < 0.001]. Post hoc analyses indicated that htDVA performance for the SC (SCDS affected and normal control right ear) was significantly worse than performance in remaining canal planes. There was also a main effect of group [F (1, 60) = 197.767, p < 0.001] with post-SCDS surgery acute and non-acute demonstrating significantly worse performance when compared to both normal control subjects and pre-SCDS surgical repair groups. Notably, there was no significant difference between the post-SCDS surgery acute and non-acute groups. Finally, there was a significant interaction with htDVA and group [F (15, 300) = 5.757, p < 0.001, MSe = 0.002]. The interaction indicates that performance on htDVA was not consistent between groups and across canals. Figure 1 demonstrates DVA performance was worse for the affected SC in both the post-SCDS surgery acute and non-acute groups, but that performance in all remaining canals across all groups was normal. SC htDVA across groups, Figure 2, show that all but 1 post-SCDS surgery subject has htDVA above (worse than) the mean for age-matched normal controls (solid line).
Table 2.
Passive canal plane htDVA (logMAR mean, (SD)) for SCDS groups compared with age-matched controls, where *denotes significance at alpha < 0.05
| Pre-SCD Surgery | Post-SCD Surgery Acute | Post-SCD Surgery Non-acute | Normal Controls | |
|---|---|---|---|---|
| Horizontal Ipsilateral (Normal Right) | 0.0555 (0.044) | 0.1072 (0.067) | 0.072 (0.041) | 0.0683 (0.065) |
| Horizontal Contralateral (Normal Left) | 0.0655 (0.038) | 0.1055 (0.046) | 0.0772 (0.046) | 0.0493 (0.054) |
| Superior Ipsilateral (Normal Right) | 0.0455 (0.043) | 0.1974* (0.112) | 0.2303* (0.126) | 0.0755 (0.066) |
| Superior Contralateral (Normal Left) | 0.0686 (0.056) | 0.0933 (0.07) | 0.1056 (0.035) | 0.0733 (0.056) |
| Posterior Ipsilateral (Normal Right) | 0.0576 (0.037) | 0.123 (0.083) | 0.0856 (0.044) | 0.0727 (0.062) |
| Posterior Contralateral (Normal Left) | 0.0683 (0.059) | 0.1040 (0.058) | 0.0864 (0.062) | 0.0641 (0.061) |
Figure 1.
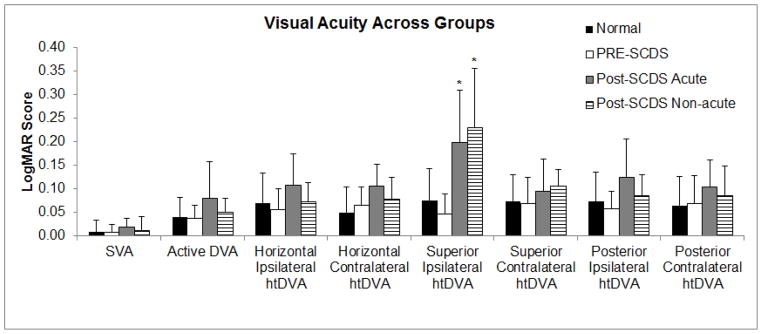
Visual acuity outcomes across all groups. Significant mean differences between groups are noted by asterisks (*). Ipsilateral refers to canals on the same side as superior canal plugging and contralateral refers to canals opposite to the surgical side.
Figure 2.
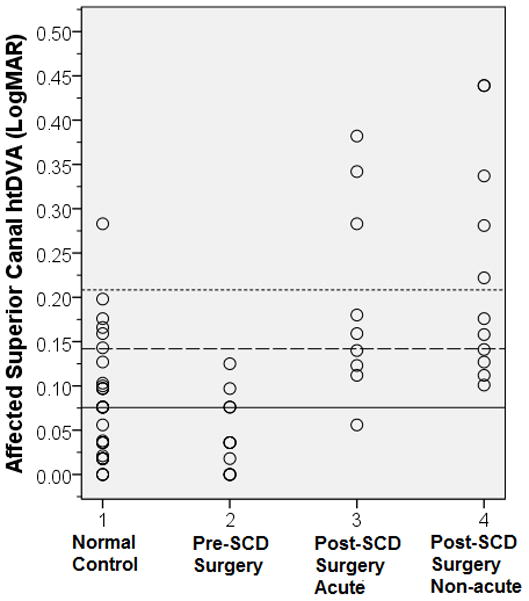
Affected Superior Canal htDVA across Groups. Group 1 = Age- matched normal control, right superior canal, 2 = Pre-SCD surgical repair, 3 = Post-SCD Surgery Acute, 4 = Post-SCD Surgery Non-acute. Mean (solid line) = 0.0755, +1 standard deviation = 0.142 and +2 standard deviation = 0.208 (hashed lines) from age-matched normal control group, right superior canal, superimposed for direct comparison.
