SUMMARY
Ultraviolet light is an established carcinogen yet evidence suggests that UV-seeking behavior has addictive features. Following UV exposure, epidermal keratinocytes synthesize Proopiomelanocortin that is processed to Melanocyte Stimulating Hormone, inducing tanning. We show that in rodents another POMC-derived peptide, β-endorphin, is coordinately synthesized in skin, elevating plasma levels after low-dose UV. Increases in pain-related thresholds are observed, and reversed by pharmacologic opioid antagonism. Opioid blockade also elicits withdrawal signs after chronic UV exposure. This effect was sufficient to guide operant behavioral choices to avoidance of opioid withdrawal (conditioned place aversion). These UV-induced nociceptive and behavioral effects were absent in β-endorphin knockout mice and in mice lacking p53-mediated POMC induction in epidermal keratinocytes. While primordial UV addiction, mediated by the hedonic action of β-endorphin and anhedonic effects of withdrawal, may theoretically have enhanced evolutionary vitamin D biosynthesis, it now may contribute to the relentless rise in skin cancer incidence in man.
INTRODUCTION
Despite widespread awareness that UV exposure is a major risk factor for all common cutaneous malignancies, skin cancer incidence relentlessly increases by ~3% per year (de Gruijl, 1999; Gandini et al., 2011; Robinson et al., 1997). UV-seeking behavior is a recognized risk factor, but it is incompletely understood whether the popularity of sunbathing represents a biological addiction or an aesthetic preference for tanned skin. Studies have reported that many UV-seekers meet CAGE and DSM-IV criteria for a substance-related disorder with respect to UV (Harrington et al., 2011; Kourosh et al., 2010; Lazovich et al., 2010; Mosher and Danoff-Burg, 2010; Warthan et al., 2005), UV-seekers were capable of distinguishing between true UV and mock treatment in blind tanning bed experiments (Feldman et al., 2004), and two studies of small cohorts of frequent tanners revealed that acute administration of the opioid antagonist naltrexone can induce withdrawal-like symptoms (Kaur et al., 2005; Kaur et al., 2006b). While a mechanism for such addiction has been lacking, these studies are consistent with the possibility of endogenous opioid-mediated addictive behavioral effects.
In the cutaneous response to UV exposure, epidermal keratinocytes respond to DNA damage via p53-mediated transcriptional induction of the proopiomelanocortin (POMC) gene (Cui et al., 2007). POMC is post-translationally cleaved into biologically active peptides, one of which is α-Melanocyte Stimulating Hormone (MSH) that mediates the tanning process by stimulating adjacent melanocytes to produce the brown/black pigment eumelanin (D'Orazio et al., 2006). The endogenous opioid β-endorphin is also post-translationally generated in skin by cleavage of the POMC pro-peptide in response to UV radiation (Cui et al., 2007; Skobowiat et al., 2011; Slominski and Wortsman, 2000). β-endorphin is the most abundant endogenous opioid, with basal plasma levels of 1pM-12pM (Bender et al., 2007; Fassoulaki et al., 2007; Leppaluoto et al., 2008), and intravenous administration of β-endorphin has been shown to cause analgesia, (Tseng et al., 1976). It binds with high affinity to the μ-opioid receptor (Schoffelmeer et al., 1991), although some evidence suggests that it may also act through other mechanisms that are, at present, incompletely characterized (Nguyen et al., 2012). Exogenous opioids with similar mechanisms are analgesic, and have reinforcing properties that make them addictive when administered systemically. Chronic opioid exposure results in tolerance (increasing dose requirement to achieve comparable efficacy) and physical dependence (opioid antagonism produces withdrawal). β-endorphin plays a role in analgesia (Ibrahim et al., 2005; Kastin et al., 1979) as well as in the reinforcement and reward that underlie addiction (Gianoulakis, 2009; Olive et al., 2001; Racz et al., 2008; Roth-Deri et al., 2003; Trigo et al., 2009). Here we asked whether UV exposure may stimulate changes in systemic β-endorphin levels that result in opioid-related behaviors, including alterations in nociceptive thresholds, tolerance to exogenous opioids, and dependence, as measured by withdrawal signs and conditioned place preference.
RESULTS
Systemic β-endorphin elevations following chronic UV exposure
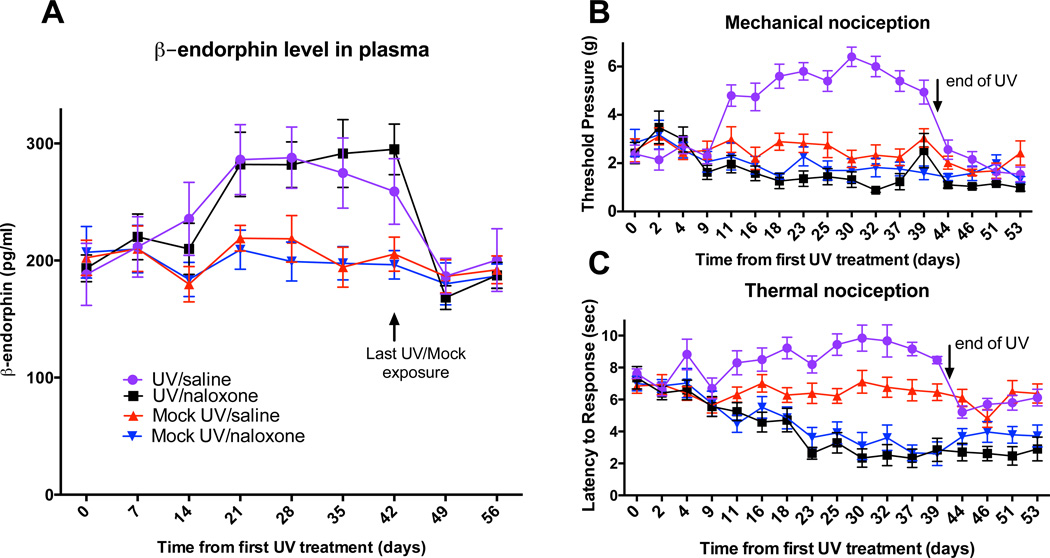
We developed a UV-exposure mouse model in which dorsally-shaved mice received a dose of 50mJ/cm2 of UVB, 5 days per week for 6 weeks, an empirically-derived sub-erythemic dose which is approximately equal to 20–30 minutes of ambient midday sun exposure in Florida during the summer for a fair-skinned person of average tanning ability (Fitzpatrick skin phototypes 2–3) (D'Orazio et al., 2006; Technology-Planning-and-Management-Corporation, 2000; US-EPA, 1994). After one week, significant elevations in circulating plasma β-endorphin were observed (Fig. 1A). Circulating β-endorphin levels remained elevated for the duration of the 6-week exposure regimen and returned within 7 days to near baseline levels after cessation of UV exposure. No significant changes in plasma β-endorphin were observed in mock UV-treated mice (Fig. 1A). Analgesic thresholds can be increased by peripheral administration of exogenous opioids or β-endorphin (Kastin et al., 1979). We quantified mechanical and thermal nociceptive thresholds over six weeks of daily UV exposure. Mechanical nociception was measured by the von Frey test (Kwan et al., 2006), which exposes fibers of increasing tensile strength to the plantar paw surface to elicit a paw withdrawal response. Thermal nociception was tested using the hot plate (52°C) test (Mogil et al., 1999) in which time to response (paw licking, paw flutter, jumping) was measured. UV-irradiated mice exhibited significant increases both in mechanical (Fig. 1B) and thermal (Fig. 1C) nociceptive thresholds. These elevated analgesic thresholds paralleled the UV-induced elevations in plasma β-endorphin (Fig. 1A). Mock-treated control mice displayed no significant elevations in pain thresholds (Fig. 1B and Fig. 1C). Treatment with naloxone, an opioid antagonist, 15min prior to analgesic testing suppressed the UV-induced increases in mechanical and thermal nociceptive thresholds (Fig. 1B and Fig. 1C) despite maintained elevations in plasma β-endorphin (Fig. 1A). These data demonstrate opioid receptor mediated analgesia as a consequence of UV, that parallels the elevation of circulating blood β-endorphin.
