Abstract
Calreticulin is a calcium-binding chaperone that has several functions in the immune response. In the endoplasmic reticulum (ER), calreticulin facilitates the folding of major histocompatibility complex (MHC) class I molecules and their assembly factor tapasin, thereby influencing antigen presentation to cytotoxic T cells. Although calreticulin is normally ER-resident, it is found at the cell surface of living cancer cells and dying cells. Here, calreticulin promotes cellular phagocytic uptake. In tumor vaccine models, drugs that induce cell-surface calreticulin confer enhanced tumor protection in an extracellular calreticulin-dependent manner. Much remains to be understood about the roles of calreticulin in these distinct functions. Further investigations are important towards advancing basic knowledge of glycoprotein folding pathways, and towards developing new cancer therapeutic strategies.
Keywords: Calreticulin, Protein Folding, Phagocytosis, Anti-tumor immunity, MHC class I, Tapasin
Calreticulin, a multi-functional protein with diverse sub-cellular localizations
The ER is the organelle in which proteins destined for secretion are first deposited for their folding and assembly (Box 1). For assistance with folding of the diverse cellular secretome, a number of chaperones and folding factors are present in the ER, each with different specificity for substrate recognition. Calreticulin is a highly conserved chaperone protein of the ER that has specificity towards glycoprotein substrates (reviewed in [1]). Calreticulin is important for the assembly and cell surface expression of MHC class I molecules and hence for CD8 T cell recognition of antigens presented by MHC class I molecules [2]. Recent studies have provided many insights into how calreticulin is recruited into the MHC class I assembly pathway [3–8]. Calreticulin enhances the stabilities of components of the MHC class I assembly pathway [5, 6] and functions in the retrieval of MHC class I molecules from post-ER compartments for their optimal assembly [9].
Text Box 1. Folding of proteins in the ER.
The ER is enriched in protein chaperones, oxido-reductases, proline isomerases and other folding factors. These chaperones and folding factors facilitate the correct folding and assembly of nascent proteins. Folded secretory proteins are packaged into vesicles for forward transport to Golgi compartments. Whereas properly folded proteins proceed along the cellular secretory pathway into post-Golgi compartments, ER chaperones and sub-optimally assembled proteins are retrieved from the Golgi back to the ER. Specific sequence motifs such as the KDEL motif are required for the retrieval of ER chaperones from the cis-Golgi to the ER. In a process called ER-associated degradation (ERAD), terminally misfolded proteins are targeted for degradation.
Although calreticulin is best known for its ER chaperone functions, recent studies demonstrate that calreticulin can also be expressed on the cell surface. Induction of cell surface calreticulin in dying cells appears to be a feature of some apoptotic and pre-apoptotic cells [10–14] and of cancer cells [15]. Cell-surface calreticulin contributes to the phagocytic uptake of cancer cells and dying cells [10, 11, 13, 15]. Pre-apoptotic exposure of calreticulin has been linked to enhanced immunogenicity of dying tumor cells. The pro-phagocytic function of cell-surface calreticulin is suggested to relate to the immunogenicity of extracellular calreticulin (reviewed in [16, 17]).
Here we examine the multifunctional roles of calreticulin at diverse sub-cellular sites. We examine the role of calreticulin in the folding and assembly of MHC class I molecules and discuss how structural features of calreticulin allow interactions with MHC class I molecules and with components of the MHC class I assembly pathway. Also examined is the role of calreticulin phagocytic uptake of dying cells and tumor cells and data showing that pre-apoptotic cell surface exposure of calreticulin on dying tumor cells is linked to enhanced tumor protection in vaccine models.
Structural features of calreticulin
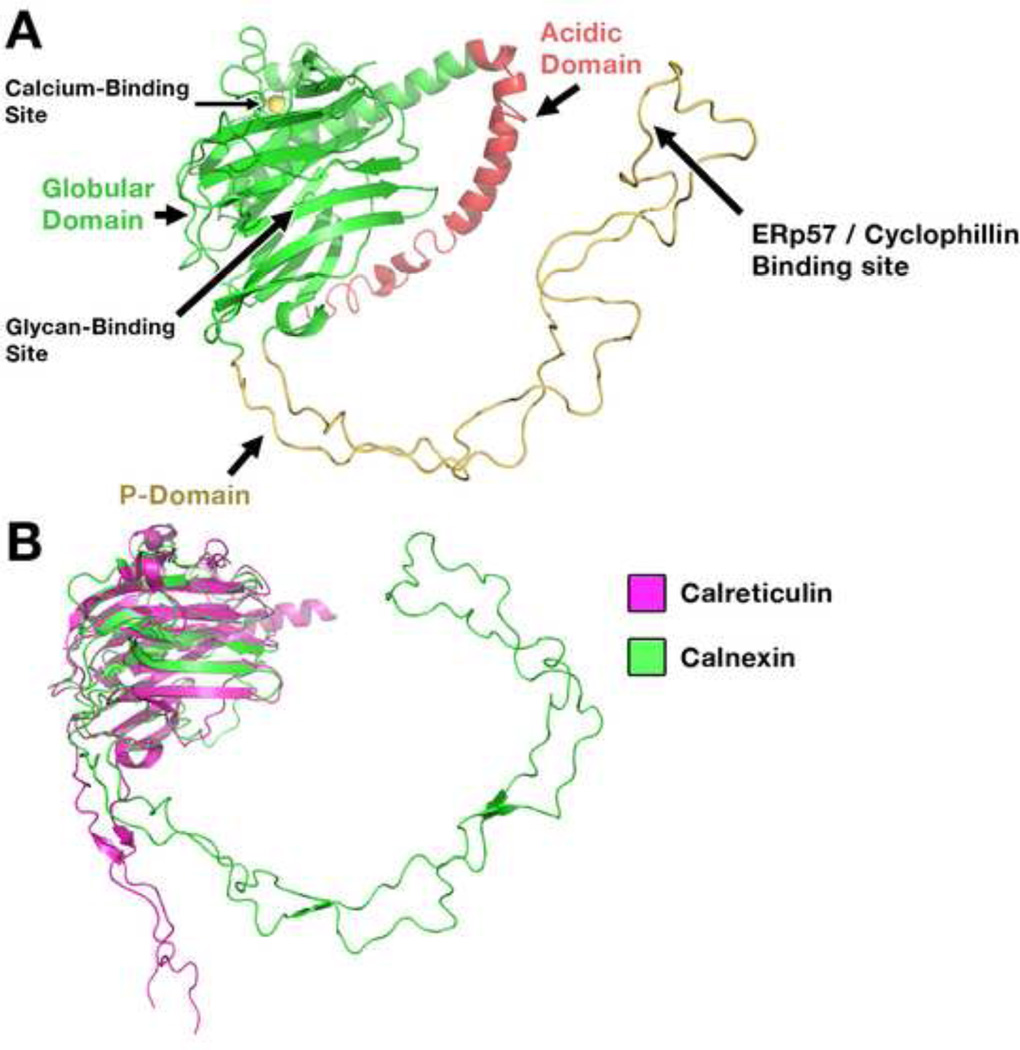
Calreticulin is a structural homolog of the ER chaperone calnexin, although calnexin is membrane-anchored, whereas calreticulin is soluble. The structures of the ER lumenal region of calnexin, and of individual calreticulin domains, have been solved [18–21]. Both calreticulin and calnexin contain a globular domain arranged in legume lectin fold (Figure 1) [18, 20, 21]. Each globular domain comprises a concave β-sheet together with a convex sheet. A C-terminal α-helix is also present in the globular domain. A single conserved high affinity calcium-binding site in the globular domains of both calreticulin and calnexin confers structural stability to the domains [18, 20, 21]. Glycan binding sites are present on the concave β-sheets of calnexin and calreticulin [18, 20], both of which have specificities for mono-glucosylated glycans. A number of studies also suggest that calreticulin and calnexin can interact with polypeptide substrates in a glycan-independent manner (for example, [22, 23]). The precise locations of the polypeptide binding sites in calreticulin and calnexin have remained elusive, although recent structural studies suggest that the periphery of the glycan binding site of calreticulin could be involved in glycan-independent interactions [21]. However, further studies are needed to better understand whether this putative binding site functions as a generic polypeptide recognition site.