Clinical Features
Pearson product-moment correlation was used to determine if SCDS size in the surgical group (n = 21, mean area = 3.34 mm2) was related to post-surgical visual acuity outcomes. Dehiscence area was inversely associated with htDVA for the affected SC (r = −0.813, p < 0.001), indicating worse htDVA in the surgically-repaired SC as dehiscence area decreased.
To determine if visual acuity outcomes improved over time following surgery, the relationship between visual acuity outcomes and duration (in days) from surgery-date was calculated. Duration was positively correlated with SVA (r = 0.628, p = 0.002), indicating that static acuity (head stable) steadily improved over time. However, there was no correlation between duration after surgery and either active DVA (r = −0.276, p = 0.226) or htDVA in any canal plane (p = 0.153 – 0.693), indicating the permanency of the effect of canal plugging on htDVA in the plugged SC.
Discussion
Our study showed that all visual acuity outcomes (SVA, active DVA, and passive htDVA) in the control population significantly worsened with age. This finding was expected as (1) visual acuity is known to decline with age, and (2) vestibular function outcomes have shown a similar decline with aging (15–17). Age has previously been related to poorer active DVA in individuals regardless of degree of vestibular loss (i.e., normal, unilateral or bilateral peripheral vestibular involvement) (8;11;13;18). Likewise, Viciana et al. (19) also demonstrated that htDVA significantly worsens with age. Schubert et al. (5) originally proposed that htDVA scores falling outside 0.158 LogMAR (mean + 2 SD in normal controls, n = 19) be considered the cut off for pathologically abnormal htDVA. However, this htDVA cutoff is likely inappropriate for normal individuals over the age of 60 as the 95th percentile reference value projected by Viciana et al. (19) ranges from 0.230 – 0.330 LogMAR. Our findings continue to support that visual acuity performance is dependent on age, and that age-based normative data are necessary for determining a diagnostic cutoff, particularly for individuals greater than 60 years of age.
The use of htDVA in diagnosing peripheral vestibular disorders has achieved mixed results. For diagnosing vestibular neuritis, htDVA has a low sensitivity and high specificity in the horizontal canal (19); however, Vital et al (11) report excellent sensitivity for htDVA in diagnosing both unilateral and bilateral vestibular involvement. In SCDS, the hydrodynamic function of the SC is usually only impaired with a large dehiscence (>5 mm length) (20); therefore, htDVA was not predicted to be abnormal sufficiently often to be used as a screening tool for diagnosis. Our results agree, in that visual acuity outcomes are not significantly affected with a patent dehiscence (i.e., in Pre-SCDS surgical repair patients). In fact, SVA and active DVA – products of inputs from the horizontal canals - remain relatively stable as expected following surgical repair of the dehiscent SC. However, reduced htDVA in the surgically plugged SC occurs in most patients and appears to persist beyond the acute stage postoperatively. Similarly, Schubert et al. (5) reported normal htDVA in SCDS patients (n = 5) pre-surgery, with worse htDVA in the affected SC post-surgery. The present findings are in agreement, demonstrating that htDVA is not helpful in diagnosing SCDS; however, post-surgically htDVA is significantly reduced in the plane of the affected SC, and this reduction persists beyond 6 weeks. Thus, our data indicate that this should be explained to patients as a permanent effect of surgical plugging.
The relatively stable active-DVA and htDVA in remaining canals suggest that function of the residual labyrinth remains intact following surgical plugging of the dehiscent SC. Using search coils, aVOR gain is similar between canals prior to surgical repair; however, post-surgically a significant reduction in canal gain in the affected SC (44%) and contralateral posterior canal (10%) was found, with no significant change in the remaining canals (6). These findings are consistent with the present perceptual findings of normal visual acuity performance in the pre-surgery group, with the only abnormality following surgical repair being decreased htDVA in the affected SC. While mild aVOR gain reductions have been noted in either ipsilateral or contralateral posterior canals (6;21), post hoc analyses of our data indicate only htDVA from the affected SC was significantly worse.
Reduced htDVA in the surgically-repaired SC was related to dehiscence size, with a smaller dehiscence being related to greater reductions in htDVA. Prior to surgical repair, weak correlations between aVOR gain and dehiscence length have been noted (6), particularly when the SCD diameter was greater than 5 mm (20). This size-related reduction in function of the dehiscent SC was presumed to reflect hydrodynamic dampening of endolymph motion as a result of dural herniation into the canal (“auto-plugging”) and size-related increases in the degree of compression of the membranous canal. Because the odds of vestibular loss have been shown to increase with increased dehiscence size (21), our present finding that post-operative htDVA was degraded more with smaller dehiscences is intriguing. Our present findings in a larger group show that degradation of htDVA function is greater for smaller SCD size (and thus shorter plugging length) even when htDVA for the other canals is not degraded. For example, patients with larger dehiscences and some degree of auto-plugging pre-operatively may have the benefit of better compensation for decreased SC function, which might manifest itself as better post-operative htDVA in the plane of the plugged SC.