Fig. 1. Plasma β-endorphin increases with chronic UV exposure and parallels Naloxone reversible changes in pain tolerance.
A) Plasma β-endorphin in C57Bl6 mice receiving daily UV or Mock irradiation. Mice were treated twice a week with either Naloxone or Saline as indicated. Data are represented as the mean +/− SEM, 2way ANOVA analysis with Bonferroni’s multiple comparisons test gives p<0.05 for both UV treated groups compared to both Mock treated groups (during UV treatment, days 14–42), and no significant effect of Naloxone treatment within either group. B) von Frey thresholds and C) Hot Plate thresholds in chronically UV-irradiated and mock-irradiated C57Bl6 mice (mean +/− SEM). Half of each group was pre-treated with naloxone (10mg/kg) 15 minutes prior to nociceptive testing, while the remainder received saline (n=10 per group). Analgesic thresholds were further monitored for 2 additional weeks after cessation of UV/Mock treatment. 2way ANOVA with Bonferroni’s multiple comparisons test reveals p<0.0001 for the UV/Saline treated group compared to all other groups during UV treatment, days 9 to 39).
Quantifiable opioid-mediated behaviors occur with chronic UV exposure
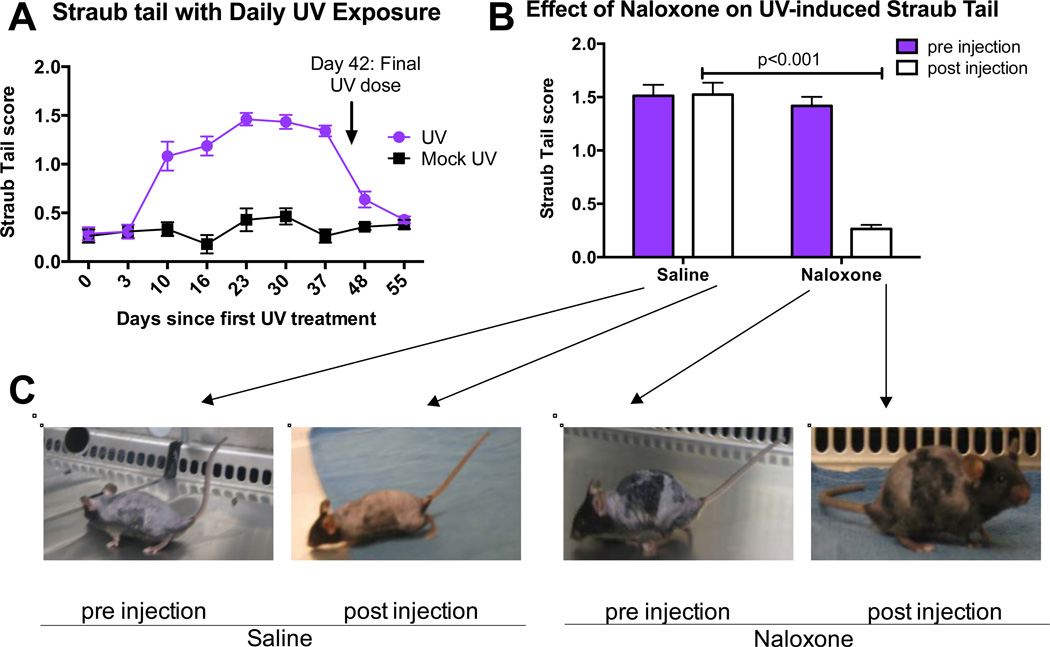
Exogenous opioids produce a dose-dependent, μ-opioid receptor-mediated contraction of the sacrococcygeus dorsalis muscle at the tail base in rodents, resulting in rigidity and elevation of the tail, a phenomenon called “Straub Tail” (Bilbey et al., 1960). Straub Tail was evident in UV-irradiated mice by the second week of daily UV exposure, persisted for the six-week exposure regimen, and diminished over two weeks after cessation of UV (Fig. 2A). Treatment with the opioid antagonist naloxone (day 23 of the UV exposure regimen) reversed the Straub Tail phenotype (Fig. 2B, Fig. 2C).
Fig. 2. Straub Tail in UV-irradiated mice is reversed by naloxone.
A) Straub Tail in C57Bl6 mice over the course of 42 days of UV irradiation (n=13) or mock-irradiation (n=6). Data are represented as the mean +/− SEM, for days 10–37 p<0.0001 by 2way Anova analysis. B) Straub Tail in at day 17 before (Pre) and 15 minutes after (Post) injection of naloxone (n=7) or saline (n=6). Data are represented as the mean +/− SEM, p<0.001 by Student’s t-test. C) Representative animals from each group in part (B). The beginning of black fur re-growth produces a patchy appearance.