Figure 1. Structural features of calreticulin and their functional relevance A.
A) The globular domain of calreticulin is depicted in green and contains a glycan binding site that interacts with monoglucosylated glycans of glycoproteins, including those present on MHC class I heavy chains and tapasin. The P domain of calreticulin is depicted in yellow, the tip of which contains a co-chaperone binding site. The predicted structure of the C-terminal acidic region is shown in red. The acidic region contains multiple low affinity calcium binding sites that are important for the maintenance of ER calcium homeostasis. The model was built based on the crystal structures of the globular domain of calreticulin [23] and the NMR structure of the P-domain [19]. Each domain (globular and P-) was modeled independently and combined to generate the final model for calreticulin. The acidic domain was modeled de novo using the loop-building feature in I-TASSER [76]. B) Overlay of the structures of lumenal domain of calnexin [18] and the globular domain of calreticulin with a truncated P-domain [23] depicting the change in angle between the globular and P-domains of calreticulin compared to calnexin. The changes in the orientation of the P domain relative to the globular domain in the two structures suggests flexibility in the conformation of the P domain which may be important to accommodate different substrates.
Both calreticulin and calnexin contain flexible arm-like, proline-rich, P-domains that interrupt and extend from the globular domain [18, 19] (Figure 1A). The P-domains of calreticulin and calnexin include three (calreticulin) or four (calnexin) pairs of repeating sequences that form a paired β-hairpin structure [18, 19]. Crystal structures of soluble calnexin and a truncated calreticulin show variable orientations of the P domain relative to the globular domain (Figure 1B) [18, 23], suggesting that flexible P domain orientations are possible, which may be important during protein folding. The tips of the P-domains of calreticulin and calnexin contain sites for interactions with the thiol oxido-reductase ERp57 [24] as well as the peptidyl-prolyl cis-trans isomerase, cyclophilin-B [25]. Both ERp57 and cyclophilin-B function as co-chaperones for calnexin and calreticulin.
Calreticulin contains a highly acidic C-terminal region (residues 351–359) that binds multiple calcium ions with low affinity [26–28]. The counterpart of this region is absent in the lumenal domains of calnexin. The acidic C-terminus of calreticulin is important for maintenance of cellular calcium homeostasis, and cells deficient in calreticulin have reduced calcium storage capacity in the ER [27]. In mice, total calreticulin deficiency is embryonic lethal due to alterations in cellular calcium homeostasis [29, 30]. The acidic region of calreticulin also plays a role in ER-retention of the protein, as a calreticulin construct lacking this domain is secreted despite the presence of a C-terminal KDEL sequence [31]. The KDEL sequence of calreticulin is conserved in other soluble ER-resident chaperones such as binding immunoglobulin protein (BiP) and protein disulfide isomerase (PDI). The presence of a KDEL motif specifies binding to the KDEL receptor, which functions in the retrieval of KDEL-containing proteins from ER-Golgi intermediate compartments and Golgi back to the ER (reviewed in [32]). The acidic region is also important for the translocation of calreticulin from the ER to the cytosol (retro-translocation) [33], a process that typically occurs with misfolded proteins undergoing degradation (Box 1). In the case of calreticulin, retro-translocation is suggested to be non-degrading [33]. A number of functions are have been proposed for cytosolic calreticulin, including the facilitation of cellular adhesion (reviewed in [34]).
Calreticulin in the folding and assembly of MHC class I molecules
MHC class I molecules comprise a heterotrimer of heavy chain, light chain (β2-microglobulin or β2m) and peptide. Assembly of these components occurs within the ER and is facilitated by specific MHC gene complex-encoded proteins, such as transporter associated with antigen processing (TAP) and tapasin, as well generic ER chaperones including calreticulin, calnexin, and ERp57 (reviewed in [35, 36]). Calnexin is thought to interact with MHC class I heavy chains prior to association of heavy chain with β2m (reviewed in [36]). Peptides that bind to MHC class I heterodimers typically derive from the cytosol, and are transported by TAP into the ER lumen. Tapasin binds to MHC class I molecules, and this interaction is enhanced by ERp57 and calreticulin [7, 8, 37]. Additionally, the transmembrane region of tapasin, which binds TAP, increases the efficiency of tapasin-MHC class I binding [37, 38]. The protein complex comprising TAP, tapasin, ERp57, calreticulin and MHC class I is termed the peptide loading complex (PLC). The PLC facilitates the binding of optimal peptides to MHC class I molecules (reviewed in [39, 40]).
After peptide binding, MHC class I molecules dissociate from the PLC and traffic to the cell surface, where they are available for immune surveillance by cytotoxic T cells (CTL) and natural killer (NK) cells (reviewed in [41]). The assembly of MHC class I molecules with an optimal peptide is strongly correlated to the thermostability of MHC class I, which in turn determines the efficiency and fidelity of the immune response. CTLs recognize complexes of MHC class I molecules bound to antigenic peptides. Unstable MHC class I molecules would be inefficient activators of CTL responses. Additionally, as MHC class I molecules engage NK cell inhibitory receptors, inappropriate cell surface instability of MHC class I could trigger a NK cell response. Thus, there is considerable interest in factors that maintain the overall quality control of MHC class I–peptide assembly.
