Abstract
Background
Many health care systems face barriers to implementing resource-intensive care management programs for patients with poorly controlled diabetes. Mobilizing patients to provide reciprocal peer support may enhance care management and improve clinical outcomes.
Objective
To compare the effectiveness of a reciprocal diabetes peer support program (RPS) with nurse care management (NCM) in improving glycemic control in real-world clinical settings.
Design
Six-month parallel randomized controlled effectiveness study from 2007–2010 (Trial Registration NCT00320112)
Setting
Two U.S. Veterans’Affairs (VA) health care facilities
Participants
244 male diabetes patients with a hemoglobin A1c (HbA1c) in the prior 6 months of 7.5% or more.
Primary Funding Source
VA Health Services Research and Development (HSR&D)
Measurements
The primary outcome was change in HbA1c between baseline and six months. Secondary outcomes were new insulin starts and intensification, blood pressure, diabetes-specific social support, emotional distress, and medication adherence.
Intervention
Participants in both arms attended an initial session led by a nurse care manager to review and discuss their point-of-service HbA1c and blood pressure values, and most recent medical record cholesterol values. RPS patients then participated in a group session to set diabetes-related behavioral goals, receive brief training in peer communication skills, and be paired with another age-matched participant. Paired peer partners were encouraged to talk weekly using a telephone platform that recorded call frequency and duration and provided automated reminders promoting peer contact. Intervention participants were also offered three optional 1.5 hour patient-driven group sessions at months 1, 3, and 6 to share concerns, questions, strategies, and progress on goals. Patients in the NCM arm attended a 1.5 hour session to receive education on care manager services and diabetes educational materials and be assigned to a nurse care manager with whom they were encouraged to follow up regularly.
Results
Of the 244 patients enrolled, at six months 216 (89%) completed the HbA1c and 231 (95%) the survey assessments. RPS participants had a mean HbA1c of 8.02% at baseline, which improved to 7.73% at six months (−0.29%) compared with an average increase in HbA1c among NCM participants (7.93 to 8.22 [SD 0.29]). The difference between groups was 0.58% (p=0.004). Among patients with a baseline HbA1c >8.0%, RPS participants had a mean decrease of 0.88% compared with a 0.07% decrease among NCM participants (p<0.001). Eight RPS patients started insulin compared to one NCM patient (p=0.02), and RPS participants reported greater increases in diabetes social support than NCM participants (+11.4 vs. +4.5, p=0.01). There were no differences between groups at follow-up in blood pressure, self-reported medication adherence, or diabetes-specific distress.
Limitations
The study was limited to male veterans. It will be important to replicate this study in gender-mixed samples of patients who share the same chronic condition and other shared characteristics (e.g., religion, ethnicity, culture) in different settings.
Conclusions
Participants in the reciprocal peer support intervention had improved HbA1c levels, insulin starts, and diabetes-specific support after six months compared to usual nurse care management. Peer-based models are effective in bridging service gaps while increasing the quality and quantitity of self-care support.
Keywords: diabetes, peer support, randomized controlled trial, glycemic control, self-management, nurse case management
Introduction
Many patients with diabetes would benefit from self-management assistance between clinic visits. In efficacy trials, nurse-led care management programs improve diabetes self-care and risk factor control (1–3). Real-life practices, however, especially in low-resource settings, face multiple barriers to delivering these services (4).
As recognized in initiatives such as the United Kingdom’s Expert Patient Program (5), peer support among patients with the same chronic health problem is a promising approach to increasing the quality and quantity of support (6). Peer support could allow patients to share experiences and receive reinforcement unavailable from time-pressed clinicians and may be especially beneficial when patients are tackling challenging medical tasks, such as insulin management. Many patients with poor glycemic control require either insulin initiation or intensification yet resist these due to concerns about the additional self-management burdens (7, 8), resulting in neuropathic and microvascular complications (9).
While few peer support models have been evaluated in randomized controlled trials (RCTs), effective tested models combine peer support with a more structured program of education and assistance (10, 11). Face-to-face peer- and clinician-led group visits (12–14) and training sessions (15–17) improve some outcomes. Yet, many participants assigned to face-to-face peer support or group sessions do not attend sessions (14, 15). It is thus important to identify novel delivery mechanisms to extend the reach (18) of evidence-based peer support models.
To build on the potential benefits of face-to-face peer support while circumventing access barriers, we designed and piloted a novel intervention supplementing optional periodic nurse-led group sessions with telephone-based peer support between paired diabetes patients (19). The model was intended to be egalitarian, i.e., encouraging both peers to receive and provide support, with no designation of a ‘helper’ or ‘helpee’. Although one mechanism by which peer support may work is to “activate” patients by having them help others (20), such reciprocal models had not been examined in chronic disease management. In the current study, we compared this reciprocal peer support (RPS) program with nurse care management (NCM) in an RCT in a real-life clinical setting. We hypothesized that helping and receiving help from other diabetes patients in group sessions and in one-on-one telephone conversations would bolster patients’ autonomous motivation (21) and self-efficacy (22) to execute diabetes self-care tasks to improve glycemic control.
Methods
Setting and Identification of Patients
From April 2007-April 2009, we identified veterans with diabetes and poor glycemic control receiving care at two Midwestern Veterans’ Affairs (VA) facilities from electronic medical records with a validated algorithm (23). Patients had to have a most recent recorded HbA1c of at least 7.5% within the prior 6 months. Exclusion criteria were an International Classification of Diseases (ICD)-9 diagnosis of post-traumatic stress disorder, bipolar disorder, dementia, schizophrenia, or personality disorder.