Opioid tolerance and physical dependence after chronic UV exposure
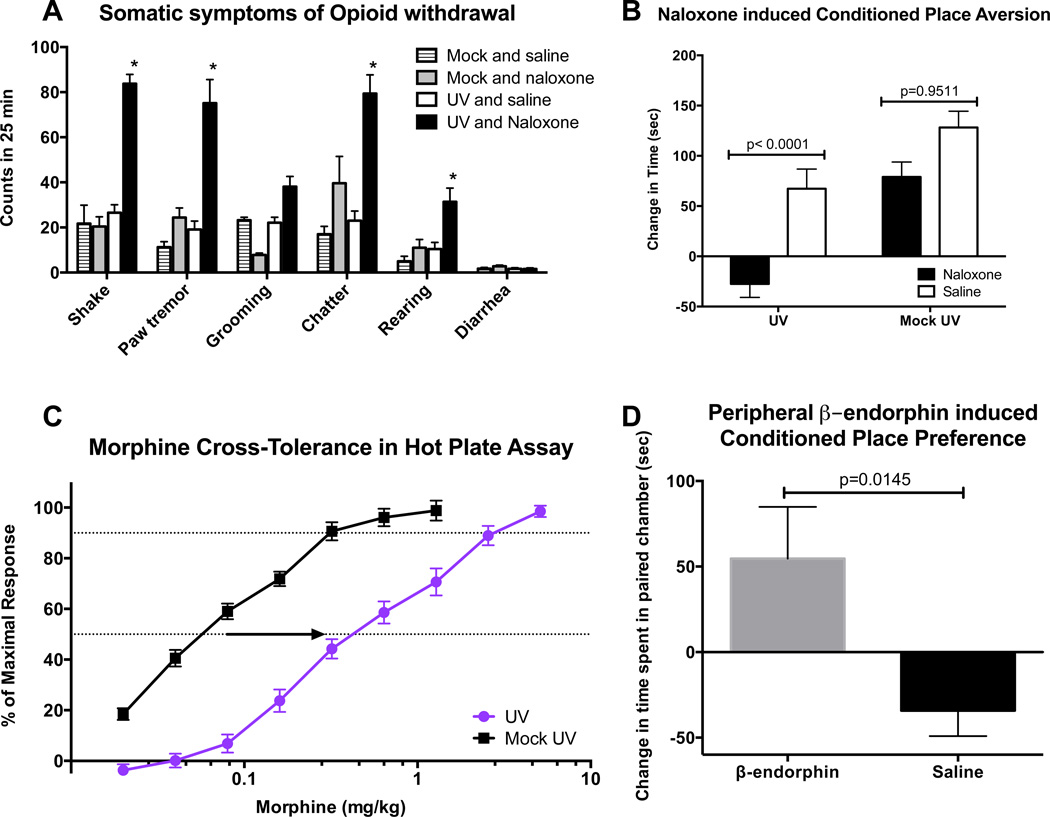
We next asked whether chronic UV exposure may be accompanied by detectable opioid dependence, in which opioid cessation or antagonism produces withdrawal symptoms, and tolerance in which increasing doses are required to achieve comparable analgesia (Drdla et al., 2009). Following chronic daily UV exposure, administration of naloxone elicited many of the classic murine signs of opioid withdrawal (wet dog shake, paw tremor, teeth chatter, rearing) (Olson et al., 2006) (Fig. 3A).
Fig. 3. Chronically UV-exposed mice show symptoms of opioid dependence.
A) Signs of opioid withdrawal in mice under experimental conditions described in Figure 3. UV / saline (n=9), mock / saline (n=7), UV / naloxone (n=15), and mock / naloxone (n=7). Data are represented as the mean +/− SEM, *p<0.05 compared to UV/Saline group by 2way ANOVA with Bonferroni’s multiple comparisons test. B) Conditioned place aversion testing in UV treated mice conditioned to the naloxone-paired box (black box) with an injection of naloxone or saline (white box) following 42 days of UV or mock treatment. Mice were permitted to freely move between naloxone-paired and saline-paired boxes prior to (pretest, n=8) and after 4 days of conditioning (test), and place preferences were assessed as change in time spent in the naloxone-paired box (postconditioning – preconditioning). Data are represented as the mean +/− SEM, p values were generated by 2way ANOVA with Bonferroni’s multiple comparisons test. C) Morphine dose-response curves in mice following 42 days UV irradiation (n=31) or mock exposure (n=29). Data are represented as the mean +/− SEM, p<0.0001 by 2way ANOVA. D) Conditioned place preference testing in mice administered i.v. β-endorphin or saline through the tail vein. Mice were conditioned to β-endorphin (6) or Saline (8) in the white box and Saline in the black box. Place preferences were assessed as change in time spent in the white (β-endorphin -paired box), postconditioning – preconditioning. Data are represented as the mean +/− SEM, p=0.0145 by Student’s t-test.
Because the magnitude of the measured withdrawal symptoms, while significant, was smaller than that commonly observed with exogenously administered opioids (Broseta et al., 2002), we wished to determine whether these withdrawal signs would be sufficient to elicit alterations in pro-active/operant behavioral choices. We utilized a conditioned place aversion assay (Skoubis et al., 2001; Weitemier and Murphy, 2009) to test whether a specific environment, paired with naloxone administration during conditioning, would be avoided in favor of a different environment paired with a neutral stimulus (saline) during conditioning in chronically UV-irradiated animals. Due to the kinetics of the UV response we chose to use naloxone as it allowed an acute effect of limited duration. Naloxone induces conditioned place aversion in exogenous opioid-dependent mice (Glass et al., 2008; Kenny et al., 2006). Following conditioning, mice are permitted to move freely between the two environments, and changes in place preference are measured in the absence of additional naloxone or saline administration. Our conditioning environments were black and white boxes with dim and bright lighting, respectively, and to minimize apparatus bias we assigned the black box as the naloxone (withdrawal stimulus)-paired box and the white box as the saline (neutral stimulus)-paired box, as rodents prefer dark environments to light environments in the absence of conditioning (Roma and Riley, 2005).
We observed that chronically UV-irradiated mice conditioned with naloxone in the black box, avoided the black box in post-conditioning preference testing. Naloxone conditioning had no effect on mock-treated (non-UV irradiated) control mice, and saline conditioning in the black box had no effect on UV-irradiated or mock-treated mice (Fig. 3B). Here, naloxone was sufficient to induce conditioned place aversion in UV-irradiated mice, suggesting that chronic UV exposure imparts an opioid-like physical dependence of sufficient magnitude to guide pro-active behavior choices.
To test for the other principle feature of chronic opioid exposure, tolerance, after chronic UV treatment, we asked whether there is cross tolerance between chronic UV exposure and morphine, altering the dose required to produce analgesia (Mao et al., 2000). After chronic UV exposure, mice required significantly higher doses of morphine than mock-treated controls to achieve comparable thermal analgesia in the hot plate test, as reflected by a rightward shift in the dose-response curve and an increase in EC50 from 57 µg/kg in the mock-treated group to 270 µg/kg in the UV-exposed group (Fig. 3C). The analgesic effect of UV exposure that we detected could be a result of systemic β-endorphin acting both through the peripheral and central nervous systems, however the withdrawal effects and conditioned place aversion point to a central nervous system effect. It has been reported that radiolabeled β-endorphin peptides cross the blood brain barrier, (Banks and Kastin 1990). To test whether it is plausible that skin-derived β-endorphin may cause central effects we decided to assess whether peripherally administered β-endorphin injected i.v. into the tail vein could cause conditioned place preference. To attempt to match an acute iv administered drug dose with a chronic elevation, we chose a β-endorphin concentration reported to cause a similar analgesic response to that we observed in our UV exposure experiments, (Tseng et al., 1976). β-endorphin or saline was injected into the tail vein of mice which were then conditioned to the white side of the CPP apparatus. The mice that had been conditioned with saline spent less time in the white box on the final day than on the initial day (Figure 3D); this was expected, as mice naturally prefer a dark environment. However, the mice that had received β-endorphin in the white box spent more time in the white box after conditioning (Figure 3D), indicating a conditioned place preference for the environment where they experienced β-endorphin. This shows that peripherally administered β-endorphin can cause conditioned place preference, presumably through the central nervous system.
These findings show that chronic UV exposure stimulates and sustains sufficient endogenous opioid release and opioid receptor activity to develop both opioid tolerance and physical dependence.