Calreticulin contributes to the quality control of MHC class I assembly in several ways. First, calreticulin is important for the overall stability of the PLC, as MHC class I recruitment into the PLC is rather inefficient in calreticulin-deficient cells [2, 3, 5]. Second, calreticulin functions in the folding and stabilization of tapasin, as calreticulin can bind tapasin in a glycan-dependent manner, and steady state levels of tapasin are low in calreticulin-deficient cells [5, 6, 8]. Third, calreticulin functions in the retrieval of sub-optimally assembled MHC class I from post-ER compartments, as accumulation of MHC class I in the cis-Golgi is dependent on the KDEL sequence of calreticulin [9]. MHC class I molecules traffic out of the ER at a more rapid rate in calreticulin-deficient cells compared to calreticulin-sufficient cells [2, 3, 6], consistent with a role for calreticulin in the retrieval for sub-optimally assembled MHC class I molecules from post-ER compartments. Calreticulin can bind MHC class I molecules independently of the PLC [7], and this mode of interaction may be relevant to the MHC class I retrieval function of calreticulin [9]. The combined effect of calreticulin’s activity leads to a net reduction in steady state levels of cell-surface MHC class I in calreticulin-deficient cells compared to wild type cells and reduced efficiency of antigen presentation to CTLs, at least for some antigens [2]. It remains to be examined whether immune responses mediated by sub-optimal peptide antigens are enhanced under conditions of calreticulin deficiency.
Optimal function of calreticulin in MHC class I assembly has been reported to require both the glycan and ERp57 binding sites of calreticulin (Figure 1A) [5, 7]. Other studies, however, suggest that these sites are non-crucial for the function of calreticulin in MHC class I assembly [3, 4]. The choice of mutation sites or specific conditions used for the binding assays could explain discrepancies between the studies. Additionally, it is likely that multiple interactions (involving glycan-based, ERp57-based and polypeptide-based binding) are relevant to the functions of calreticulin within the MHC class I assembly pathway, another possibility that could explain the discrepancies between the different studies.
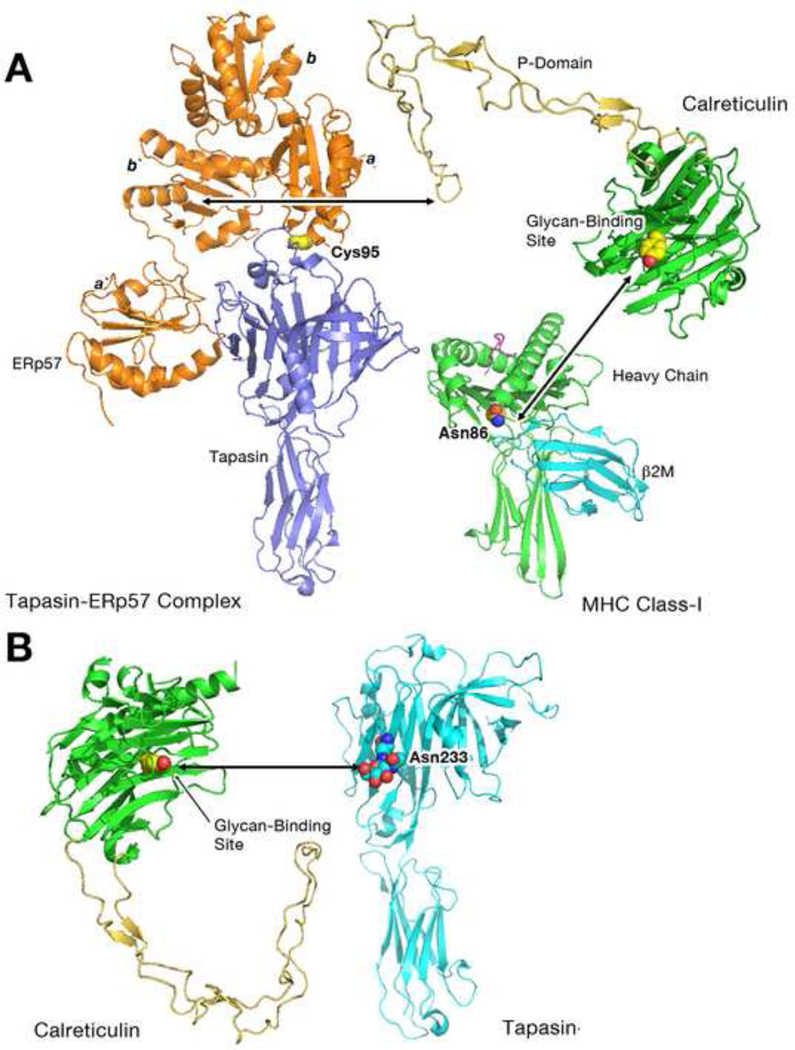
As already described, calreticulin and calnexin have specificity for mono-glucosylated glycans. Mono-glucosylated glycans are transiently present on newly synthesized glycoproteins during their early folding and maturation (reviewed in [1]). MHC class I molecules contain a conserved N-linked glycosylation site at the C-terminal end of their α1 helix (at asparagine 86 (N86)). Tapasin is also a glycoprotein that contains a single N-linked glycosylation site at asparagine 233 (N233). Additionally, tapasin contains a free thiol residue, cysteine 95 (C95), which forms a disulfide-linked conjugate with ERp57 ([42] and reviewed in [36]) (Figure 2). Recruitment of calreticulin to human TAP/tapasin complexes is fully dependent upon C95 and N233 of tapasin [8]. Detailed studies of binding interactions in tapasin-deficient cells that are reconstituted with wild type or mutant forms of tapasin indicate that calreticulin forms two distinct sets of interactions with TAP/tapasin complexes; indirectly, via tapasin(C95)-linked ERp57, and directly, via tapasin(N233) [8] (Figure 2). The former interactions are more important for MHC class I recruitment to tapasin/TAP complexes, while tapasin(N233)-dependent interactions may help stabilize tapasin, and/or PLC precursors, prior to MHC class I binding [8].
Figure 2. Modes of calreticulin recruitment to the PLC and to tapasin.
A) Structure of the tapasin-ERp57 [42] and MHC class-I heterodimers [77] to illustrate locations of the MHC class I glycan (N86) and site of conjugation of tapasin to ERp57. In a proposed model for the PLC [42], calreticulin is suggested to engage the glycan of MHC class I via its glycan binding site, and to ERp57 (of tapasin-ERp57 conjugates) via the tip of the P domain. Arrows denote the predicted sites of interaction between the P-domain of calreticulin and the b` domain of ERp57 as well as between the glycan-binding site of calreticulin and the N-linked glycan moiety of MHC class I (linked to N86 and denoted as spheres). B) Putative glycan-mediated interaction [8] between calreticulin and the N-linked glycan of tapasin (N233) [42] (denoted as spheres).