Telephone Screening
The protocol was approved by the VA Ann Arbor Healthcare System institutional review board. Lists of patients were generated every four to six weeks. All providers except one (N=49) allowed their patients to participate, were sent lists of potentially eligible patients prior to contact by research staff, and signed the initial information letter sent to the patients. A research associate then telephoned patients, described the study as a comparison of two diabetes self-management support models (to avoid possible expectation bias), and administered a screening questionnaire. We excluded those reporting active substance abuse, severe depression, hearing loss, terminal illnesses, and participation in other diabetes studies. Eligible and interested patients were scheduled for a face-to-face initial session in groups of 4 to 18. We alternated recruitment between cohorts aged 45–66 with cohorts aged 65 or older to facilitate group cohesion in the group sessions and help pair participants with an age-matched peer partner.
Recruitment and Randomization
At the initial group session, participants completed written informed consent, a self-administered survey, blood pressure, and HbA1c tests with a Bayer DCA2000+ Analyzer (Bayer AG, Keverkusen, Germany) (24), and were randomized to RPS or NCM arms. Participants were provided $20 for the baseline and six-month assessments.
Random sequence generation and treatment group assignment were determined centrally just prior to the initial session. Sequence was concealed until interventions were assigned. Patients, research staff, and care managers were blinded to randomization results until completion of baseline surveys and physiologic measures. Data assessors remained blinded to group assignment throughout the study. Because an even number of participants in the RPS group was required to pair participants, randomization algorithms ensured allocation of an even number to that group. After randomization, based on evidence that peers closer in age increase the likelihood of effective peer support (6), participants in the RPS group were paired based on age with a peer partner attending the same initial session.
Description of the Intervention
Initial Case Manager Training
As this was an effectiveness study, all care managers (9 at one site, 6 at the other) facilitated intervention group sessions as part of their assigned VA work duties with no additional salary support. The study was explained to care managers and other providers as comparing two different diabetes self-management support models, with no mention of specific hypotheses. Care managers completed a 4-hour training in Motivational Interviewing (MI) (25) and Empowerment-based approaches (26) to facilitate group discussion and encourage patients to identify diabetes-related behavioral goals consistent with their goals and values and to develop a short-term ‘action plan’ of specific steps to meet these goals (27). Care managers also attended two one-hour booster sessions. Because VA clinical leaders wanted all care managers to participate in both programs, we actively encouraged the care managers to use these same behavioral approaches in their interactions with patients in both arms to be able to assess the additional value of peer support interactions in the RPS group. Participants in both arms were given the same instructions about algorithms for self-adjusting insulin as per usual care and were not instructed to self-adjust oral anti-hyperglycemic medications.
Nurse Care Management (NCM)
All study participants received point-of-service HbA1c and blood pressure results and most recent medical record cholesterol values at baseline and six-month follow-up. NCM participants then attended a 1.5 hour session led by a care manager to review their lab and blood pressure results, ask questions, and receive information on VA care management services. They were provided contact information of their assigned care manager and encouraged to schedule follow-up (telephone calls and/or face-to-face visits) with that care manager. Each participant was also provided with diabetes self-management educational materials. NCM patients thus received enhanced usual care, because although they all would be eligible for nurse case manager support at the study sites, in practice unless their physician refers them to nurse case management, many patients are not aware and do not avail themselves of this service.
Reciprocal Peer Support (RPS) Group
After the baseline assessment, RPS participants attended a 3-hour group session facilitated by a care manager and research associate. In the first half, participants’ lab and blood pressure results were reviewed and action planning was introduced. In the second half, participants received brief training in basic peer communication skills and were paired with another age-matched intervention participant in their cohort. Peer partners were encouraged to call each other at least once a week using an interactive voice response-facilitated telephone platform that recorded call initiation, frequency, and duration; enabled partners to telephone without exchanging telephone numbers and to set time periods in which calls could be blocked; and generated automated reminders every 7 days if no peer calls were attempted. During a reminder call, participants could be transferred automatically to their peer-partner’s number. The system also had functions enabling participants to leave voice messages for research staff or care managers. At the end of the initial session, intervention participants were given a DVD demonstrating peer communication skills and a diabetes self-management workbook that they could use to help guide their peer telephone calls.
Intervention participants were also offered three optional 1.5 hour group sessions at months 1, 3, and 6. These were completely patient-driven sessions in which participants were encouraged to share concerns, questions, strategies, and progress on their action plans. Sessions were facilitated by a care manager and a research associate. Research associates were present to help maintain intervention fidelity by encouraging non-directive facilitation of group discussions and to complete a check list of key areas covered and communication skills used in each session.
Outcomes and Measurements
The primary outcome was change between baseline and six-month HbA1c, measured with a Bayer DCA 2000+ point-of-care analyzer (24). The assay has a test coefficient of variation <5% as required by the National Diabetes Data Group (24). A subsample of HbA1c results was compared with those from the VA’s laboratory services with no significant discrepancies. Blood pressure was recorded according to American Heart Association guidelines (28) with an Omron Intellisense Blood Pressure Monitoring System (Omron Corporation, Kyoto, Japan). We had intended to include change in point-of-service cholesterol levels, but after trial commencement determined the quality of measurement was poor (in comparisons of a subsample of assays with VA laboratories), so dropped this measure. Secondary self-report outcomes measured via survey at baseline and 6 months included validated measures of medication adherence (29), diabetes-related emotional distress (30), and diabetes-specific social support (31). Finally, we reviewed medical records to determine all primary care and diabetes-related subspecialty clinic visits, care manager contacts (phone or face-to-face), and anti-hyperglycemic and blood pressure medication increases.