β-endorphin knockout abolishes UV induced behavioral changes
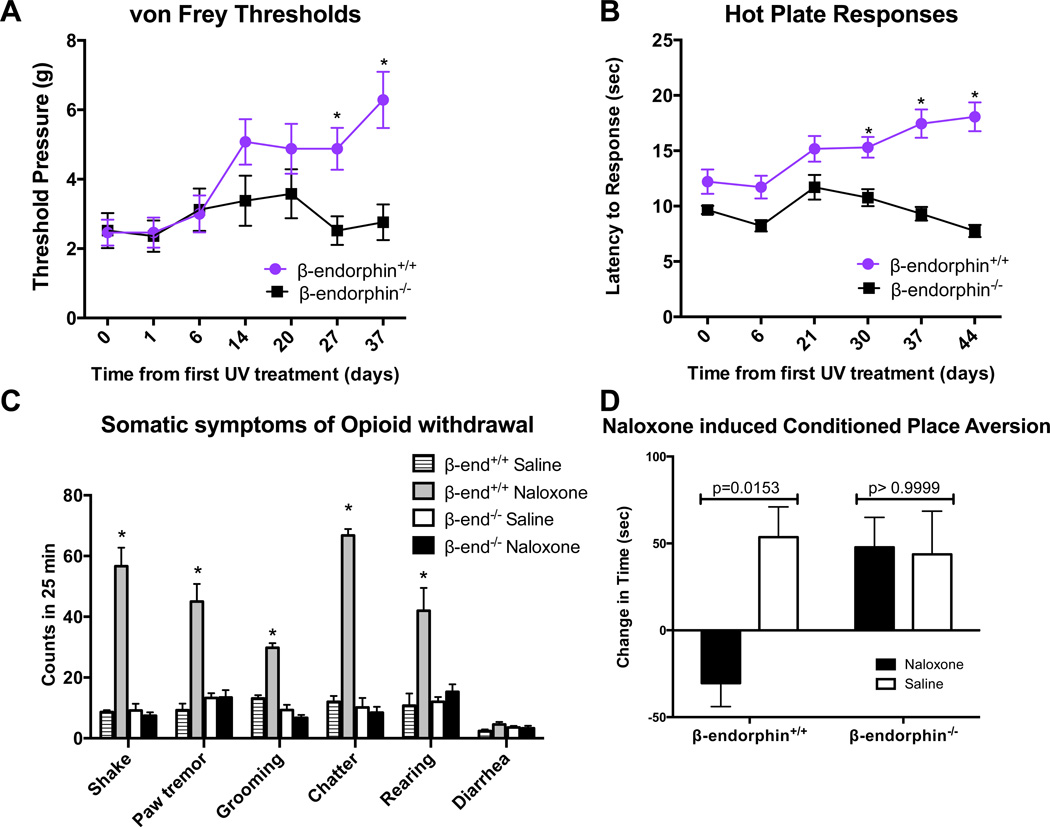
To specifically examine the functional requirement for β-endorphin in these UV-associated behavioral changes, we employed β-endorphin knockout mice (lacking the C-terminus of the POMC gene) (Rubinstein et al., 1996), and found that they exhibited no significant changes in thermal or mechanical nociceptive thresholds with chronic UV exposure (Fig. 4A and Fig. 4B). The β-endorphin null mice also failed to develop signs of opioid withdrawal (Fig. 4C) and when subjected to the conditioned-place aversion test, exhibited no measurable change in place preference (Fig. 4D).
Fig. 4. Genetic lack of β-endorphin abolishes changes in pain tolerance and opioid dependence with chronic UV exposure.
A) von Frey test and B) thermal analgesic thresholds in wild type (n=11) and β-endorphin −/− (n=13) mice over 35 day UV exposure. Data are represented as the mean +/− SEM, *p<0.05 by 2way ANOVA with Bonferroni’s multiple comparisons test. C) Signs of naloxone precipitated opioid withdrawal in control and β-endorphin null mice after 6 weeks of UV. Data are represented as the mean +/− SEM, *p<0.0001 compared to β-endorphin−/−/Naloxone group by 2way ANOVA with Bonferroni’s multiple comparisons test. D) Conditioned place aversion testing in UV treated control and β-endorphin null mice conditioned to the naloxone-paired box (black box) with an injection of naloxone or saline. All mice were conditioned to saline in the white box, n>10 for all groups. Mice were permitted to freely move between naloxone-paired and saline-paired boxes prior to and after 4 days of conditioning, and place preferences were assessed as change in time spent in the naloxone-paired box (postconditioning – preconditioning). Data are represented as the mean +/− SEM, p values were generated by 2way ANOVA with Bonferroni’s multiple comparisons test.
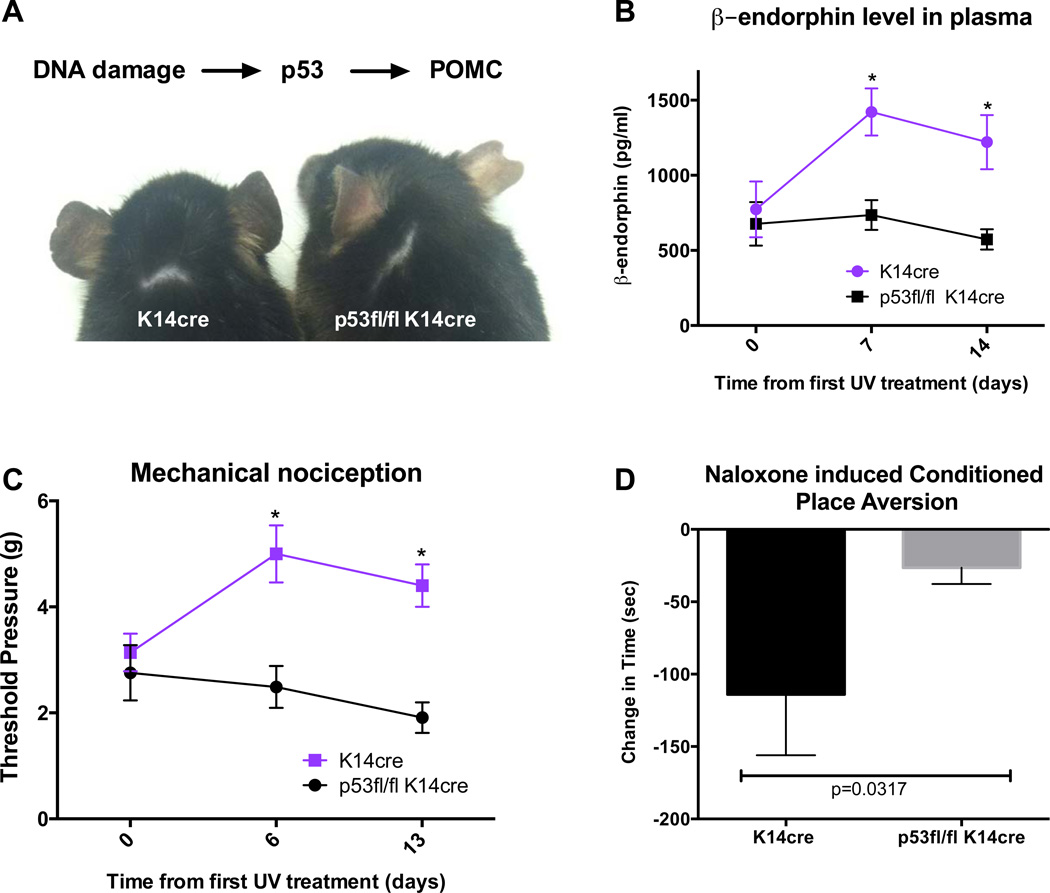
Keratinocyte expression of p53 is required for elevated β-endorphin levels and pain thresholds
The UV induced cutaneous upregulation of POMC, the precursor to both α-MSH and β-endorphin, is mediated by the tumor suppressor p53, which directly activates POMC gene transcription in keratinocytes (Cui et al., 2007). To test whether keratinocyte expression of p53 is required for UV-mediated increases in circulating β-endorphin, we crossed a mouse strain with a floxed allele of p53 with a strain containing cre under the control of the keratin 14 promoter, which is selective to keratinocytes. We subjected the p53fl/fl K14cre and control p53+/+ K14cre mice to the UV irradiation regimen and assayed plasma β-endorphin levels, mechanical nociception and naloxone induced conditioned place aversion. Consistent with the known role of p53 in the tanning response there was an absence of any tanning on the ears of the p53fl/fl K14cre animals (Figure 5A). Further we observed no increase in circulating β-endorphin (Fig. 5B) or in mechanical nociception threshold (Figure 5C). Moreover, the K14cre control mice showed significant naloxone conditioned place aversion compared to the p53fl/fl K14cre animals, (Figure D). These data indicate that keratinocyte-derived β-endorphin is a key factor in mediating UV-induced addiction.