A proposed model for the PLC involves the simultaneous engagement of the MHC class I glycan (in its mono-glucosylated form) and tapasin-ERp57 by calreticulin, via its glycan and ERp57 binding sites respectively [7, 42] (Figure 2A). However there is evidence that that calreticulin and β2m may interact within the PLC, even under heavy chain-deficient conditions ([8] and additional references cites therein). β2m is the non-glycosylated light chain of the MHC class I molecule, and it remains unclear how β2m is stabilized within intermediate PLCs that are deficient in heavy chains. Recent in vitro studies implicate the P-domain in protein-protein interactions mediated by calreticulin [23]. Additionally, whereas mutation of W244 (the ERp57 binding site) on the tip of the P-domain reduces the efficiency of incorporation of calreticulin into the PLC, a complete P domain deletion renders calreticulin recruitment into the PLC undetectable [5]. Given its flexibility and unusual structure, the P-domain is likely to play a role in tethering protein-protein interactions within the PLC. It is possible that the P domain of calreticulin facilitates an interaction between β2m and calreticulin or β2m and tapasin, providing a platform for recruitment of heavy chains and their assembly with β2m. Additional studies will be needed to understand the precise nature of PLC interactions mediated by the calreticulin P domain.
Calreticulin can inhibit the in vitro heat-induced precipitation of many proteins including soluble non-glycosylated recombinant MHC class I molecules [43, 44]. Efficient suppression of non-glycosylated MHC class I precipitation by calreticulin requires heat shock (42 – 50 °C) or calcium-depleting conditions [45]. Calreticulin can also suppress precipitation of other proteins in vitro at 37 °C (for example, [5, 6, 23], but high stoichiometric ratios of calreticulin relative to substrate protein are needed. A number of point mutations in calreticulin, including Y92A and W244A that disrupt glycan and ERp57 binding, respectively, induce the ability of calreticulin to suppress protein precipitation, but interfere with the function of calreticulin in MHC class I assembly in cells [5, 6]. Thus, the ability of calreticulin to suppress protein precipitation may reflect interactions relevant to cell stress conditions rather than those relevant to calreticulin-dependent protein folding under normal (non-stress) conditions. The secondary structure of calreticulin has been shown to be remarkably thermostable, even with heating to high temperatures (60 °C) [28]. Rather than melting, heat-induced tertiary structural changes in calreticulin expose hydrophobic residues and reduce protein rigidity. Heat-shock (42–50 °C) and other destabilizing conditions also induce the formation of ordered protein oligomers of calreticulin both in vitro and in cells [44–46]. These unusual structural features of calreticulin are undoubtedly relevant to the functional activity of calreticulin in the suppression of protein precipitation/aggregation under conditions of cellular stress, such as those that arise from protein misfolding in the ER (reviewed in [47]), a process that can eventually disrupt cellular calcium homeostasis.
It appears likely that calreticulin contributes to MHC class I assembly through a combination of interactions involving glycan-dependent and glycan-independent modes of binding, as well as ERp57-linked interactions. These interactions contribute to the stability of calreticulin recruitment to the PLC, and to the stabilizing functions of calreticulin within the PLC. The specific sequence of binding events and the precise nature of protein-protein interactions sites remain to be elucidated. Under conditions such as heat-shock and calcium depletion calreticulin undergoes structural changes that enhance its ability to interact with and suppress precipitation of proteins, independently of their glycan content. Although more studies are required to understand the nature of physiological and stress-induced protein-protein interaction sites used by calreticulin, it is likely that distinct protein-protein interaction surfaces are involved.
Mechanisms of cell surface expression of calreticulin
Calreticulin can also be expressed on the cell surface and is relevant for phagocytic uptake and immunogenicity of cells. It is intriguing how calreticulin, a soluble ER chaperone, arrives at and is retained at the cell surface. In 2005, it was described that calreticulin is up-regulated and redistributed on the cell surface of UVB light-induced apoptotic cells, to become co-localized with phosphatidylserine (PS) [10]. Recent in vitro binding experiments indicate that calreticulin can also bind to PS in a calcium-dependent manner [48, 49]. As described above, a fraction of the total cellular calreticulin is thought to be localized in the cytosol following its retro-translocation from the ER [33]. One suggested mechanism for the surface exposure of calreticulin involves association of cytosolic calreticulin with PS on the inner leaflet of the plasma membrane, thereby allowing calreticulin to become exposed during apoptosis [49].
Other pathways have also been proposed for the cell-surface expression of calreticulin. Several studies have reported expression of calreticulin-ERp57 complexes on the surface of pre-apoptotic cells after exposure to anthracyclin chemotherapeutics (such as mitoxantrone) and platinum-based chemotherapeutics (such as oxaliplatin) [11, 12, 14, 50, 51]. A vesicular transport mechanism involving the cellular secretory pathway appears to be involved in calreticulin externalization by anthracyclin chemotherapeutics [14]. Cell surface expression of calreticulin is suggested to involve activation of pancreatic ER kinase (PERK), induction of reactive oxygen species, pre-apoptotic cleavage of caspase 8, activation of pro-apoptotic molecules, Bcl-2-associated X protein (BAX) and Bcl-2-homologous antagonist/killer (BAK), and ER calcium efflux [14].