Statistical Analysis
We estimated the sample size to provide 80% power with a two-sided alpha of 0.05 to detect an HbA1c difference of 0.5% between groups. The unit of analysis was the individual, but we allowed for a possible correlation of outcomes between members of the same pair (intraclass correlation [ICC], or rho) of 0.05 in the intervention group, using the methods of Cohen (32, 33). We estimated a standard deviation of HbA1c of 1.2 based on our prior RCTs (34).
Analyses
As per international guidelines for analysis and reporting of clinical trials (35), we visually examined baseline data for clinically important differences between groups for study endpoints and other potential prognostic indicators. To assess the primary endpoint of change in mean HbA1c we used STATA 11’s xtmixed command, which fits multi-level mixed-effects linear regression models, with clustering by assigned pairs (33, 36). All models evaluating changes between baseline and six-month values included as independent variables participants’ baseline value and intervention arm. Although there were no differences between arms in baseline characteristics at <0.1 level, further analyses adjusted for variables that hypothetically could influence the outcome (e.g., insulin use, age, comorbidities). As there were no differences in results, unadjusted analyses are reported.
The intra-pair ICC for changes in HbA1c in our sample was .092, and there were no differences in our results in analyses accounting for clustering (xtmixed) and using simple linear multiple regression (regress). To maintain the integrity of our a priori study design in which pairing of peer partners was a key element, we conducted all analyses clustering by assigned pairs in the RPS arm and by sham pairs created by matching age-matched participants in the NCM arm (37). If RPS patients requested reassignment to another peer (2 cases), they were analyzed according to their initial pairing. All analyses were intention-to-treat. We also conducted alternative analyses adjusting for potential clustering by cohort and by site with no differences in our results.
Treatment of Missing Data
Six-month HbA1c data were missing for 28 randomized participants (11%). We therefore conducted a second analysis that imputed missing data (38, 39). In a third sensitivity analysis we examined the worst-case scenario that baseline HbA1c values of participants lacking 6-month HbA1cs remained unchanged at 6 months. Our results in both of these were unchanged. The funding sources had no role in study design, conduct, analysis or decision to submit findings for publication.
Results
Participant flow, baseline data, level of engagement and other outpatient health care utilization
The CONSORT diagram in Figure 1 shows participant flow. Of the 1171 patients who were contacted, 244 (21%) were enrolled: 126 were randomized to RPS, and 119 to NCM. One RPS participant incorrectly completed written informed consent and then refused to correct it so did not receive the intervention. Participants’ baseline characteristics and assessment results are reported in Table 1. There were no statistically significant differences between groups in any measure.
Figure 1.
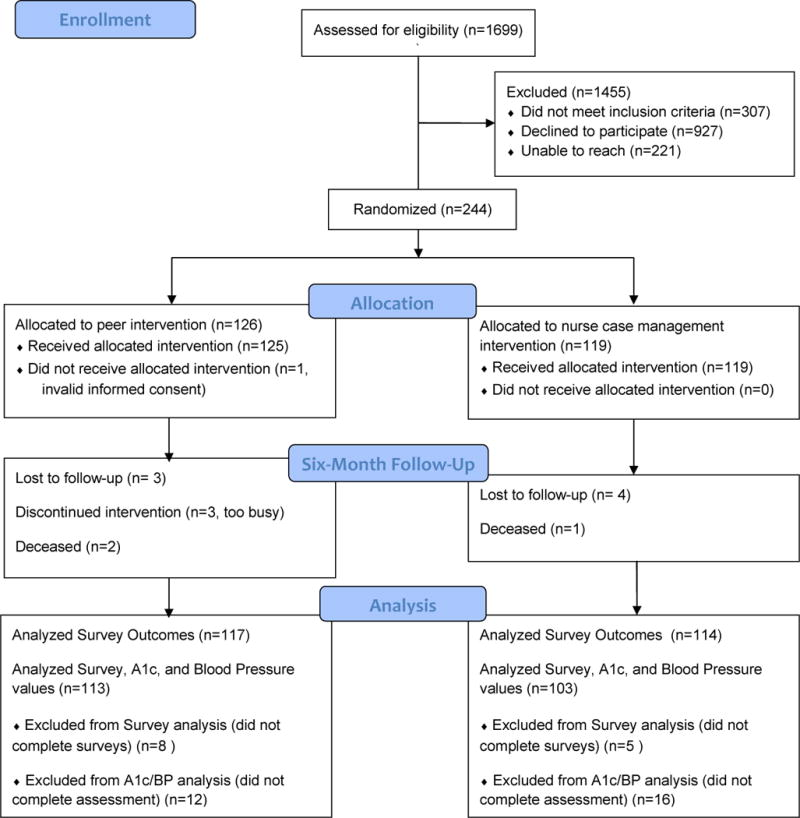
CONSORT 2010 Flow Diagram
Table 1.