Fig. 5. Keratinocyte expression of p53 is required for elevated β-endorphin levels and pain thresholds.
A) Representative K14cre and p53fl/fl K14cre mice after 4 weeks of daily UV treatment. B) Plasma β-endorphin in mice in K14cre and p53fl/fl K14cre mice receiving daily UV irradiation. Data are represented as the mean +/− SEM, *p<0.05 by 2way ANOVA analysis with Bonferroni’s multiple comparisons test. C) Mechanical analgesic thresholds in K14cre (n=10) and p53fl/fl K14cre (n=9) mice over 13 days UV exposure. Data are represented as the mean +/− SEM, *p<0.05 by 2way ANOVA with Bonferroni’s multiple comparisons test. D) K14cre and p53fl/fl K14cre mice were conditioned to naloxone in the black box after 3 weeks of daily UV. Place preferences were assessed as change in time spent in the naloxone-paired box. Data are represented as the mean +/− SEM, p=0.0317 by Student’s t-test. The change in time spent in the black box was not significant when the postconditioning and preconditioning times were compared by Student’s t-test (p=0.26).
DISCUSSION
Our findings suggest that repeated UV exposure produces an opioid receptor-mediated addiction due to elevations in circulating β-endorphin, leading to increased nociceptive thresholds that are reversed by naloxone or ablated in β-endorphin null mice. Measurable withdrawal symptoms are elicited by naloxone, and pro-active place-preference behaviors were strongly induced, based on prior conditioning between opioid receptor antagonism and cage color. Further a skin specific knockout of p53, a critical step in the UV response pathway, prevented both the β-endorphin elevation and the behavioral responses. There remains a formal possibility that skin specific p53 knockout could affect β-endorphin expression outside of the skin.
While these studies were performed in a nocturnal and furred animal model, significant evidence supports a strong relationship between UV exposure and addictive behaviors in humans (Feldman et al., 2004; Harrington et al., 2011; Kaur et al., 2005; Kaur et al., 2006b; Kourosh et al., 2010; Mosher and Danoff-Burg, 2010; Warthan et al., 2005; Zeller et al., 2006). Several small studies have attempted to measure β-endorphin changes with UV exposure in man (Gambichler et al., 2002; Kaur et al., 2006a; Levins et al., 1983; Wintzen et al., 2001), with some showing UV-induced elevations and others not, although significant confounding variables complicate such measurements, such as diurnal variations in β-endorphin (McMurray et al., 1990) and stressors which also influence β-endorphin levels (Aravich et al., 1993; Gianoulakis et al., 1996; Petraglia et al., 1990; Welch et al., 1996). While the effects of sunscreen have not been reported in this context, it does appear likely that sunscreen use would protect against UV induced addictive behaviors.
Despite the carcinogenicity of UV and hence the serious maladaptive consequences of addiction to UV exposure, our results may also imply a potential evolutionary benefit of an endogenous mechanism that reinforces UV-seeking behavior, one that may operate by creating an opioid-mediated hedonic experience followed by dependence on the behavior to avoid the anhedonic consequences of withdrawal. Further studies may shed light on the site/sites of β-endorphin action within the brain; and whether the brain regions and neuronal subpopulations involved in sun seeking behavior are the same as those involved the analgesic response or in exogenous opioid addiction. A recent study has shown activation of known reward centers in the brains of volunteers during UV exposure, (Harrington et al., 2012).
The current studies suggest that UV exposure is biologically addictive, but dangerous due to UV’s mutagenic activities towards formation of all common forms of skin cancer. This calls into question the perceived safety of tanning beds and current benign views of indoor tanning, reflected in the United States’ current FDA classification of UV-emitting devices as Class I, and therefore minimally regulated. It may be necessary, therefore, to more pro-actively protect individuals, including teens, from the risks of an avoidable, potentially life-threatening, exposure and to view recreational tanning and opioid drug abuse as engaging in the same biological pathway.
EXPERIMENTAL PROCEDURES
Mice
All mice used were on a C57Bl/6 background. For select experiments, mice with homozygous deletion of the C-terminus of the POMC gene, resulting in lack of β-endorphin (β-endorphin −/−) (Rubinstein et al., 1996), and mice with a floxed allele of p53, (Marino et al., 2000), and a Keratin 14 promoter driven Cre recombinase strain, (Dassule et al., 2000), were used.
UV irradiation and blood draws
Mice were dorsally shaved two days prior to the start of radiation exposure, then exposed to 50mJ/cm2/day of UVB, an empirically determined sub-erythematic dose, 5 days per week (Monday – Friday) for 6 weeks. Mice were re-shaved once every two weeks if there were patches of fur re-growth.
For blood draws, mice were placed in a standard restrainer and tail vein blood was collected in EDTA microvette tubes containing 0.6TIU aprotinin. Mice underwent blood draws prior to the stat of radiation exposure, once per week during the radiation exposure regimen, and once per week for two weeks following cessation of the UV regimen. Blood was drawn in the mornings prior to radiation exposure on Fridays.
Tubes of collected blood were maintained on ice until centrifugation at 3500RPM for 20 minutes at 4°C. Plasma was isolated, and samples were stored at −80°C until β-endorphin measurement. β-endorphin was quantified by radioimmunoassay (Phoenix Pharmaceuticals, Burlingame, CA).