Cellular stress conditions (such as protein misfolding) that interfere with ER homeostasis and function are collectively referred to as ER stress. PERK is a component of the three-pronged unfolded protein response (UPR) pathway that is induced by ER stress [47]. The suggestion that PERK and intracellular calcium are involved in cell surface calreticulin translocation [14], raises the question of whether other forms of ER stress can induce calreticulin translocation to the extracellular space. Although some studies implicate intracellular calcium depletion in cell surface calreticulin translocation [52], other studies from the same group report poor induction of cell-surface calreticulin in response to ER stress-inducing and ER calcium-depleting drugs [11]. In addition, early studies showed that ER calcium depletion results in the secretion of ER resident proteins, including calreticulin [53]. A recent study attempted to clarify these different findings by examining the influences of different forms of ER stress upon calreticulin cell surface expression. Treatment of mouse embryonic fibroblasts with thapsigargin, an inhibitor of the sarcoplasmic reticulum endoplasmic reticulum calcium ATPase (SERCA-1) [54], induces pre-apoptotic cell-surface exposure of calreticulin and the secretion of calreticulin, PDI, BiP and gp96 from the ER. Calreticulin cell surface expression is detected at early time points after thapsigargin exposure on cells that retain membrane integrity (non-necrotic cells) and lack PS exposure (a marker of apoptotic cells [55]) [13]. The calreticulin-ERp57 interaction is not required for surface calreticulin exposure induced by thapsigargin, and release of ER chaperones from thapsigargin-treated cells appears to involve the secretory pathway, as secretion is inhibited by Brefeldin A, and glycosylated forms of gp96 are detectable in cell supernatants (a cytosolic exposure pathway would cleave the glycans) [13]. The chaperone activity of calreticulin that is induced by calcium-depleting conditions appears to be relevant to cell-surface expression of calreticulin in thapsigargin-treated cells [6]. An ERp57-independent secretory pathway is also relevant to calreticulin cell surface expression in cells treated with hypericin, an ER-associated photosensitizer that inactivates SERCA-2 and induces disruption of ER calcium homeostasis [56]. Calreticulin surface exposure in hypericin-treated cells was also observed on cells that largely retained plasma membrane integrity.
Thus, three main mechanisms have been proposed for cell surface calreticulin exposure on dying and stressed cells: cell surface translocation of cytosolic calreticulin during apoptosis [49], and ERp57-dependent [14], or ERp57-independent [13, 56] translocation of calreticulin into the extracellular environment via the secretory pathway. The influence of thapsigargin treatment upon secretion of calreticulin and other ER chaperones suggests that ER chaperone externalization may be a more general feature of some physiological forms of ER stress [13]. Cell surface expression of calreticulin was detected on a number of different primary human cancer cells [15]. Understanding the mechanisms for calreticulin cell surface expression in human cancers could further the design of therapies to enhance uptake of cancer cells by phagocytes. In another example of extracellular calreticulin expression that is relevant for the immune system, calreticulin is a component of lytic granules of cytotoxic T cells. Following cellular activation, calreticulin becomes exocytosed together with perforin and granzymes [57]. Further studies are needed to understand how calreticulin translocates to lytic granules from the ER, as well as the precise role of calreticulin in modulating the activity of lytic granules.
Calreticulin in cellular phagocytic uptake
Cell-surface expression of calreticulin promotes the phagocytic uptake of dying, stressed and cancer cells [10, 11, 13, 15]. The pro-phagocytic function of calreticulin is inhibited by CD47 (integrin-associated protein) [10] (Figure 3), which was previously described as an anti-phagocytic signal on red blood cells [58]. Both calreticulin and CD47 are up-regulated on the surface of live primary human or murine cancer cells [15, 59, 60]. In pre-clinical studies, anti-CD47 antibodies were therapeutic in a number of cancer progression models [61–64]. Calreticulin is suggested to be the dominant pro-phagocytic signal in several human cancers that allows for the therapeutic function of anti-CD47 [15] (Figure 3). Human monocyte-derived macrophages mediate calreticulin-dependent uptake of a number of primary cancer cells and cancer cell lines in the presence of anti-CD47. Conversely, as CD47 expression is lower on normal cells, the presence of exogenous recombinant calreticulin promotes phagocytic uptake of normal bone marrow cells by human macrophages. Low density lipoprotein-related protein 1 (LRP-1 or CD91) is the postulated macrophage receptor that mediates calreticulin-dependent uptake of cancer cells [15]. Together, these studies indicate that the calreticulin-CD47 balance is an important determinant of cellular phagocytic uptake and manipulating this balance may be an important route to induce stronger anti-tumor immunity. Further studies are needed to understand calreticulin and CD47 expression in different human cancers, and the different ways in which the balance can be shifted. There may also be differences in the plasma membrane localization and mobility of CD47 between human and mouse cells that could impact on phagocytosis [65], a possibility that needs further investigations.
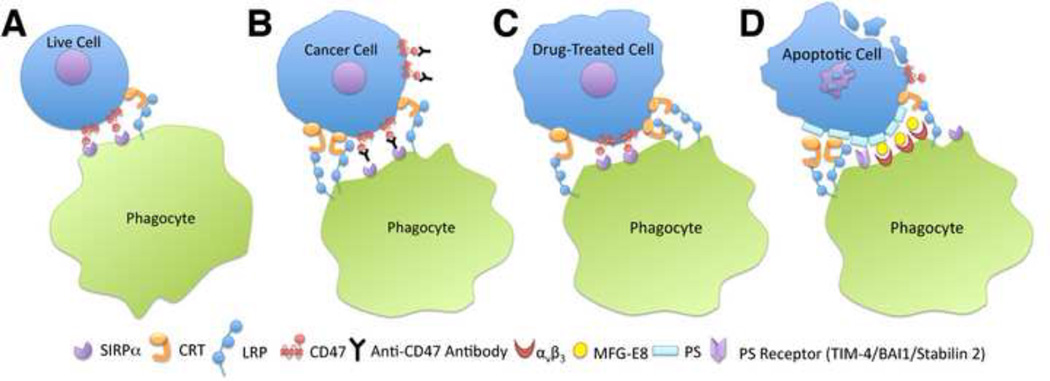
Figure 3. “Eat-me” and “don’t-eat-me” signals in cellular phagocytic uptake.
A) Normal healthy cells express low amounts of the “eat me” signal calreticulin, and higher amounts of the “don’t eat me” signal CD47. Thus, live cells are not phagocytosed [10]. B) Live cancer cells up-regulate calreticulin relative to normal healthy cells. However, cancer cells also up-regulate CD47 to evade phagocytic uptake [15]. Anti-CD47 antibodies are shown to have therapeutic effects in different types of cancers, and enhance cancer cell phagocytic uptake. C) Treatments of cells with certain chemotherapeutic drugs and ER stress-inducing drugs causes up-regulation of calreticulin, and enhances phagocytic uptake by dendritic cells [11, 13] via a mechanism that is at least partially calreticulin-dependent. D) Calreticulin is also detectable in certain apoptotic cell contexts, where it is shown to co-localize with PS [10, 68]. Additionally, apoptotic cells down-regulate or re-distribute CD47. A number of membrane-linked receptors (such as TIM-4, brain angiogenesis inhibitor 1 (BAI1)) recognize PS directly. Soluble extracellular bridging molecules, such as milk fat globule EGF factor 8 (MFG-E8), can also bind PS and facilitate uptake of apoptotic cells by bridging to receptors (αvβ3 integrin) on phagocytes (reviewed in [66, 67]). The balance is shifted towards “eat me,” and a phagocyte engulfs the dying cell. CD91 and SIRPα are suggested receptors for calreticulin and CD47, respectively.
