Abstract
Background
Previous observational studies examining outcomes associated with the timing of dialysis initiation in the US have often been limited by lead time and survivor bias.
Study Design
Retrospective cohort study comparing the effectiveness of early versus later (conventional) dialysis initiation in advanced chronic kidney disease (CKD). The analysis employed inverse probability weighting to account for an individual’s contribution to different exposure groups over time in a pooled logistic regression model. Patients contributed risk to both exposure categories (early and later initiation) until there was a clear treatment strategy [i.e. dialysis was initiated early, or estimated glomerular filtration rate (eGFR) fell below 10 ml/min per 1.73 m2].
Setting & Participants
CKD patients who had at least one face-to-face outpatient encounter with a Cleveland Clinic health care provider as of January 1, 2005 and at least two estimated eGFRs in the range of 20 to 30 ml/min per 1.73m2 measured at least 180 days apart.
Predictors
Timing of dialysis initiation as determined using model-based interpolation of eGFR trajectories over time. Timing was defined as early (interpolated eGFR at dialysis initiation ≥10 ml/min per 1.73m2) or later (eGFR < 10), and was time-varying.
Outcomes
Death from any cause occurring from the time that eGFR was equal to 20 ml/min per 1.73m2 through September 15, 2009.
Results
The study population consisted of 652 patients meeting inclusion criteria. The majority of the study population (71.3%) did not initiate dialysis during follow up. Patients who did not initiate dialysis (n=465) were older, more likely to be Caucasian, and had more favorable laboratory profiles than those who initiated. Overall, 146 initiated early, and 80 had eGFR fall below 10 ml/min per 1.73 m2. Many participants (n=426) were censored prior to attaining a clear treatment strategy and were considered undeclared. There was no statistically significant survival difference for the early compared to later initiation strategies (odds ratio 0.85, 95% confidence interval 0.65–1.11).
Limitations
Interpolated eGFR, moderate sample size, and likely unmeasured confounders.
Conclusions
Among patients with advanced CKD, timing of dialysis initiation was not associated with mortality when accounting for lead time bias and survivor bias.
INTRODUCTION
Over the past decade, there has been a trend towards initiation of dialysis at higher levels of kidney function1,2. Definitive clinical trials of early versus later timed dialysis initiation have been difficult to conduct in part due to the unpredictable clinical course that often accompanies renal function decline. Following the Initiating Dialysis Early and Late (IDEAL) trial,3 questions still remain about the relevance to populations and care settings not well represented in the trial. Thus, substantial debate continues regarding the effectiveness of initiating dialysis early versus later.
Observational studies conducted to date have largely suggested greater mortality risk associated with early dialysis initiation4–18, although some found no different19 or improved survival associated with earlier initiation20–22. Most studies have been limited by observations of survival time originating from dialysis initiation rather than a common eGFR, except for a few studies that have addressed this using inverse probability weighting or imputation to fill in “lead times” for individuals initiating dialysis later18,19. Therefore, studies may have been subject to lead time bias, which tends to favor earlier dialysis initiation because patients beginning dialysis at a higher eGFR enter the analysis earlier in the course of their disease than those beginning later and accordingly gain a spurious residual lifetime advantage. Importantly, prior studies have also been subject to survivor bias, where healthier individuals may have been able to survive long enough to become later initiators, but others may not have survived.
In the absence of additional clinical trials, observational studies employing data in advance of dialysis initiation and methods accounting for lead-time and survivor bias could offer clinicians greater confidence in treatment decisions. We employed such data and a novel methodological strategy explicated by Sjölander and colleagues19, which employs inverse probability weighting, allows survival time to originate at a common level of kidney function, and allows patients who die before starting dialysis to contribute to the analysis. We also describe characteristics of patients who did or did not initiate dialysis to inform future studies of advanced chronic kidney disease (CKD).