Straub Tail measurement
Straub Tail measurement was performed as described (Bilbey et al., 1960). Scoring was on a scale of 0–2 according to the angle of elevation of the tail from the horizontal plane (0 = tail relaxed and no elevation; 1 = tail is rigid and elevated 1–10° from horizontal; 1.5 = 11–45° elevation and rigidity at the base of the tail; 2 = 46–90° elevation with rigidity at the base of the tail). For each time point, each mouse was scored every 10 seconds for 1 minute and the final score was the average of these six values. Mice undergoing the six-week UV exposure regimen or mock treatment were scored prior to the start of the regimen, once per week during the regimen, and once per week for 2 weeks following cessation of UV/mock treatment. On day 23 of the regimen after weekly Straub Tail scoring, mice were injected (ip) with either 10mg/kg naloxone hydrochloride (Sigma, St. Louis, MO) or saline. Mice underwent Straub Tail scoring again 15 minutes following injection.
Analgesic threshold testing
Mice underwent mechanical and thermal analgesic testing during UV/mock treatment regimens using the von Frey test (Kwan et al., 2006) and the hot plate test respectively (Mogil et al., 1999). In the von Frey test, mice were placed in individual enclosures on an elevated wire mesh rack and the plantar surface of the left hind paw was serially poked with fibers of increasing tensile strength (10 times per fiber at a rate of 1/second) until a paw withdrawal response was elicited on 2/10 pokes. In the hot plate test, mice were placed on a 52°C hot plate and time to response (paw flutter, paw licking, jumping) was measured.
Mice were habituated to the wire mesh rack for 30 minutes per day and to the hot plate at room temperature for 2 minutes per day for 3 days prior to measuring baseline nociceptive thresholds. Mice underwent nociceptive testing twice per week on nonconsecutive days during and for two weeks following cessation of UV/mock treatment. Mice received an injection (ip) of 10mg/kg naloxone or saline 15 minutes prior to nociceptive testing.
Somatic symptoms of opiate withdrawal
Mice that had undergone 6 weeks of daily UV exposure or mock exposure were injected (ip) with either 2mg/kg naloxone or saline and signs of opioid withdrawal were tabulated as described (Olson et al., 2006). Mice were observed in an open-topped Plexiglas 30cm×15cm×15cm rectangular container for 25 minutes each following injection, and signs of opioid withdrawal were tabulated. Wet dog shake, teeth chatter, and bouts of grooming were measured as occurrence in each 15-second interval. Individual rearing events were counted. Number of fecal pellets at the end of the 25-minute interval was used to quantify diarrhea.
Conditioned place aversion testing
The apparatus used consisted of a box with black interior and dim lighting and a box with white interior and bright lighting connected by a smaller gray “neutral” box, and procedures were followed as described (Skoubis et al., 2001; Weitemier and Murphy, 2009). Mice that had undergone 6 weeks of daily UV exposure or mock exposure were tested for baseline place preferences prior to conditioning (10 minute testing time per mouse). Over the following 4 days conditioning took place in which mice were either conditioned with naloxone (10mg/kg ip injection) or saline (ip injection) in the black box, and all animals were conditioned with saline (ip injection) in the white box. Conditioning time in each box was 30 minutes following injection. For each animal there were four hours between conditioning in one box and condition in the other box each day. On the day following the final day of conditioning, place preferences were again tested (post-conditioning, 10 minute testing time per mouse).
Morphine cross-tolerance testing
Morphine dose-response curves in the hot plate test were measured as described (Mao et al., 2000) in mice that had undergone 6 weeks of UV exposure or mock treatment. Morphine was injected at a starting dose of 0.02mg/kg ip, and was increased logarithmically in cumulative dose increments of 0.3 log units. Thermal analgesic thresholds were tested 15 minutes after each morphine injection until there was failure to respond in the hot plate test (cutoff time was 20 seconds) or until there was no change in response time from one dose to the next. There were 30 minutes between injections, and 30 minutes between hot plate testings for each mouse. Percent of maximal effect was calculated based on the equation: (test latency-baseline latency) / (maximal latency-baseline latency) × 100% (Mao et al., 2000).
HIGHLIGHTS.
Ultraviolet (UV) exposure leads to elevated blood levels of β–endorphin in mice
UV causes systemic analgesia which is reversible with opioid receptor blockade
Chronic UV causes dependency and ‘addiction’ like behavior
‘Addiction’ like behaviors require keratinocyte signaling and β–endorphin expression
ACKNOWLEDGMENTS
The authors gratefully acknowledge Dr. Charles Berde, Dr. Jeff Mogil, Ms. Vivien Igras, Mr. John Dellorusso, Ms. Teri Herbert, Ms. Leeza Hargreaves, Dr. Roydon Price, and Dr. Grewo Lim for productive discussions during the early phases of this project and for technical assistance with the experiments and manuscript. The authors thank Dr. Mary Jeanne Kreek for useful discussions. The authors also acknowledge Douglas Hayden from the MGH Biostatistics Center for consultation regarding the statistical analysis. This work (lab of DEF) was supported by NIH grants R01-AR043369-16 and R01-CA150226-03, and grants from the Melanoma Research Alliance, the US-Israel Binational Science Foundation, and the Dr. Miriam and Sheldon Adelson Medical Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aravich PF, Rieg TS, Lauterio TJ, Doerries LE. Beta-endorphin and dynorphin abnormalities in rats subjected to exercise and restricted feeding: relationship to anorexia nervosa? Brain Res. 1993;622:1–8. doi: 10.1016/0006-8993(93)90794-n. [DOI] [PubMed] [Google Scholar]
- Bender T, Nagy G, Barna I, Tefner I, Kadas E, Geher P. The effect of physical therapy on beta-endorphin levels. Eur J Appl Physiol. 2007;100:371–382. doi: 10.1007/s00421-007-0469-9. [DOI] [PubMed] [Google Scholar]
- Bilbey DL, Salem H, Grossman MH. The anatomical basis of the straub phenomenon. British journal of pharmacology and chemotherapy. 1960;15:540–543. doi: 10.1111/j.1476-5381.1960.tb00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broseta I, Rodriguez-Arias M, Stinus L, Minarro J. Ethological analysis of morphine withdrawal with different dependence programs in male mice. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:335–347. doi: 10.1016/s0278-5846(01)00277-9. [DOI] [PubMed] [Google Scholar]