Drug treatments that induce cell surface exposure of calreticulin on pre-apoptotic cells also enhance cellular phagocytic uptake by splenic or bone marrow derived dendritic cells. Phagocytic uptake is calreticulin-dependent, at least to some extent [11, 13]. Several phagocyte receptors have been characterized that interact with PS on the surface of apoptotic cells, either directly, or via soluble bridging molecules (reviewed in [66, 67]). After UV treatment of MEFs, calreticulin is up-regulated and redistributed to become co-localized with PS, and contributes to the phagocytic uptake of apoptotic cells [10]. Calreticulin is also redistributed on the surface of apoptotic Drosophila S2 cells, and plays a role upon in vivo phagocytosis in Drosophila embryos [68]. Other studies indicate that up-regulation of cell-surface CRT in dying cells is dependent upon the specific cell death inducer used, and additionally that pre-apoptotic cells display cell surface calreticulin [11–14]. At least under some conditions, the increased amount of non-specific protein binding to apoptotic cells makes it difficult to observe the specific induction of apoptotic cell surface calreticulin by a magnitude detectable by flow cytometry [13]. Nonetheless, recent in vitro binding experiments indicate that calreticulin can bind to PS in a calcium-dependent manner [48, 49]. Additionally, extracellular calreticulin has been observed in a number of physiological and pathological contexts [69, 70]. Thus, apoptotic cell-surface calreticulin could derive either from an exogenous extracellular source, become endogenously translocated to the cell surface, or become re-distributed from the surface of live or pre-apoptotic cells. In the context of several other PS-dependent phagocytic uptake signals, the precise role for calreticulin in apoptotic cell phagocytosis, as well as the selectivity or universality of calreticulin induction on apoptotic cells remains to be defined. How the PS-binding function of calreticulin relates to the phagocytosis of apoptotic cells is an important area for further investigations. LRP-1 is the proposed phagocytic receptor for calreticulin-dependent uptake of apoptotic cells [10], as well as live cancer cells [15]. In mice, peritoneal macrophages and splenic dendritic cells are shown to mediate calreticulin-dependent uptake of dying cells [10, 11]. A role for LRP-1 in calreticulin-dependent phagocytic uptake remains to be verified in in vivo.
Immunogenicity of calreticulin
There is much interest in the signals conveyed to the immune system by dying cells, and in identification of molecular determinants of immunogenic and tolerogenic forms of cell death (reviewed in [16, 71]). Calreticulin is a key determinant of immunogenic forms of cell death [11, 12, 14, 51, 72]. In tumor vaccine models, subcutaneous immunizations with dying tumor cells that express cell-surface calreticulin results in reduced tumor burden upon subsequent live tumor challenge [11, 12, 14, 51, 72]. Tumor protection requires cell surface calreticulin as well as CD8 and CD4 T cells [11]. Via its pro-phagocytic function, cell-surface calreticulin (induced by chemotherapeutic drugs such as mitoxantrone, and oxaliplatin) is suggested to aid cross-priming of tumor cell antigens to generate CD8 T cell responses. However, calreticulin’s role in phagocytic uptake of dying tumor cells by lymph node antigen-presenting cells remains to be demonstrated in vivo. It is noteworthy that thapsigargin treatment does not confer immunogenicity to dying tumor cells [11, 12], despite thapsigargin-mediated induction of cell-surface and extracellular calreticulin [13].As noted above, there are multiple mechanisms described for calreticulin externalization, and it is possible that the specific mode of calreticulin exposure accounts in part for reported immunogenicity differences between different drug treatments [11]. Furthermore, ATP and/or HMGB-1 are absent in thapsigargin-treated cell supernatants [13], but may be required co-factors for immunogenicity of dying tumor cells (reviewed in [17]). Calreticulin primes Th17 cell responses via a LRP-1-dependent mechanism [73], which could relate to the immunogenicity of extracellular calreticulin. It is also possible that the PS-binding function of calreticulin influences immunogenicity by blocking other PS-dependent interactions, such as those involving T cell/transmembrane, immunoglobulin, and mucin 4 (TIM-4), which are known to be tolerance inducing (reviewed in [74]). Additional studies are needed to fully understand the link between extracellular calreticulin in cell-based vaccines and enhanced tumor protection. In separate studies involving therapeutic tumor models, DNA vaccines encoding calreticulin linked to human papillomavirus E7 antigen confer enhanced tumor protection [75]. The molecular mechanisms are unclear, but could relate to an effect of calreticulin on E7 antigen stability.
Concluding remarks
The intracellular form of calreticulin is important for protein folding and assembly and in maintenance of ER calcium homeostasis. Calreticulin contributes to the quality control of MHC class I assembly, and has multiple roles within the MHC class I pathway. Further studies are needed to understand the contributions of glycan-independent modes of binding to the cellular chaperone activity of calreticulin (towards MHC class I molecules and other substrates). Calreticulin translocates to the cell surface under conditions of cell stress and tumorigenesis, and cell-surface calreticulin is an “eat-me” signal. Further understanding of molecular mechanisms underlying the phagocytic clearance of cancer cells and dying cells and the calreticulin-dependence of these processes could be fundamentally important towards developing new cancer treatment regimens. Finally, cell-surface calreticulin confers immunogenicity to dying cells. Much remains to be understood about the underlying mechanisms, which is another important area for further studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rutkevich LA, Williams DB. Participation of lectin chaperones and thiol oxidoreductases in protein folding within the endoplasmic reticulum. Curr Opin Cell Biol. 2011;23:157–166. doi: 10.1016/j.ceb.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 2.Gao B, et al. Assembly and antigen-presenting function of MHC class I molecules in cells lacking the ER chaperone calreticulin. Immunity. 2002;16:99–109. doi: 10.1016/s1074-7613(01)00260-6. [DOI] [PubMed] [Google Scholar]