METHODS
Overview
This was an observational cohort study comparing the effectiveness of early versus later dialysis initiation among adults with advanced CKD, and was part of the Agency for Healthcare Research and Quality Developing Evidence to Inform Decisions about Effectiveness (DEcIDE) Network Patient Outcomes in ESRD Study23.
Data Source and Study Population
We used data from the Cleveland Clinic Foundation (CCF) CKD Registry24 which includes 54,351 patients who had at least one face-to-face outpatient encounter with a Cleveland Clinic health care provider and (1) had two eGFR values ≤60 ml/min per 1.73 m2 90 days apart between January 1, 2005 and September 15, 2009 and/or (2) had at least two outpatient International Classification of Diseases (ICD-9) diagnosis codes for CKD, polycystic kidney disease, glomerulonephritis, diabetic nephropathy, hypertensive nephrosclerosis, or renovascular disease as of January 1, 2005. Patients less than 18 years of age or with ESRD were excluded.24
We linked CCF data to the US Renal Data System (USRDS) to determine initiation of treatment for ESRD. We followed patients through September 15, 2009. The institutional review boards of the CCF and Johns Hopkins University approved the study.
Data Collection
We extracted patients’ demographic, clinical and laboratory status from the CCF ambulatory electronic health records (EHRs).24 We obtained patients’ serum creatinine, cause of ESRD, and comorbidities at the time of dialysis initiation from the Centers for Medicare and Medicaid Services (CMS) form 2728 for those patients who initiated dialysis.
Study Population
We included patients based on their observed eGFRs prior to initiating renal replacement and our estimation that their kidney function was undergoing a decline.
Serum Creatinine Measurements
Serum creatinine measurements were performed during the course of patients’ routine clinical care at CCF and varied in number and timing. Measurements were obtained from the same clinical laboratory using integrated database management-system traceable samples to minimize calibration bias. All creatinine measurements were performed by the modified kinetic Jaffe reaction, using a Hitachi 747–200 Chemistry Analyzer (1996 to 2001) or a Hitachi D 2400 Modular Chemistry Analyzer thereafter (Roche Diagnostics, Indianapolis, IN) in the Cleveland Clinic laboratory. Estimated GFR was calculated using the four-variable Modification of Diet in Renal Disease study equation25.
Cohort Inclusion Criteria
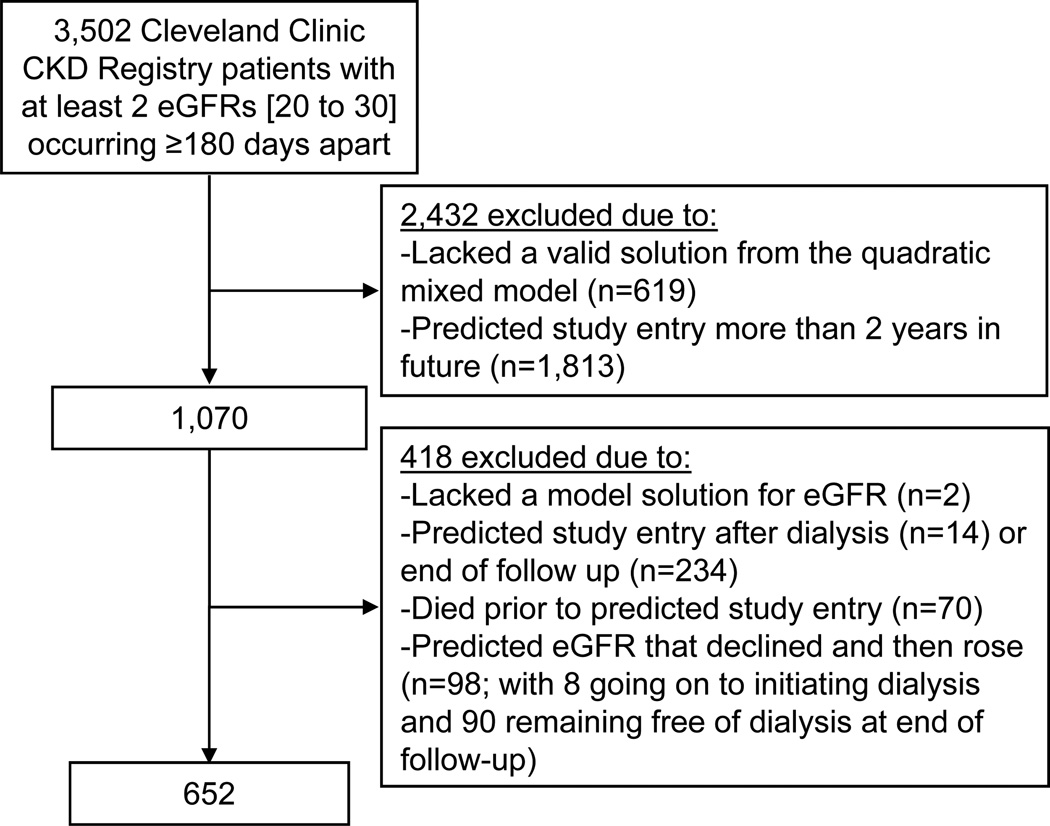
Conceptually, our study aimed to mimic a clinical trial randomizing patients at risk for ESRD to early or later dialysis initiation. As a first step, we defined a cohort of patients who could reasonably be considered “at risk” of progressive kidney function decline that might ultimately require dialysis initiation. We constructed “spaghetti” plots26 reflecting longitudinal trajectories of patients’ observed kidney function. Here, we observed that many patients’ eGFRs remained stable at a level much higher