Participant Baseline Characteristics (n=244)*
| Characteristic | Reciprocal Peer Support n = 125 n(%) or mean(SD) |
Nurse Case Management n = 119 n(%) or mean(SD) |
Between-Group Difference P-value | Total n = 244 n(%) or mean(SD) |
|---|---|---|---|---|
| Age in years | 61.8(6.1) | 62.3(6.6) | 0.26 | 62.0(6.3) |
| Male | 125(100%) | 119(100%) | – | 244(100%) |
| Race or Ethnicity | 0.54 | |||
| White, non-Hispanic | 99(80%) | 98(84%) | 197(82%) | |
| Hispanic | 5(4%) | 2(2%) | 7(3%) | |
| Black | 11(9%) | 11(9%) | 22(9%) | |
| Other | 9(7%) | 5(4%) | 14(6%) | |
| Education | 0.67 | |||
| Some High School, High School Graduate, or General Educational Development (GED) | 34(27%) | 35(30%) | 69(28%) | |
| ≥ Some college, technical, or vocational | 91(73%) | 83(70%) | 174(72%) | |
| Annual Income, $ | 0.94 | |||
| ≤ 30,000 | 78(63%) | 71(63%) | 149(63%) | |
| ≥ 31,000 | 46(37%) | 41(37%) | 87(37%) | |
| Social Support | ||||
| Lives with a spouse | 82(66%) | 84(71%) | 0.40 | 166(68%) |
| Lives alone | 29(23%) | 22(18%) | 0.37 | 51(21%) |
| Travels an hour or more to appointments | 33(26%) | 37(31%) | 0.42 | 70(29%) |
| Diabetes Social Support† | 55.1(24.5) | 53.3(25.4) | 0.72 | 54.2(24.9) |
| Diabetes Distress‡ | 26.5(16.4) | 26.4(19.8) | 0.52 | 26.4(18.1) |
| Anti-hyperglycemic Medications | 0.93 | |||
| Only oral diabetes medication | 55(44%) | 53(45%) | 108(44%) | |
| Insulin, +/− oral medication | 70(56%) | 66(55%) | 136(56%) | |
| Medication Adherence | ||||
| Misses ≥1 insulin dose per week | 52(74%) | 48(73%) | 0.84 | 100(74%) |
| Misses ≥1 oral med dose per week | 71(72%) | 66(66%) | 0.38 | 137(69%) |
| Self-Rated General Health Status | ||||
| Fair or Poor health | 59(47%) | 56(47%) | 0.98 | 115(47%) |
| Physiological Tests | ||||
| HbA1c | 8.02(1.32) | 7.93(1.40) | 0.70 | 7.98(1.40) |
| Systolic Blood Pressure | 140.3(18.6) | 136.4(16.9) | 0.96 | 138.4(17.9) |
| Diastolic Blood Pressure | 77.1(11.5) | 75.8(10.7) | 0.81 | 76.5(11.1) |
Baseline characteristics are the results from surveys and blood draws taken during the first group session.
“Diabetes Support” was assessed using 6 questions from the Diabetes Support Scale (40). Each question had 6 answer choices, ranging from “Strongly Disagree” to “Strongly Agree” The answers were scored from 0 to 5 points, with higher scores indicating higher levels of diabetes social support, and the total score was calculated as a percentage of possible points.
“Diabetes Distress” was assessed using 14 questions from the Diabetes Distress Scale (41). Each question had 5 answer choices, ranging from “Not a problem” to “Serious problem.” The answers were scored from 0 to 4 points, with higher scores indicating higher levels of distress, and the total score was calculated as a percentage of possible points. T-test used for continuous measures of age, physiological tests, Diabetes Distress and Diabetes Social Support.
χ2 Used for all other categorical variables.
Figure 2 shows average duration and number of recorded calls in each month among the 90% of peer pairs who had at least one conversation. 20 pairs reported talking without using the system. 61% of RPS participants attended the month 1 group session, 59% the month 3 session, and 63% the six-month session. 40% attended all three optional sessions, 26% attended two, and 12% attended one. During the intervention period, there were no significant differences between groups in additional case manager contacts (mean RPS 1.24 [SD 1.58] vs. mean NCM 1.48 [SD 1.50] or physician visits (mean RPS 1.70 [SD 1.23] vs. mean NCM 1.57 [SD 0.99]). The range of case manager encounters was 0–7 in RPS and 0–8 in NCM. There were no adverse events including severe hypoglycemia reported in either group.
Figure 2.
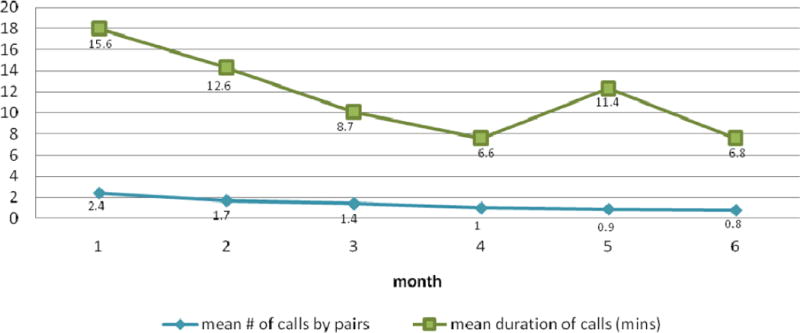
Distribution of Interactive Voice Response (IVR) Telephone Calls Data
Six-month outcomes
Table 2 reports changes in study measures from baseline to six-month follow-up. RPS participants had a mean baseline HbA1c of 8.02%, which improved to 7.73% at six months (−0.29%). NCM participants had on average an increase in HbA1c (7.93 to 8.22 [+0.29]). The difference in the change in HbA1c between the two groups was −0.58% (p=0.004). In stratified analyses, among patients with baseline HbA1c >8.0%, RPS participants had a mean decrease of 0.88% at six months compared with 0.07% among NCM participants (p<0.001) (analyses not shown). Mean blood pressures at baseline in both groups were relatively good (mean systolic blood pressure 138.4, SD 17.9) (42) and decreased slightly, with no significant differences between groups (see Table 2).
Table 2.