- 3.Ireland BS, et al. Lectin-deficient Calreticulin Retains Full Functionality as a Chaperone for Class I Histocompatibility Molecules. Mol Biol Cell. 2008;19:2413–2423. doi: 10.1091/mbc.E07-10-1055. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Zhang Y, et al. ERp57 does not require interactions with calnexin and calreticulin to promote assembly of class I histocompatibility molecules, and it enhances peptide loading independently of its redox activity. J Biol Chem. 2009;284:10160–10173. doi: 10.1074/jbc.M808356200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Del Cid N, et al. Modes of calreticulin recruitment to the major histocompatibility complex class I assembly pathway. J Biol Chem. 2010;285:4520–4535. doi: 10.1074/jbc.M109.085407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeffery E, et al. The polypeptide binding conformation of calreticulin facilitates its cell surface expression under conditions of ER stress. J Biol Chem. 2011;286:2402–2415. doi: 10.1074/jbc.M110.180877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wearsch PA, et al. Essential glycan-dependent interactions optimize MHC class I peptide loading. Proc Natl Acad Sci U S A. 2011;108:4950–4955. doi: 10.1073/pnas.1102524108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizvi SM, et al. Distinct function for glycans of tapasin and heavy chains in the assembly of MHC class I molecules. J. Immunol. 2011;186:2309–2320. doi: 10.4049/jimmunol.1002959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howe C, et al. Calreticulin-dependent recycling in the early secretory pathway mediates optimal peptide loading of MHC class I molecules. EMBO J. 2009;28:3730–3744. doi: 10.1038/emboj.2009.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardai SJ, et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 11.Obeid M, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 12.Tesniere A, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29:482–491. doi: 10.1038/onc.2009.356. [DOI] [PubMed] [Google Scholar]
- 13.Peters LR, Raghavan M. Endoplasmic reticulum calcium depletion impacts chaperone secretion, innate immunity, and phagocytic uptake of cells. J Immunol. 2011;187:919–931. doi: 10.4049/jimmunol.1100690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panaretakis T, et al. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J. 2009;28:578–590. doi: 10.1038/emboj.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chao MP, et al. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci Transl Med. 2010;2:63ra–94ra. doi: 10.1126/scitranslmed.3001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zitvogel L, et al. Decoding cell death signals in inflammation and immunity. Cell. 2010;140:798–804. doi: 10.1016/j.cell.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 17.Kepp O, et al. Molecular determinants of immunogenic cell death elicited by anticancer chemotherapy. Cancer Metastasis Rev. 2011;30:61–69. doi: 10.1007/s10555-011-9273-4. [DOI] [PubMed] [Google Scholar]
- 18.Schrag JD, et al. The Structure of calnexin, an ER chaperone involved in quality control of protein folding. Mol Cell. 2001;8:633–644. doi: 10.1016/s1097-2765(01)00318-5. [DOI] [PubMed] [Google Scholar]
- 19.Ellgaard L, et al. NMR structure of the calreticulin P-domain. Proc Natl Acad Sci U S A. 2001;98:3133–3138. doi: 10.1073/pnas.051630098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kozlov G, et al. Structural basis of carbohydrate recognition by calreticulin. J Biol Chem. 2010;285:38612–38620. doi: 10.1074/jbc.M110.168294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chouquet A, et al. X-ray structure of the human calreticulin globular domain reveals a Peptide-binding area and suggests a multi-molecular mechanism. PLoS One. 2011;6:e17886. doi: 10.1371/journal.pone.0017886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duus K, et al. Interaction of the chaperone calreticulin with proteins and peptides of different structural classes. Protein Pept Lett. 2009;16:1414–1423. doi: 10.2174/092986609789353772. [DOI] [PubMed] [Google Scholar]
- 23.Pocanschi CL, et al. Structural and functional relationships between the lectin and arm domains of calreticulin. J Biol Chem. 2011;286:27266–27277. doi: 10.1074/jbc.M111.258467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frickel EM, et al. TROSY-NMR reveals interaction between ERp57 and the tip of the calreticulin P-domain. Proc Natl Acad Sci U S A. 2002;99:1954–1959. doi: 10.1073/pnas.042699099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kozlov G, et al. Structural basis of cyclophilin B binding by the calnexin/calreticulin P-domain. J Biol Chem. 2010;285:35551–35557. doi: 10.1074/jbc.M110.160101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baksh S, Michalak M. Expression of calreticulin in Escherichia coli and identification of its Ca2+ binding domains. J Biol Chem. 1991;266:21458–21465. [PubMed] [Google Scholar]
- 27.Nakamura K, et al. Functional specialization of calreticulin domains. J Cell Biol. 2001;154:961–972. doi: 10.1083/jcb.200102073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wijeyesakere SJ, et al. Calreticulin is a thermostable protein with distinct structural responses to different divalent cation environments. J Biol Chem. 2011;286:8771–8785. doi: 10.1074/jbc.M110.169193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mesaeli N, et al. Calreticulin is essential for cardiac development. J Cell Biol. 1999;144:857–868. doi: 10.1083/jcb.144.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo L, et al. Cardiac-specific expression of calcineurin reverses embryonic lethality in calreticulin-deficient mouse. J Biol Chem. 2002;277:50776–50779. doi: 10.1074/jbc.M209900200. [DOI] [PubMed] [Google Scholar]
- 31.Sonnichsen B, et al. Retention and retrieval: both mechanisms cooperate to maintain calreticulin in the endoplasmic reticulum. J Cell Sci. 1994;107(Pt 10):2705–2717. doi: 10.1242/jcs.107.10.2705. [DOI] [PubMed] [Google Scholar]
- 32.Dancourt J, Barlowe C. Protein sorting receptors in the early secretory pathway. Annu Rev Biochem. 2010;79:777–802. doi: 10.1146/annurev-biochem-061608-091319. [DOI] [PubMed] [Google Scholar]
- 33.Afshar N, et al. Retrotranslocation of the chaperone calreticulin from the endoplasmic reticulum lumen to the cytosol. Mol Cell Biol. 2005;25:8844–8853. doi: 10.1128/MCB.25.20.8844-8853.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gold LI, et al. Calreticulin: non-endoplasmic reticulum functions in physiology and disease. FASEB J. 2010;24:665–683. doi: 10.1096/fj.09-145482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raghavan M, et al. MHC class I assembly: out and about. Trends Immunol. 2008;29:436–443. doi: 10.1016/j.it.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wearsch PA, Cresswell P. The quality control of MHC class I peptide loading. Curr Opin Cell Biol. 2008;20:624–631. doi: 10.1016/j.ceb.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rizvi SM, Raghavan M. Mechanisms of function of tapasin, a critical major histocompatibility complex class I assembly factor. Traffic. 2010;11:332–347. doi: 10.1111/j.1600-0854.2009.01025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simone LC, et al. Influence of the tapasin C terminus on the assembly of MHC class I allotypes. Immunogenetics. 2009;61:43–54. doi: 10.1007/s00251-008-0335-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Purcell AW, Elliott T. Molecular machinations of the MHC-I peptide loading complex. Curr Opin Immunol. 2008;20:75–81. doi: 10.1016/j.coi.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Sadegh-Nasseri S, et al. The convergent roles of tapasin and HLA-DM in antigen presentation. Trends Immunol. 2008;29:141–147. doi: 10.1016/j.it.