than those at which dialysis is initiated in standard practice, and some patients improved over time. Because we hypothesized that patients most at risk of requiring dialysis would experience a sustained eGFR decline (rather than a fall to a low eGFR followed by recovery), we restricted our analysis to the cohort of n=3502 patients with at least two eGFRs in the range of 20 to 30 ml/min per 1.73m2 and measured at least 180 days apart. This also may have excluded patients with acute kidney injury wherein there is less of a ‘choice’ regarding dialysis initiation. We defined patient age (in days) at the first of the measures in the 20 to 30 ml/min per 1.73m2 range as the patient’s “ascertainment time.”
In a hypothetical clinical trial, patients might be enrolled and randomized to early or later initiation upon reaching a certain threshold of disease severity. We defined this threshold as eGFR equal to 20 ml/min per 1.73m2, based on prior literature27. Because we rarely observed the actual crossing of this threshold in our study, we interpolated or extrapolated patients’ crossing times. To do so, we described patients’ eGFR trajectories as a quadratic function of (age at measurement–ascertainment time) using a mixed effects model with random effects for intercept (patient-specific mean eGFR at ascertainment), slope (linear rate of change), and acceleration (quadratic term). Upon visual inspection, the fitted model characterized the observed eGFR data closely for a large majority of patients. It employed all available eGFR measurements beginning with each patient’s ascertainment time. Person-specific quadratic eGFR trajectories were obtained [using “best linear unbiased predictions” (BLUPs) from the model]. From these, we solved for age satisfying eGFR = 20 ml/min per 1.73m2 (hereafter, termed “study entry”—the time origin for survival analysis) using the quadratic formula. We excluded patients lacking a real-valued solution to this equation, which typically occurred for patients with a precipitous decline in eGFR that improved quickly. To see why such cases exist, consider a U-shaped trajectory with nadir exceeding 20: no crossing of eGFR=20 would ever occur for such a trajectory. We further excluded patients who (1) had a predicted study entry occurring after dialysis initiation, (2) had a predicted study entry after the end of administrative follow-up, or (3) died before predicted study entry. To avoid excessive extrapolation, we also excluded patients with a predicted crossing of eGFR equal to 20 ml/min per 1.73m2 occurring greater than two years after their last observed eGFR in the CCF. (Figure 1) This allowed for a reasonable window during which to ascertain covariates. In implementing predicted assignments to “early” and “later” dialysis initiation, we discovered that a small fraction of individuals’ predicted trajectories ultimately improved following an initial decline, which in turn induced errors in making the predicted assignments. These individuals were also excluded from the analysis. (Figure 1)
Figure 1.