Changes in Outcome Measures from Baseline to 6months
| Reciprocal Peer Support (RPS)* | Nurse Care Management (NCM)† | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | 6 month | Change | Baseline | 6 month | Change | Between-group differences (p-value) | |
|
|
|
||||||
| A. Physiological Measures | |||||||
| Hemoglobin HbA1c (mean[SD]) | 8.02(1.32) | 7.73(1.32) | −0.29 | 7.93(1.40) | 8.22(1.74) | 0.29 | .004 |
| Systolic Blood Pressure (mean[SD]) | 140.3(18.6) | 136.9(16.8) | −3.4‡ | 136.4(16.9) | 135.0(17.7) | −1.4 | .91 |
| Diastolic Blood Pressure (mean[SD]) | 77.1(11.5) | 76.8(11.9) | −0.3 | 75.8(10.7) | 76.1(10.6) | 0.3 | .10 |
| B. Medication Change Measures (from Medical Chart Review) | |||||||
| Insulin Starts (n)‖ | – | – | +8 | – | – | +1 | .02 |
| % participants with insulin dose increase | 38.5 | 43.3 | .56 | ||||
| % participants with increase in oral anti-hyperglycemic medications (dose or number) | 16.5 | 17.2 | .90 | ||||
| % participants with increase in blood pressure medications (dose or number) | 24.0 | 24.4 | .95 | ||||
| Mean # of insulin dose increases (SD) | 1.50(.78) | 1.31(.54) | .28 | ||||
| Mean # of oral anti-hyperglycemic increases (SD) | 1.41(.62) | 1.00(.00) | .01 | ||||
| Mean # blood pressure medication increases (SD) | 1.40(.86) | 1.41(.78) | .95 | ||||
| C. Self-Reported Measures (from Surveys) | |||||||
| Diabetes Social Support¶ (mean[SD]) | 55.1(24.5) | 66.5(19.5) | 11.4‡ | 53.3(25.4) | 57.8(25.8) | 4.5 | .01 |
| Diabetes Distress** (mean [SD]) | 26.5(16.4) | 22.8(17.2) | −4.0‡ | 26.4(19.8) | 20.8(18.8) | −5.6§ | .24 |
| Medication Adherence | |||||||
| Misses ≥1 insulin dose per week | 52(74%) | 49(69%) | −3(−5%) | 48(73%) | 38(63%) | −10(−10%) | .67 |
| Misses ≥1 oral medication dose per week | 71(72%) | 65(70%) | −6(−2%) | 66(66%) | 59(63%) | −7(−3%) | .54 |
For RPS participants, baseline values for all measures reflect the total number of 125 participants. For measures related to six month follow-up, 117 RPS participants completed a six month follow-up patient survey and 113 provided six month follow-up physiologic measures.
For NCM participants, baseline values for all measures reflect the total number of 119 participants. For measures related to six month follow-up, 114 NCM participants completed a six month follow-up patient survey and 103 provided six month follow-up physiologic measures.
Statistically significant difference at p<.05 within the RPS group.
Statistically significant difference at p<.05 within the NCM group.
Data from both medical chart review and patient survey report
“Diabetes Social Support” was assessed using 6 questions from the Diabetes Support Scale (40). Each question had 6 answer choices, ranging from “Strongly Disagree” to “Strongly Agree”. The answers were scored from 0 to 5 points, with higher numbers signifying higher social support, and the total score was calculated as a percentage of possible points.
“Diabetes Distress” was assessed using 14 questions from the Diabetes Distress Scale (41). Each question had 5 answer choices, ranging from “Not a problem” to “Serious problem.” The answers were scored from 0 to 4 points, with higher scores signifying more distress, and the total score was calculated as a percentage of possible points.
As Table 2 shows, there were more insulin starts among RPS than NCM participants (8 vs. 1, p=0.02), and on average among patients on oral anti-hyperglycemic medications more intensification events (1.41 vs. 1.00, p=0.01). RPS participants reported greater increases in diabetes-specific social support at six-months (+11.4 vs. +4.5, p=0.01). Besides insulin starts, there were no differences between groups at follow-up in number of other medication intensification events, self-reported medication adherence, or diabetes-specific quality of life (Table 2).
COMMENT
Participants randomized to reciprocal peer support achieved HbA1cs on average 0.58% lower than those randomized to nurse care management. RPS patients with baseline HbA1cs >8.0% achieved a mean decrease of 0.88% compared with a 0.07% decrease among NCM participants. These are both statistically and clinically significant differences. The UK Prospective Diabetes Study found that a 0.5% mean difference in HbA1c translates into a 2.8% absolute risk reduction in diabetes events over a 10 year period, or a number needed to treat of 36 (43). There were more insulin starts among RPS participants and higher reported diabetes-specific social support at six months, but no differences in blood pressure, self-reported medication adherence or diabetes distress.
By mobilizing patients to help each other, peer support interventions are less resource-intensive than many diabetes management programs. When six-month outcomes were assessed, besides for the peer telephone calls, the 46% of RPS participants who attended the initial, 1-month and 3-month group sessions had 4.5 hours in face-to-face meetings over the six-month period more than what all NCM participants received. This is far less time-intensive than other diabetes self-management programs that achieved similar or smaller improvements in glycemic control (1, 44, 45). Moreover, introduction of most oral medications as monotherapy leads to HbA1c decreases of 0.5 to 1.0%, similar to the reductions achieved in this intervention (41).
These findings reinforce evidence from observational and non-randomized studies suggesting health benefits from both receiving and giving social support (20, 40, 46, 47) and address the call by two recent Cochrane Reviews for high-quality research on the clinical effectiveness of peer support in chronic disease management (10, 11). This RCT is the first to demonstrate benefits from a reciprocal model of peer support. In addition, because we conducted our trial as an effectiveness trial with nurse case managers providing care as part of their normal duties, the results are more likely to be replicated in other real-life clinical settings.