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li XC, Raghavan M. Structure and function of major histocompatibility complex class I antigens. Curr Opin Organ Transplant. 2010;15:499–504. doi: 10.1097/MOT.0b013e32833bfb33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dong G, et al. Insights into MHC class I peptide loading from the structure of the tapasin-ERp57 thiol oxidoreductase heterodimer. Immunity. 2009;30:21–32. doi: 10.1016/j.immuni.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saito Y, et al. Calreticulin functions in vitro as a molecular chaperone for both glycosylated and non-glycosylated proteins. Embo J. 1999;18:6718–6729. doi: 10.1093/emboj/18.23.6718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mancino L, et al. Calreticulin recognizes misfolded HLA-A2 heavy chains. Proc Natl Acad Sci U S A. 2002;99:5931–5936. doi: 10.1073/pnas.092031799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rizvi SM, et al. A polypeptide-binding conformation of calreticulin is induced by heat-shock, calcium depletion, or by mutation of the C-terminal acidic domain. Mol. Cell. 2004;15:913–923. doi: 10.1016/j.molcel.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 46.Jorgensen CS, et al. Dimerization and oligomerization of the chaperone calreticulin. Eur J Biochem. 2003;270:4140–4148. doi: 10.1046/j.1432-1033.2003.03808.x. [DOI] [PubMed] [Google Scholar]
- 47.Todd DJ, et al. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat Rev Immunol. 2008;8:663–674. doi: 10.1038/nri2359. [DOI] [PubMed] [Google Scholar]
- 48.Paidassi H, et al. Investigations on the C1q-calreticulin-phosphatidylserine interactions yield new insights into apoptotic cell recognition. J Mol Biol. 2011;408:277–290. doi: 10.1016/j.jmb.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 49.Tarr JM, et al. A mechanism of release of calreticulin from cells during apoptosis. J Mol Biol. 2010;401:799–812. doi: 10.1016/j.jmb.2010.06.064. [DOI] [PubMed] [Google Scholar]
- 50.Obeid M. ERP57 membrane translocation dictates the immunogenicity of tumor cell death by controlling the membrane translocation of calreticulin. J Immunol. 2008;181:2533–2543. doi: 10.4049/jimmunol.181.4.2533. [DOI] [PubMed] [Google Scholar]
- 51.Panaretakis T, et al. The co-translocation of ERp57 and calreticulin determines the immunogenicity of cell death. Cell Death Differ. 2008;15:1499–1509. doi: 10.1038/cdd.2008.67. [DOI] [PubMed] [Google Scholar]
- 52.Tufi R, et al. Reduction of endoplasmic reticulum Ca2+ levels favors plasma membrane surface exposure of calreticulin. Cell Death Differ. 2008;15:274–282. doi: 10.1038/sj.cdd.4402275. [DOI] [PubMed] [Google Scholar]
- 53.Booth C, Koch GL. Perturbation of cellular calcium induces secretion of luminal ER proteins. Cell. 1989;59:729–737. doi: 10.1016/0092-8674(89)90019-6. [DOI] [PubMed] [Google Scholar]
- 54.Thastrup O, et al. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)−ATPase. Proc Natl Acad Sci U S A. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fadok VA, et al. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 56.Garg AD, et al. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. EMBO J. 2012;31:1062–1079. doi: 10.1038/emboj.2011.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dupuis M, et al. The calcium-binding protein calreticulin is a major constituent of lytic granules in cytolytic T lymphocytes. J Exp Med. 1993;177:1–7. doi: 10.1084/jem.177.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oldenborg PA, et al. Role of CD47 as a marker of self on red blood cells. Science (New York, N Y ) 2000;288:2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 59.Jaiswal S, et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eric-Nikolic A, et al. Overexpression of calreticulin in malignant and benign breast tumors: relationship with humoral immunity. Oncology. 2012;82:48–55. doi: 10.1159/000335267. [DOI] [PubMed] [Google Scholar]
- 61.Chao MP, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142:699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Majeti R, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Edris B, et al. Antibody therapy targeting the CD47 protein is effective in a model of aggressive metastatic leiomyosarcoma. Proc Natl Acad Sci U S A. 2012;109:6656–6661. doi: 10.1073/pnas.1121629109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Willingham SB, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A. 2012;109:6662–6667. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Subramanian S, et al. Membrane mobility and clustering of Integrin Associated Protein (IAP, CD47)--major differences between mouse and man and implications for signaling. Blood Cells Mol Dis. 2006;36:364–372. doi: 10.1016/j.bcmd.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 66.Nagata S, et al. Autoimmunity and the clearance of dead cells. Cell. 2010;140:619–630. doi: 10.1016/j.cell.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 67.Ravichandran KS. Find-me and eat-me signals in apoptotic cell clearance: progress and conundrums. J Exp Med. 2010;207:1807–1817. doi: 10.1084/jem.20101157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuraishi T, et al. Identification of calreticulin as a marker for phagocytosis of apoptotic cells in Drosophila. Exp Cell Res. 2007;313:500–510. doi: 10.1016/j.yexcr.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 69.Somogyi E, et al. Calreticulin--an endoplasmic reticulum protein with calcium-binding activity is also found in the extracellular matrix. Matrix Biol. 2003;22:179–191. doi: 10.1016/s0945-053x(02)00117-8. [DOI] [PubMed] [Google Scholar]
- 70.Tarr JM, et al. Extracellular calreticulin is present in the joints of patients with rheumatoid arthritis and inhibits FasL (CD95L)-mediated apoptosis of T cells. Arthritis Rheum. 2010;62:2919–2929. doi: 10.1002/art.27602. [DOI] [PubMed] [Google Scholar]
- 71.Green DR, et al. Immunogenic and tolerogenic cell death. Nat Rev Immunol. 2009;9:353–363. doi: 10.1038/nri2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Obeid M, et al. Calreticulin exposure is required for the immunogenicity of gamma-irradiation and UVC light-induced apoptosis. Cell Death Differ. 2007;14:1848–1850. doi: 10.1038/sj.cdd.4402201. [DOI] [PubMed] [Google Scholar]
- 73.Pawaria S, Binder RJ. CD91-dependent programming of T-helper cell responses following heat shock protein immunization. Nat Commun. 2011;2:521. doi: 10.1038/ncomms1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Freeman GJ, et al. TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol Rev. 2010;235:172–189. doi: 10.1111/j.0105-2896.2010.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tseng CW, et al. Pretreatment with cisplatin enhances E7-specific CD8+ T-Cell-mediated antitumor immunity induced by DNA vaccination. Clin Cancer Res. 2008;14:3185–3192. doi: 10.1158/1078-0432.CCR-08-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roy A, et al. I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc. 2010;5:725–738. doi: 10.1038/nprot.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bjorkman PJ, et al. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987;329:506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]