Study Population
Key Variables
Dialysis Initiation Strategy
Our definition of dialysis initiation strategy was time-varying. We defined the initiation strategy as ‘early’ if the patient began dialysis with a predicted eGFR of ≥10 ml/min per 1.73m2 (and < 20 ml/min per 1.73m2) and ‘later’ once predicted eGFR fell to <10 ml/min per 1.73m2 whether they initiated dialysis or not. So long as patients did not meet either of these criteria (i.e. predicted eGFR of ≥10 ml/min per 1.73m2 but did not initiate dialysis) we considered them ‘undeclared’ and consistent with both initiation strategies.
Covariates
All covariates in our analysis were fixed and defined based upon proximity of their measurement to the date of ‘study entry’. We defined age at the date corresponding to patients’ study entry. We defined race, gender and smoking status based on available information in the EHRs from the last documentation prior to study entry. We defined patients’ comorbid conditions at time of entry into the registry24. We included causes of CKD if ever documented. We calculated patients’ body weight from the median of weight measurements from the 365 days prior to study entry. We defined phosphate binder and vitamin D use based on outpatient prescriptions in the 365 days prior to study entry. We defined erythropoiesis stimulating agent use as present if an outpatient prescription was ever noted. We considered patients with no prescriptions listed to have missing data on all medications. We calculated serum intact parathyroid hormone and phosphorus from the mean of those available in the 365 days prior to and 90 days following study entry. We calculated hemoglobin, potassium, blood urea nitrogen, serum bicarbonate, and serum albumin as the mean of values in the 180 days prior to and 180 days following study entry. We defined the presence of abnormal laboratory values (e.g. serum albumin <3.5 g/L) based on any existing record of such. We defined proteinuria as the presence of >1+ proteinuria in dipstick studies, >30 mg/g in those who had urine albumin-to-creatinine ratio and/or urine protein-to-creatinine studies and >30 mg proteinuria in 24 hour studies.
Outcome Assessment
We defined mortality as death from any cause, ascertained from the EHRs, the Social Security death index and USRDS data.
Statistical Analyses
We used descriptive statistics to compare the demographic, clinical and laboratory characteristics of patients who met criteria for advanced CKD (predicted eGFR of 20 ml/min per 1.73m2) stratified by whether they did or did not initiate dialysis during follow up, and separately, by the final dialysis initiation strategy (later, early or undeclared) as determined by eGFR at dialysis initiation or loss to follow up as predicted by our model.
We sought to determine the effect on the hazard of mortality of initiating dialysis early versus later, in terms of time since study entry. This approach has previously been termed a “from threshold” analysis14,18. First, we fitted a Cox proportional hazards model to time between study entry and death, restricting the analysis to patients assigned to early or later initiation within the study period. This analysis addresses lead time bias, but not survivor bias. To address the latter, we implemented an approach proposed by Cain et al. and elucidated in ESRD by Sjolander et al.19,28 In this approach individuals contribute person-time to both treatment groups until they are definitively “assigned” to one of them by initiating dialysis or losing the opportunity to start early (eGFR crossing below 10 ml/min per 1.73m2), and commensurately being “censored” from the other group. Because persons censored in this way will tend to have lower eGFR than those not artificially censored, “stabilized” inverse probability weights (IPWs) are introduced to correct this induced bias.19 Specifically, we addressed mortality through a discrete-time survival analysis over one-month time intervals implemented by a weighted “pooled” logistic regression. In brief, weights for this mortality analysis were derived from a second, separate pooled logistic regression29 describing the log odds of dialysis incidence in a given interval as a linear combination of time, time squared, eGFR terms linear through quartic, age_at_crossing of eGFR=20, sex (male versus female), race/ethnicity (Caucasian versus other), smoking status (never, former, current), and indicators for baseline diagnosis of diabetes, hypertension, hyperlipidemia, coronary artery disease, congestive heart failure, and cerebrovascular disease. Using this model, the denominator of a person’s weight in the risk set for death in an interval t is formed from their predicted probabilities of remaining dialysis-free so long as dialysis does not occur, and monthly contributions of 1 thereafter, cumulatively multiplied from study entry through interval t-1. Technical details are noted in S2.