One might expect that plugging a canal to any degree would cause a large reduction in hydrodynamic sensitivity, but the frequency of the head impulse stimulus must be considered. Yakushin et al (22) found only small aVOR gain reductions for high-frequency head rotations in macaques after complete disruption and plugging of open segments of the semicircular canals. They concluded that canal plugs acted as “high-pass filters,” suggesting hydrodynamic action on the cupula must still occur for high-frequency head movements despite the disruption of the circular continuity of the canal (22). Although the disruption of the circular fluid path in the SC has a greater effect on cupular dynamics for low-frequency head rotations, it is also true that low frequency rotations are less likely to cause inhibitory saturation of the contralateral posterior canal. For these frequencies, the aVOR can be derived from the inhibitory signal from the contralateral posterior canal. Thus, across a broad spectrum of head rotations, plugging one canal in a coplanar pair appears to have modest effects on visual stabilization.
Since its first description, reports regarding SCDS have primarily centered on the sensitivity of diagnosing SCDS and the effects of surgical intervention. Improvement in patient symptoms of autophony, disequilibrium, hearing, and dizziness in response to sound and/or pressure have been documented (1–4). However, few studies have documented the functional ramifications of surgical intervention. Previous studies by our group indicate that while functional balance is reduced in the acute stage following surgical repair, overall fall risk normalizes after 6 weeks post-operatively (7). This persistent reduction of htDVA in the SC is not speculated to have a gross impact on functional balance. However, further research is needed to determine the long term direct impact and implications of reduced VOR function in the isolated SC.
One shortcoming of the current study is that not all patients were able to be tested at each time interval, eliminating the opportunity to examine true longitudinal data within subjects, and resulting in analysis of between group data at different time periods. Despite this shortcoming, findings in the between-group analysis are felt to be representative of post-operative outcomes.
Conclusion
Age-based normative data are necessary for dynamic visual acuity testing and should be accounted for in populations with both normal and abnormal vestibular function (11;23). In populations with SCDS, SVA and active-DVA are unaffected by surgical intervention. Average htDVA performance for the SC in SCDS is normal before surgery, but afterwards performance is reduced, and that reduction is likely permanent. Patients with larger dehiscences may actually have better htDVA performance after surgery, but all patients should be counseled about the permanency of mildly reduced visual acuity for specific rapid head movements in the plane of the plugged SC.
Acknowledgments
Research study supported by the following mechanisms:
NIH/NIDCD R01 DC005040: Evaluation of vestibular function in Ménière’s disease; PI: John Carey, M.D.
NIDCD K23-007926: Incremental VOR Adaptation and utility of saccades as rehabilitation strategies; PI: Michael Schubert, Ph.D.
NIH Training Grant T32DC000023; PI: Eric Young, Ph.D.
Footnotes
Conflict of Interest: There are no conflicts of interest to report for this manuscript.
Reference List
- 1.Crane BT, Lin FR, Minor LB, et al. Improvement in autophony symptoms after superior canal dehiscence repair. Otol Neurotol. 2010;31:140–146. doi: 10.1097/mao.0b013e3181bc39ab. [DOI] [PubMed] [Google Scholar]
- 2.Minor LB. Clinical manifestations of superior semicircular canal dehiscence. Laryngoscope. 2005;115:1717–1727. doi: 10.1097/01.mlg.0000178324.55729.b7. [DOI] [PubMed] [Google Scholar]
- 3.Agrawal SK, Parnes LS. Transmastoid superior semicircular canal occlusion. Otol Neurotol. 2008;29:363–367. doi: 10.1097/mao.0b013e3181616c9d. [DOI] [PubMed] [Google Scholar]
- 4.Crane BT, Minor LB, Carey JP. Superior canal dehiscence plugging reduces dizziness handicap. Laryngoscope. 2008;118:1809–1813. doi: 10.1097/MLG.0b013e31817f18fa. [DOI] [PubMed] [Google Scholar]