- Cui R, Widlund HR, Feige E, Lin JY, Wilensky DL, Igras VE, D'Orazio J, Fung CY, Schanbacher CF, Granter SR, et al. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell. 2007;128:853–864. doi: 10.1016/j.cell.2006.12.045. [DOI] [PubMed] [Google Scholar]
- D'Orazio JA, Nobuhisa T, Cui R, Arya M, Spry M, Wakamatsu K, Igras V, Kunisada T, Granter SR, Nishimura EK, et al. Topical drug rescue strategy and skin protection based on the role of Mc1r in UV-induced tanning. Nature. 2006;443:340–344. doi: 10.1038/nature05098. [DOI] [PubMed] [Google Scholar]
- Dassule HR, Lewis P, Bei M, Maas R, McMahon AP. Sonic hedgehog regulates growth and morphogenesis of the tooth. Development. 2000;127:4775–4785. doi: 10.1242/dev.127.22.4775. [DOI] [PubMed] [Google Scholar]
- de Gruijl FR. Skin cancer and solar UV radiation. Eur J Cancer. 1999;35:2003–2009. doi: 10.1016/s0959-8049(99)00283-x. [DOI] [PubMed] [Google Scholar]
- Drdla R, Gassner M, Gingl E, Sandkuhler J. Induction of synaptic long-term potentiation after opioid withdrawal. Science. 2009;325:207–210. doi: 10.1126/science.1171759. [DOI] [PubMed] [Google Scholar]
- Fassoulaki A, Paraskeva A, Kostopanagiotou G, Tsakalozou E, Markantonis S. Acupressure on the extra 1 acupoint: the effect on bispectral index, serum melatonin, plasma beta-endorphin, and stress. Anesth Analg. 2007;104:312–317. doi: 10.1213/01.ane.0000250911.43942.4e. [DOI] [PubMed] [Google Scholar]
- Feldman SR, Liguori A, Kucenic M, Rapp SR, Fleischer AB, Jr, Lang W, Kaur M. Ultraviolet exposure is a reinforcing stimulus in frequent indoor tanners. J Am Acad Dermatol. 2004;51:45–51. doi: 10.1016/j.jaad.2004.01.053. [DOI] [PubMed] [Google Scholar]
- Gambichler T, Bader A, Vojvodic M, Avermaete A, Schenk M, Altmeyer P, Hoffmann K. Plasma levels of opioid peptides after sunbed exposures. Br J Dermatol. 2002;147:1207–1211. doi: 10.1046/j.1365-2133.2002.04859.x. [DOI] [PubMed] [Google Scholar]
- Gandini S, Autier P, Boniol M. Reviews on sun exposure and artificial light and melanoma. Prog Biophys Mol Biol. 2011;107:362–366. doi: 10.1016/j.pbiomolbio.2011.09.011. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C. Endogenous opioids and addiction to alcohol and other drugs of abuse. Curr Top Med Chem. 2009;9:999–1015. doi: 10.2174/156802609789630956. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C, de Waele JP, Thavundayil J. Implication of the endogenous opioid system in excessive ethanol consumption. Alcohol. 1996;13:19–23. doi: 10.1016/0741-8329(95)02035-7. [DOI] [PubMed] [Google Scholar]
- Glass MJ, Hegarty DM, Oselkin M, Quimson L, South SM, Xu Q, Pickel VM, Inturrisi CE. Conditional deletion of the NMDA-NR1 receptor subunit gene in the central nucleus of the amygdala inhibits naloxone-induced conditioned place aversion in morphine-dependent mice. Exp Neurol. 2008;213:57–70. doi: 10.1016/j.expneurol.2008.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington CR, Beswick TC, Graves M, Jacobe HT, Harris TS, Kourosh S, Devous MD, Sr, Adinoff B. Activation of the mesostriatal reward pathway with exposure to ultraviolet radiation (UVR) vs. sham UVR in frequent tanners: a pilot study. Addict Biol. 2012;17:680–686. doi: 10.1111/j.1369-1600.2010.00312.x. [DOI] [PubMed] [Google Scholar]
- Harrington CR, Beswick TC, Leitenberger J, Minhajuddin A, Jacobe HT, Adinoff B. Addictive-like behaviours to ultraviolet light among frequent indoor tanners. Clin Exp Dermatol. 2011;36:33–38. doi: 10.1111/j.1365-2230.2010.03882.x. [DOI] [PubMed] [Google Scholar]
- Ibrahim MM, Porreca F, Lai J, Albrecht PJ, Rice FL, Khodorova A, Davar G, Makriyannis A, Vanderah TW, Mata HP, et al. CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc Natl Acad Sci U S A. 2005;102:3093–3098. doi: 10.1073/pnas.0409888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastin AJ, Jemison MT, Coy DH. Analgesia after peripheral administration of enkephalin and endorphin analogues. Pharmacol Biochem Behav. 1979;11:713–716. doi: 10.1016/0091-3057(79)90268-5. [DOI] [PubMed] [Google Scholar]
- Kaur M, Liguori A, Fleischer AB, Jr, Feldman SR. Side effects of naltrexone observed in frequent tanners: could frequent tanners have ultraviolet-induced high opioid levels? J Am Acad Dermatol. 2005;52:916. doi: 10.1016/j.jaad.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Kaur M, Liguori A, Fleischer AB, Jr, Feldman SR. Plasma beta-endorphin levels in frequent and infrequent tanners before and after ultraviolet and non-ultraviolet stimuli. J Am Acad Dermatol. 2006a;54:919–920. doi: 10.1016/j.jaad.2006.01.062. [DOI] [PubMed] [Google Scholar]
- Kaur M, Liguori A, Lang W, Rapp SR, Fleischer AB, Jr, Feldman SR. Induction of withdrawal-like symptoms in a small randomized, controlled trial of opioid blockade in frequent tanners. J Am Acad Dermatol. 2006b;54:709–711. doi: 10.1016/j.jaad.2005.11.1059. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Chen SA, Kitamura O, Markou A, Koob GF. Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. J Neurosci. 2006;26:5894–5900. doi: 10.1523/JNEUROSCI.0740-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourosh AS, Harrington CR, Adinoff B. Tanning as a behavioral addiction. Am J Drug Alcohol Abuse. 2010;36:284–290. doi: 10.3109/00952990.2010.491883. [DOI] [PubMed] [Google Scholar]
- Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Lazovich D, Vogel RI, Berwick M, Weinstock MA, Anderson KE, Warshaw EM. Indoor tanning and risk of melanoma: a case-control study in a highly exposed population. Cancer Epidemiol Biomarkers Prev. 2010;19:1557–1568. doi: 10.1158/1055-9965.EPI-09-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppaluoto J, Westerlund T, Huttunen P, Oksa J, Smolander J, Dugue B, Mikkelsson M. Effects of long-term whole-body cold exposures on plasma concentrations of ACTH, beta-endorphin, cortisol, catecholamines and cytokines in healthy females. Scand J Clin Lab Invest. 2008;68:145–153. doi: 10.1080/00365510701516350. [DOI] [PubMed] [Google Scholar]
- Levins PC, Carr DB, Fisher JE, Momtaz K, Parrish JA. Plasma beta-endorphin and beta-lipoprotein response to ultraviolet radiation. Lancet. 1983;2:166. doi: 10.1016/s0140-6736(83)90150-2. [DOI] [PubMed] [Google Scholar]