Following Sjolander et al. and others, weights were truncated at 10. Standard errors were obtained via bootstrapping (1,000 samples). In adjusted analyses, we included a parsimonious group of covariates informed by prior studies of dialysis patients and noted above. We did not include laboratory measures as covariates in our primary analysis as not all patients had the laboratory tests of interest measured during their care.
Sensitivity and Diagnostic Analyses
To test the robustness of our findings to the choice of truncation threshold, we examined various thresholds for truncating IPWs. Weights were truncated such that all persons were included in the analysis, but those with weights exceeding the threshold were given a weight at the truncation point. Additionally, to address discrepancy between dialysis initiation strategy as defined by predicted eGFR and as ascertained from the USRDS record of the observed eGFR at dialysis initiation, we repeated our primary analysis (weight truncation=10) defining initiation strategy according to observed eGFR for those known to initiate dialysis and according to predicted eGFR (including “undeclared” as a possibility) for all others. In a third sensitivity analysis, we adjusted our primary model for the year of predicted crossing of an eGFR=20 to account for temporal trends. Fourth, we adjusted our primary model for serum albumin among the 524 patients with available data. Finally, we checked the proportionality assumption of our Cox model by testing for interaction between treatment groups and a time-varying indicator of time since study entry > 18 months, a cut point motivated by Kaplan-Meier plotting.
RESULTS
Patient Characteristics by Dialysis Status
Our final cohort consisted of 652 patients meeting inclusion criteria. (Figure 1) Patients had undergone a median of 15 (interquartile range, 9 to 25) eGFR measurements and 91% had an observed eGFR <20 ml/min per 1.73m2during follow up. The majority (71.3%) did not initiate dialysis during follow up, and these patients differed from those who did initiate dialysis (N=187) on a number of characteristics (Table 1).
Table 1.
Patient Characteristics, Overall and by Dialysis Outcome
| Characteristic | N | Overall N=652 n(%) |
No Dialysis N=465 n (%) |
Dialysis N=187 n (%) |
P-value |
|---|---|---|---|---|---|
| Age, mean (SD) | 652 | 69.5±15.0 | 71.7±14.7 | 64.0±14.4 | <0.001 |
| Male gender | 652 | 302(46.3) | 200(43.0) | 102(54.5) | 0.008 |
| Race | 652 | <0.001 | |||
| Caucasian | 485(74.4) | 369(79.4) | 116(62.0) | ||
| African American | 153(23.5) | 90(19.4) | 63(33.7) | ||
| Other | 14(2.1) | 6(1.3) | 8(4.3) | ||
| Comorbid conditions | 652 | ||||
| Diabetes | 240(36.8) | 162(34.8) | 78(41.7) | 0.1 | |
| Hypertension | 558(85.6) | 402(86.5) | 156(83.4) | 0.3 | |
| Hyperlipidemia | 513(78.7) | 367(78.9) | 146(78.1) | 0.8 | |