Further work is necessary to disentangle the relative contributions of the different intervention components, i.e., the group sessions versus the one-on-one peer telephone calls, to the intervention’s success. Future research also will need to examine correlates of successful peer partner pairings as well as assess mediators and moderators of intervention effects in both quantitative and qualitative assessments. The success of peer support is hypothesized to be due in part to the nonhierarchical, reciprocal relationship created through sharing similar life experiences (6). Many sources of patient resistance to initiating and intensifying insulin therapy lend themselves to peer support (8, 48–50). Our findings of higher rates of insulin starts in RPS compared to NCM suggests that patients’ experiential concerns about insulin may indeed be best addressed with another person also coping with insulin management. We did not gather data on changes in diet, exercise, or weight that may have also contributed to the HbA1c improvements.
Our study had a number of limitations. First, it only included male patients. While peer support interventions have been found to be more acceptable to women than men (6, 51), research also suggests that more similar peers are more likely to have mutually supportive peer relationships (6, 51). Peer support initiatives may thus be especially effective among participants with common identity bonds, such as shared experiences, cultural and ethnic backgrounds, or religious faith. We targeted veterans in the same age cohort, who often have a common sense of ‘veteran identity’ due to shared military experience within a specific socio-historical context (52). It will thus be important to replicate this study in other gender-mixed samples of patients. It will also be important to examine the effectiveness of different peer support models among patients facing a range of self-management challenges. The success of this intervention can help guide other efforts to combine periodic group sessions, care management support and peer communication to initiate and support other behavior changes in diabetes and other conditions requiring high levels of self-management (e.g., obesity, heart failure, chronic pain, physical inactivity).
Second, this intervention only lasted six months. It will be important to test peer support interventions such as the one we tested over longer periods of time. One advantage of peer support interventions is their potential to provide flexible, longer-term self-management support (53). Third, while nurse care managers and all other providers were blinded to the study’s hypotheses, the nature of the intervention prevented blinding to treatment group. Moreover, because the same care managers provided care to patients in both arms, treatment bias cannot be excluded. However, the nurse care managers might be expected to be invested in showing that their current provision of care was superior to between-patient support, so the most likely effect of any treatment bias would be toward the null. It is also important to note that this intervention focused exclusively on activating patients to improve their self-management. A more powerful intervention would also target provider behaviors (e.g., encourage provider medication initiation and intensification). A final limitation is the relatively low rate of uptake of the intervention. Peer-based programs such as this would need to be part of a menu of options available to patients. It is also possible that with greater acceptance of novel programs such as this, uptake rates would increase.
In conclusion, periodic nurse-facilitated, patient-driven group sessions supplemented with one-on-one peer-support telephone calls between age-matched partners improved glycemic control and other key outcomes more than provision of nurse care management services alone among male diabetes patients. Because many chronically-ill patients need more support for self-care than most healthcare systems can provide, models such as this that increase the quality and intensity of assistance through peer support deserve further exploration. Reciprocal peer models can be an effective and efficient approach for helping patients help each other and themselves.
Acknowledgments
The Principal Investigators Michele Heisler and John Piette had full access to all of the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. We thank the very dedicated VA nurse care managers who participated in this intervention as part of their VA work assignments: Debra Beaulieu, Amanda Benadum, Christine Bulifant, Marion Cooper, Joanne Donovan, Janette Elwing, Kelly Johnston, Jacqueline Hurd, Wendy Morrish, Alex Paul, Joe Pawelezyke, Carol Peterson, Maria Reno, Ken Sizemore, and Diane Sobecki-Ryniak. We also thank VA clinical leaders Denise Ramsey, Jennifer Walch, Adam Tremblay, Thomas Gross, and Susan Meade. Many thanks also to Barbara Stanislawski and Jennifer Burgess who successfully conducted all participant recruitment, helped with facilitation and successful execution of the group sessions, and with data collection. Martha Funnell and Mary Lou Gillard provided initial training to the VA nurse care managers in group facilitation, assisted in the creation of the peer partner workbooks, and documented intervention fidelity through periodic observation of group sessions. Many thanks to Mary Rogers, PhD for technical assistance with study design and analysis.
Role of Funding Sources
This research was supported by the VA HSR&D Grant No. IIR 04–239, the Michigan Diabetes Research and Training Center (NIH Grant 5P60-DK20572), the Robert Wood Johnson Foundation Clinical Scholars Program, and the Michigan Institute for Clinical and Health Research (NIH #UL1RR024986). John Piette is a VA Research Career Scientist. The authors have no conflict of interest or financial disclosures. The funding sources had no role in the study design; data collection; administration of the interventions; analysis, interpretation, or reporting of data; or decision to submit the findings for publication.
References
- 1.Norris SL, Nichols PJ, Caspersen CJ, et al. The effectiveness of disease and case management for people with diabetes. A systematic review. Amer J Prev Med. 2002;22(4 Suppl):15–38. doi: 10.1016/s0749-3797(02)00423-3. [DOI] [PubMed] [Google Scholar]
- 2.Shojania KG, Ranji SR, McDonald KM, et al. Effects of Quality Improvement Strategies for Type 2 Diabetes on Glycemic Control: A Meta-Regression Analysis. JAMA. 2006;296(4):427–440. doi: 10.1001/jama.296.4.427. [DOI] [PubMed] [Google Scholar]
- 3.Stanford University, UCSF Evidence-based Practice Center. Evidence-based Practice Center Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies. Vol. Volume 2 – Diabetes Mellitus. AHRQ. 2004 http://www.ahrq.gov/downloads/pub/evidence/pdf/qualgap2/qualgap2.pdf. Accessed June 24, 2010.