- 5.Schubert MC, Migliaccio AA, la Santina CC. Dynamic visual acuity during passive head thrusts in canal planes. J Assoc Res Otolaryngol. 2006;7:329–338. doi: 10.1007/s10162-006-0047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carey JP, Migliaccio AA, Minor LB. Semicircular canal function before and after surgery for superior canal dehiscence. Otol Neurotol. 2007;28:356–364. doi: 10.1097/01.mao.0000253284.40995.d8. [DOI] [PubMed] [Google Scholar]
- 7.Janky KL, Zuniga MG, Carey JP, et al. Balance dysfunction and recovery after surgery for superior canal dehiscence syndrome. Arch Otolaryngol Head Neck Surg. 2012;138:723–730. doi: 10.1001/archoto.2012.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herdman SJ, Tusa RJ, Blatt P, et al. Computerized dynamic visual acuity test in the assessment of vestibular deficits. Am J Otol. 1998;19:790–796. [PubMed] [Google Scholar]
- 9.Herdman SJ, Schubert MC, Das VE, et al. Recovery of dynamic visual acuity in unilateral vestibular hypofunction. Arch Otolaryngol Head Neck Surg. 2003;129:819–824. doi: 10.1001/archotol.129.8.819. [DOI] [PubMed] [Google Scholar]
- 10.Herdman SJ, Hall CD, Schubert MC, et al. Recovery of dynamic visual acuity in bilateral vestibular hypofunction. Arch Otolaryngol Head Neck Surg. 2007;133:383–389. doi: 10.1001/archotol.133.4.383. [DOI] [PubMed] [Google Scholar]
- 11.Vital D, Hegemann SC, Straumann D, et al. A new dynamic visual acuity test to assess peripheral vestibular function. Arch Otolaryngol Head Neck Surg. 2010;136:686–691. doi: 10.1001/archoto.2010.99. [DOI] [PubMed] [Google Scholar]
- 12.Schubert MC, Migliaccio AA, Clendaniel RA, et al. Mechanism of dynamic visual acuity recovery with vestibular rehabilitation. Arch Phys Med Rehabil. 2008;89:500–507. doi: 10.1016/j.apmr.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schubert MC, Herdman SJ, Tusa RJ. Vertical dynamic visual acuity in normal subjects and patients with vestibular hypofunction. Otol Neurotol. 2002;23:372–377. doi: 10.1097/00129492-200205000-00025. [DOI] [PubMed] [Google Scholar]
- 14.Ferris FL, III, Kassoff A, Bresnick GH, et al. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94:91–96. [PubMed] [Google Scholar]
- 15.Ward BK, Mohammed MT, Brach JS, et al. Physical performance and a test of gaze stabilization in older adults. Otol Neurotol. 2010;31:168–172. doi: 10.1097/MAO.0b013e3181c4c3e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agrawal Y, Zuniga MG, Davalos-Bichara M, et al. Decline in semicircular canal and otolith function with age. Otol Neurotol. 2012;33:832–839. doi: 10.1097/MAO.0b013e3182545061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ward BK, Mohammad MT, Whitney SL, et al. The reliability, stability, and concurrent validity of a test of gaze stabilization. J Vestib Res. 2010;20:363–372. doi: 10.3233/VES-2010-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herdman SJ, Schubert MC, Tusa RJ. Role of central preprogramming in dynamic visual acuity with vestibular loss. Arch Otolaryngol Head Neck Surg. 2001;127:1205–1210. doi: 10.1001/archotol.127.10.1205. [DOI] [PubMed] [Google Scholar]
- 19.Viciana D, Ferrer J, Palma MJ, et al. Dynamic visual acuity during head-thrust test in canal planes in healthy subjects and patients with vestibular neuritis. Acta Otolaryngol. 2010;130:1260–1266. doi: 10.3109/00016481003785994. [DOI] [PubMed] [Google Scholar]
- 20.Cremer PD, Minor LB, Carey JP, et al. Eye movements in patients with superior canal dehiscence syndrome align with the abnormal canal. Neurology. 2000;55:1833–1841. doi: 10.1212/wnl.55.12.1833. [DOI] [PubMed] [Google Scholar]
- 21.Agrawal Y, Migliaccio AA, Minor LB, et al. Vestibular hypofunction in the initial postoperative period after surgical treatment of superior semicircular canal dehiscence. Otol Neurotol. 2009;30:502–506. doi: 10.1097/MAO.0b013e3181a32d69. [DOI] [PubMed] [Google Scholar]
- 22.Yakushin SB, Raphan T, Suzuki J, et al. Dynamics and kinematics of the angular vestibulo-ocular reflex in monkey: effects of canal plugging. J Neurophysiol. 1998;80:3077–3099. doi: 10.1152/jn.1998.80.6.3077. [DOI] [PubMed] [Google Scholar]
- 23.Rine RM, Schubert MC, Whitney SL, et al. Vestibular function assessment using the NIH Toolbox. Neurology. 2013;80:S25–S31. doi: 10.1212/WNL.0b013e3182872c6a. [DOI] [PMC free article] [PubMed] [Google Scholar]