| Coronary artery disease | 137(21.0) | 105(22.6) | 32(17.1) | 0.1 | |
| Congestive heart failure | 79(12.1) | 54(11.6) | 25(13.4) | 0.5 | |
| Cerebrovascular disease | 80(12.3) | 54(11.6) | 26(13.9) | 0.4 | |
| Tobacco use | 572 | 44(7.7) | 27(6.5) | 17(11.0) | 0.07 |
| Weight (kg), mean (SD) | 605 | 83.5±21.5 | 81.6±20.7 | 88.3±23.1 | <0.001 |
| Cause of kidney disease | 652 | ||||
| Diabetic nephropathy | 121(18.6) | 83(17.8) | 38(20.3) | 0.5 | |
| Polycystic kidney disease | 21(3.2) | 16(3.4) | 5(2.7) | 0.6 | |
| Glomerulonephritis | 22(3.4) | 12(2.6) | 10(5.3) | 0.08 | |
| Medications | |||||
| Phosphorus binder | 584 | 35(6.0) | 26(6.1) | 9(5.6) | 0.8 |
| Erythropoiesis-stimulating agent | 586 | 184(31.4) | 127(30.0) | 57(35.2) | 0.2 |
| Activated vitamin D | 584 | 59(10.1) | 42(9.9) | 17(10.6) | 0.8 |
| Laboratory measurements, mean (SD) | |||||
| Intact parathyroid hormone, pg/ml | 278 | 182.8±160.0 | 165.7±166.9 | 213.7±142.3 | 0.02 |
| Serum phosphorus, mg/dl | 331 | 4.2±0.68 | 4.2±0.69 | 4.2±0.68 | 0.9 |
| Serum hemoglobin, g/dl | 543 | 11.3±1.4 | 11.3±1.4 | 11.2±1.3 | 0.8 |
| Serum potassium, mmol/l | 608 | 4.6±0.53 | 4.6±0.51 | 4.5±0.56 | 0.03 |
| Blood urea nitrogen, mg/dl | 607 | 53.8±15.9 | 52.7±15.6 | 56.4±16.6 | 0.01 |
| Serum bicarbonate, mmol/l | 607 | 23.2±3.4 | 23.0±3.4 | 23.5±3.4 | 0.2 |
| Serum albumin, g/dl | 524 | 3.9±0.50 | 3.9±0.46 | 3.7±0.54 | <0.001 |
| Number of eGFR measures, median [P25, P75] | 652 | 15.0 (9.0, 25.0) | 14.0 (8.0, 23.0) | 17 (10.0, 28.0) | 0.009 |
| Abnormal laboratory measurements, n (%) | |||||
| Serum phosphorus >4.5 mg/dl | 535 | 373(69.7) | 241(65.3) | 132(79.5) | <0.001 |
| Serum hemoglobin <10.0 g/dl | 633 | 462(73.0) | 316(70.4) | 146(79.3) | 0.02 |
| Serum potassium >5.5 mmol/l | 652 | 369(56.6) | 250(53.8) | 119(63.6) | 0.02 |
| Blood urea nitrogen >100 mmol/l | 652 | 174(26.7) | 109(23.4) | 65(34.8) | 0.003 |
| Serum albumin <3.5 g/dl | 639 | 397(62.1) | 257(56.6) | 140(75.7) | <0.001 |
| Proteinuria | 593 | 533(89.9) | 365(87.1) | 168(96.6) | <0.001 |
| Insurance | 627 | ||||
| Medicare/Medicaid | 627 | 489(78.0) | 352(78.0) | 137(77.8) | 0.9 |
Chi-square tests were performed for categorical variables, and t tests for continuous variables.
Patient Characteristics by Dialysis Initiation Strategy
Our model predicted 80 patients to have undergone a later initiation strategy, 146 to have been early, and 426 undeclared at study end. These groups varied on many characteristics (Table 2), with the later initiation strategy group being the youngest and comprising the smallest proportion of males and Caucasians. Further, the later group comprised the greatest proportion of patients with diabetes and/or diabetic nephropathy and had a greater burden of abnormal laboratory findings when compared to the other two initiation strategy groups.
Table 2.