- Mao J, Price DD, Lu J, Keniston L, Mayer DJ. Two distinctive antinociceptive systems in rats with pathological pain. Neurosci Lett. 2000;280:13–16. doi: 10.1016/s0304-3940(99)00998-2. [DOI] [PubMed] [Google Scholar]
- Marino S, Vooijs M, van Der Gulden H, Jonkers J, Berns A. Induction of medulloblastomas in p53-null mutant mice by somatic inactivation of Rb in the external granular layer cells of the cerebellum. Genes Dev. 2000;14:994–1004. [PMC free article] [PubMed] [Google Scholar]
- McMurray RG, Hill D, Field KM. Diurnal variations of beta-endorphin at rest and after moderate intensity exercise. Chronobiol Int. 1990;7:135–142. doi: 10.3109/07420529009056965. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Wilson SG, Bon K, Lee SE, Chung K, Raber P, Pieper JO, Hain HS, Belknap JK, Hubert L, et al. Heritability of nociception II. 'Types' of nociception revealed by genetic correlation analysis. Pain. 1999;80:83–93. doi: 10.1016/s0304-3959(98)00196-1. [DOI] [PubMed] [Google Scholar]
- Mosher CE, Danoff-Burg S. Addiction to indoor tanning: relation to anxiety, depression, and substance use. Arch Dermatol. 2010;146:412–417. doi: 10.1001/archdermatol.2009.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen AT, Marquez P, Hamid A, Kieffer B, Friedman TC, Lutfy K. The rewarding action of acute cocaine is reduced in beta-endorphin deficient but not in mu opioid receptor knockout mice. Eur J Pharmacol. 2012;686:50–54. doi: 10.1016/j.ejphar.2012.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW. Stimulation of endorphin neurotransmission in the nucleus accumbens by ethanol, cocaine, and amphetamine. J Neurosci. 2001;21:RC184. doi: 10.1523/JNEUROSCI.21-23-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson VG, Griner NB, Heusner CL, Palmiter RD. Lack of neuropeptide Y attenuates the somatic signs of opiate withdrawal. Synapse. 2006;60:553–556. doi: 10.1002/syn.20328. [DOI] [PubMed] [Google Scholar]
- Petraglia F, Bacchi Modena A, Comitini G, Scazzina D, Facchinetti F, Fiaschetti D, Genazzani AD, Barletta C, Scavo D, Genazzani AR. Plasma beta-endorphin and beta-lipotropin levels increase in well trained athletes after competition and non competitive exercise. J Endocrinol Invest. 1990;13:19–23. doi: 10.1007/BF03348571. [DOI] [PubMed] [Google Scholar]
- Racz I, Schurmann B, Karpushova A, Reuter M, Cichon S, Montag C, Furst R, Schutz C, Franke PE, Strohmaier J, et al. The opioid peptides enkephalin and beta-endorphin in alcohol dependence. Biol Psychiatry. 2008;64:989–997. doi: 10.1016/j.biopsych.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JK, Rigel DS, Amonette RA. Trends in sun exposure knowledge, attitudes, and behaviors: 1986 to 1996. J Am Acad Dermatol. 1997;37:179–186. doi: 10.1016/s0190-9622(97)80122-3. [DOI] [PubMed] [Google Scholar]
- Roma PG, Riley AL. Apparatus bias and the use of light and texture in place conditioning. Pharmacol Biochem Behav. 2005;82:163–169. doi: 10.1016/j.pbb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Roth-Deri I, Zangen A, Aleli M, Goelman RG, Pelled G, Nakash R, Gispan-Herman I, Green T, Shaham Y, Yadid G. Effect of experimenter-delivered and self-administered cocaine on extracellular beta-endorphin levels in the nucleus accumbens. J Neurochem. 2003;84:930–938. doi: 10.1046/j.1471-4159.2003.01584.x. [DOI] [PubMed] [Google Scholar]
- Rubinstein M, Mogil JS, Japon M, Chan EC, Allen RG, Low MJ. Absence of opioid stress-induced analgesia in mice lacking beta-endorphin by site-directed mutagenesis. Proc Natl Acad Sci U S A. 1996;93:3995–4000. doi: 10.1073/pnas.93.9.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoffelmeer AN, Warden G, Hogenboom F, Mulder AH. Beta-endorphin: a highly selective endogenous opioid agonist for presynaptic mu opioid receptors. J Pharmacol Exp Ther. 1991;258:237–242. [PubMed] [Google Scholar]
- Skobowiat C, Dowdy JC, Sayre RM, Tuckey RC, Slominski A. Cutaneous hypothalamic-pituitary-adrenal axis homolog: regulation by ultraviolet radiation. American journal of physiology Endocrinology and metabolism. 2011;301:E484–E493. doi: 10.1152/ajpendo.00217.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoubis PD, Matthes HW, Walwyn WM, Kieffer BL, Maidment NT. Naloxone fails to produce conditioned place aversion in mu-opioid receptor knock-out mice. Neuroscience. 2001;106:757–763. doi: 10.1016/s0306-4522(01)00333-5. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocrine reviews. 2000;21:457–487. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- Technology-Planning-and-Management-Corporation. Report on Carcinogens Background Document for Broad-Spectrum Ultraviolet (UV) Radiation and UVA, and UVB, and UVC (U.S. Department of Health and Human Services) 2000. [Google Scholar]
- Trigo JM, Zimmer A, Maldonado R. Nicotine anxiogenic and rewarding effects are decreased in mice lacking beta-endorphin. Neuropharmacology. 2009;56:1147–1153. doi: 10.1016/j.neuropharm.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng LF, Loh HH, Li CH. Beta-Endorphin as a potent analgesic by intravenous injection. Nature. 1976;263:239–240. doi: 10.1038/263239a0. [DOI] [PubMed] [Google Scholar]
- US-EPA. The Federal experimental ultraviolet index : what you need to know (United States Environmental Protection Agency) 1994. [Google Scholar]
- Warthan MM, Uchida T, Wagner RF., Jr UV light tanning as a type of substance-related disorder. Arch Dermatol. 2005;141:963–966. doi: 10.1001/archderm.141.8.963. [DOI] [PubMed] [Google Scholar]
- Weitemier AZ, Murphy NP. Accumbal dopamine and serotonin activity throughout acquisition and expression of place conditioning: correlative relationships with preference and aversion. The European journal of neuroscience. 2009;29:1015–1026. doi: 10.1111/j.1460-9568.2009.06652.x. [DOI] [PubMed] [Google Scholar]
- Welch CC, Kim EM, Grace MK, Billington CJ, Levine AS. Palatability-induced hyperphagia increases hypothalamic Dynorphin peptide and mRNA levels. Brain Res. 1996;721:126–131. doi: 10.1016/0006-8993(96)00151-5. [DOI] [PubMed] [Google Scholar]
- Wintzen M, Ostijn DM, Polderman MC, le Cessie S, Burbach JP, Vermeer BJ. Total body exposure to ultraviolet radiation does not influence plasma levels of immunoreactive beta-endorphin in man. Photodermatol Photoimmunol Photomed. 2001;17:256–260. doi: 10.1034/j.1600-0781.2001.170602.x. [DOI] [PubMed] [Google Scholar]
- Zeller S, Lazovich D, Forster J, Widome R. Do adolescent indoor tanners exhibit dependency? J Am Acad Dermatol. 2006;54:589–596. doi: 10.1016/j.jaad.2005.12.038. [DOI] [PubMed] [Google Scholar]