- 4.American College of Physicians. Patient-Centered Medical Home. Philadelphia, PA: 2010. http://www.acponline.org/running_practice/pcmh/. Accessed June 24, 2010. [Google Scholar]
- 5.Donaldson L. Expert patients usher in a new era of opportunity for the NHS. BMJ. 2003;326:1279–1280. doi: 10.1136/bmj.326.7402.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dennis CL. Peer Support within a health care context: a concept analysis. Internat J Nurs Stud. 2003;40(3):321–32. doi: 10.1016/s0020-7489(02)00092-5. [DOI] [PubMed] [Google Scholar]
- 7.Hayward RA, Manning WG, Kaplan SH, Wagner EH, Greenfield S. Starting insulin therapy in patients with type 2 diabetes: effectiveness, complications, and resource utilization. JAMA. 1997;278(20):1663–1669. [PubMed] [Google Scholar]
- 8.Vijan S, Hayward R, Ronis DL, Hofer TP. The burden of diabetes therapy and its implications for the design of effective patient-centered treatment. JGIM. 2005;20:479–482. doi: 10.1111/j.1525-1497.2005.0117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saaddine JB, Cadwell B, Gregg EW, et al. Improvements in Diabetes Processes of Care and Intermediate Outcomes: United States, 1988–2002. Ann Intern Med. 2006;144(7):465–474. doi: 10.7326/0003-4819-144-7-200604040-00005. [DOI] [PubMed] [Google Scholar]
- 10.Dale J, Caramlau IO, Lindenmeyer A, Williams SM. Peer support telephone calls for improving health (review) The Cochrane Database of Systematic Reviews. 2008 doi: 10.1002/14651858.CD006903.pub2. The Cochrane Library(4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doull M, O’Connor AM. Peer Support Strategies. Cochrane Database of Systematic Reviews. 2005 [Google Scholar]
- 12.Clancy DE, Huang P, Okonofua E, Yeager D, Magruder KM. Group visits: promoting adherence to diabetes guidelines. J Gen Intern Med. 2007;22(5):620–4. doi: 10.1007/s11606-007-0150-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trento M, Basile M, Borgo E, et al. A randomised controlled clinical trial of nurse-, dietitian- and pedagogist-led Group Care for the management of Type 2 diabetes. J Endocrinol Invest. 2008;31(11):1038–42. doi: 10.1007/BF03345645. [DOI] [PubMed] [Google Scholar]
- 14.Wagner EH, Grothaus LC, Sandhu N, et al. Chronic care clinics for diabetes in primary care: a system-wide randomized trial. Diabetes Care. 2001;24(4):695–700. doi: 10.2337/diacare.24.4.695. [DOI] [PubMed] [Google Scholar]
- 15.Lorig KR, Sobel DS, Ritter PL, Laurent D, Hobbs M. Effect of a self-management program on patients with chronic disease. Effect Clin Pract. 2001;4(6):256–62. [PubMed] [Google Scholar]
- 16.Lorig K, Ritter PL, Villa FJ, Armas J. Community-based peer-led diabetes self-management: a randomized trial. Diabetes Educ. 2009;35(4):641–51. doi: 10.1177/0145721709335006. [DOI] [PubMed] [Google Scholar]
- 17.Griffiths C, Foster G, Ramsay J, Eldridge S, Taylor S. How effective are expert patient (lay led) education programmes for chronic disease? BMJ. 2007;334:1254–1256. doi: 10.1136/bmj.39227.698785.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glasgow RE, Nelson CC, Strycker LA, King DK. Using RE-AIM metrics to evaluate diabetes self-management support interventions. Am J Prev Med. 2006;30(1):67–73. doi: 10.1016/j.amepre.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 19.Heisler M, Piette JD. “I help you, and you help me”: facilitated telephone peer support among patients with diabetes. Diabetes Educ. 2005;31(6):869–79. doi: 10.1177/0145721705283247. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz CE, Sendor M. Helping others helps oneself: response shift effects in peer support. Soc Sci Med. 1999;48(11):1563–1575. doi: 10.1016/s0277-9536(99)00049-0. [DOI] [PubMed] [Google Scholar]
- 21.Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. Amer Psychol. 2000;55(1):68–78. doi: 10.1037//0003-066x.55.1.68. [DOI] [PubMed] [Google Scholar]
- 22.Bandura A. Social Foundations of Thought and Action. A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice-Hall; 1986. [Google Scholar]
- 23.Heisler M, Bouknight RR, Hayward RA, Smith DM, Kerr EA. The relative importance of physician communication, participatory decision making, and patient understanding in diabetes self-management. J Gen Intern Med. 2002;17(4):243–252. doi: 10.1046/j.1525-1497.2002.10905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arsie MP, Marchioro L, Lapolla A, et al. Evaluation of diagnostic reliability of DCA 2000 for rapid and simple monitoring of HbA1c. Acta Diabetol. 2000;37(1):1–7. doi: 10.1007/s005920070028. [DOI] [PubMed] [Google Scholar]
- 25.Rollnick S, Mason P, Butler C. Health behavior change: a guide for practicioners. Edinburgh and New York: Churchill Livongstone; 2008. [Google Scholar]
- 26.Funnell MM, Kruger DF, Spencer M. Self-management support for insulin therapy in type 2 diabetes. Diabetes Educ. 2004;30(2):274–80. doi: 10.1177/014572170403000220. [DOI] [PubMed] [Google Scholar]
- 27.Bodenheimer T, Handley MA. Goal-setting for behavior change in primary care: an exploration and status report. Patient Educ Couns. 2009;76(2):174–80. doi: 10.1016/j.pec.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111(5):697–716. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- 29.Heisler M, Faul JD, Hayward R, Langa K, Blaum CS, Weir D. Mechanisms for Racial and Ethnic Disparities in Glycemic Control in Middle-Aged and Older Americans in the Health and Retirement Study. Arch Intern Med. 2007;167(17):1–8. doi: 10.1001/archinte.167.17.1853. [DOI] [PubMed] [Google Scholar]
- 30.Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28(3):626–31. doi: 10.2337/diacare.28.3.626. [DOI] [PubMed] [Google Scholar]
- 31.Barrera M, Jr, Glasgow RE, McKay HG, Boles SM, Feil EG. Do Internet-based support interventions change perceptions of social support?: An experimental trial of approaches for supporting diabetes self-management. Amer J Community Psychol. 2002;30(5):637–54. doi: 10.1023/A:1016369114780. [DOI] [PubMed] [Google Scholar]
- 32.Cohen J. Statistical Power Analysis for the Behavorial Sciences. 2. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 33.StataCorp LP. Stata 11 User’s Guide. 2009. [Google Scholar]
- 34.Piette JD, Weinberger M, Kraemer FB, McPhee SJ. Impact of automated calls with nurse follow-up on diabetes treatment outcomes in a Department of Veterans Affairs Health Care System: a randomized controlled trial. Diabetes Care. 2001;24(2):202–8. doi: 10.2337/diacare.24.2.202. [DOI] [PubMed] [Google Scholar]
- 35.Campbell MK, Elbourne DR, Altman DG. CONSORT statement: extension to cluster randomised trials. BMJ. 2004;328(7441):702–8. doi: 10.1136/bmj.328.7441.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murray DM. Design and Analysis of Group Randomized Trials. New York: Oxford University Press; 1998. [Google Scholar]
- 37.Imai K, King G, Nall C. The essential role of pair matching in cluster-randomized experiments, with application to the Mexican universal health insurance evaluation. Statistical Science. 2009;24(1):29–53. [Google Scholar]
- 38.Lavori PW, Dawson R, Shera D. A multiple imputation strategy for clinical trials with truncation of patient data. Stat Med. 1995;14(17):1913–25. doi: 10.1002/sim.4780141707. [DOI] [PubMed] [Google Scholar]
- 39.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: Wiley Publishing Co; 1987. [Google Scholar]
- 40.Gallant MP. The influence of social support on chronic illness self-management: a review and directions for research. Health Educ Behav. 2003;30(2):170–95. doi: 10.1177/1090198102251030. [DOI] [PubMed] [Google Scholar]
- 41.Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355(23):2427–43. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 42.US Department of Health and Human Services. (National Institutes of Health) The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. 2004. [Google Scholar]
- 43.UKPDS. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 44.Knight K, Badamgarav E, Henning JM, et al. A Systematic Review of Diabetes Disease Management Programs. Amer J Manag Care. 2005;11(4):242–50. [PubMed] [Google Scholar]
- 45.Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self-management education for adults with type 2 diabetes: a meta-analysis of the effect on glycemic control. Diabetes Care. 2002;25(7):1159–71. doi: 10.2337/diacare.25.7.1159. [DOI] [PubMed] [Google Scholar]
- 46.Brown SL, Nesse RM, Vionkur AD, Smith DM. Providing social support may be more beneficial than receiving it: Results from a prospective study of mortality. Psychological Science. 2003;14:320–327. doi: 10.1111/1467-9280.14461. [DOI] [PubMed] [Google Scholar]
- 47.Joseph DH, Griffin M, Hall RF, Sullivan ED. Peer coaching: an intervention for individuals struggling with diabetes. Diabetes Educ. 2001;27(5):703–10. doi: 10.1177/014572170102700511. [DOI] [PubMed] [Google Scholar]
- 48.Weingarten SR, Henning JM, Badamgarav E, et al. Interventions used in disease management programmes for patients with chronic illness-which ones work? Meta-analysis of published reports. BMJ. 2002;325(7370):925. doi: 10.1136/bmj.325.7370.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Korytkowski M. When oral agents fail: practical barriers to starting insulin. Internat J Obes Relat Metab Disord. 2002;26(Suppl 3):S18–24. doi: 10.1038/sj.ijo.0802173. [DOI] [PubMed] [Google Scholar]
- 50.Hunt LM, Valenzuela MA, Pugh JA. NIDDM patients’ fears and hopes about insulin therapy. The basis of patient reluctance. Diabetes Care. 1997;20(3):292–8. doi: 10.2337/diacare.20.3.292. [DOI] [PubMed] [Google Scholar]
- 51.Helgeson VS, Cohen S, Schulz R, Yasko J. Group support interventions for women with breast cancer: who benefits from what? Health Psychol. 2000;19(2):107–14. doi: 10.1037//0278-6133.19.2.107. [DOI] [PubMed] [Google Scholar]
- 52.Harada ND, Damron-Rodriguez J, Villa VM, et al. Veteran Identity and Race/Ethnicity: Influences on VA Outpatient Care Utilization. Med Care. 2002;40(1 Supp):117–128. [PubMed] [Google Scholar]
- 53.Tang TS, Gillard ML, Funnell MM, et al. Developing a new generation of ongoing: Diabetes self-management support interventions: a preliminary report. Diabetes Educ. 2005;31(1):91–7. doi: 10.1177/0145721704273231. [DOI] [PubMed] [Google Scholar]