Patient Characteristics by Dialysis Initiation Strategy at the End of Follow Up
| Characteristic | N for analysis |
Later N=80 n (%) |
Early N=146 n (%) |
Undeclared N=426 n (%) |
P-value |
|---|---|---|---|---|---|
| Age, mean (SD) | 652 | 63.5±15.4 | 65.7±13.7 | 71.9±14.8 | <0.001 |
| Male gender | 652 | 29(36.3) | 84(57.5) | 189(44.4) | 0.004 |
| Race | 652 | <0.001 | |||
| Caucasian | 38(47.5) | 101(69.2) | 346(81.2) | ||
| African American | 39(48.8) | 40(27.4) | 74(17.4) | ||
| Other | 3(3.8) | 5(3.4) | 6(1.4) | ||
| Comorbid conditions | 652 | ||||
| Diabetes | 40(50.0) | 60(41.1) | 140(32.9) | 0.007 | |
| Hypertension | 71(88.8) | 120(82.2) | 367(86.2) | 0.4 | |
| Hyperlipidemia | 62(77.5) | 117(80.1) | 334(78.4) | 0.9 | |
| Coronary artery disease | 12(15.0) | 29(19.9) | 96(22.5) | 0.3 | |
| Congestive heart failure | 7(8.8) | 23(15.8) | 49(11.5) | 0.2 | |
| Cerebrovascular disease | 12(15.0) | 21(14.4) | 47(11.0) | 0.4 | |
| Tobacco use | 572 | 7(8.8) | 11(7.5) | 26(6.1) | 0.05 |
| Weight, mean (SD) | 605 | 84.4±21.8 | 89.4±24.0 | 81.3±20.3 | <0.001 |
| Cause of kidney disease | 652 | ||||
| Diabetic nephropathy | 25(31.3) | 23(15.8) | 73(17.1) | 0.007 | |
| Polycystic kidney disease | 2(2.5) | 5(3.4) | 14(3.3) | 0.9 | |
| Glomerulonephritis | 5(6.3) | 6(4.1) | 11(2.6) | 0.2 | |
| Medications | |||||
| Phosphorus binder | 584 | 5(6.8) | 6(4.9) | 24(6.2) | 0.8 |
| Erythropoiesis-stimulating agent | 586 | 22(29.7) | 44(35.8) | 118(30.3) | 0.5 |
| Activated vitamin D | 584 | 5(6.8) | 15(12.3) | 39(10.1) | 0.5 |
| Laboratory measurements, mean (SD) | |||||
| Intact parathyroid hormone, pg/ml | 278 | 209.6±139.1 | 203.8±137.9 | 166.1±172.8 | 0.1 |
| Serum phosphorus, mg/dl | 331 | 4.0±0.81 | 4.2±0.61 | 4.2±0.68 | 0.3 |
| Serum hemoglobin, g/dl | 543 | 11.4±1.4 | 11.2±1.3 | 11.3±1.4 | 0.8 |
| Serum potassium, mmol/l | 608 | 4.6±0.51 | 4.5±0.57 | 4.7±0.51 | 0.008 |
| Blood urea nitrogen, mg/dl | 607 | 49.0±14.5 | 58.5±16.8 | 53.0±15.5 | <0.001 |
| Serum bicarbonate, mmol/l | 607 | 22.9±3.3 | 23.7±3.6 | 23.0±3.3 | 0.1 |
| Serum albumin, g/dl | 524 | 3.7±0.59 | 3.7±0.54 | 3.9±0.45 | <0.001 |
| Abnormal laboratory measurements, n (%) | |||||
| Serum phosphorus >4.5 mg/dl | 535 | 60(81.1) | 97(77.0) | 216(64.5) | 0.002 |
| Serum hemoglobin <10.0 g/dl | 633 | 65(81.3) | 110(76.9) | 287(70.0) | 0.06 |
| Serum potassium >5.5 mmol/l | 652 | 54(67.5) | 89(61.0) | 226(53.1) | 0.03 |
| Blood urea nitrogen >100 mmol/l | 652 | 31(38.8) | 49(33.6) | 94(22.1) | <0.001 |
| Serum albumin <3.5 g/dl | 639 | 60(75.0) | 106(73.6) | 231(55.7) | <0.001 |
| Proteinuria | 593 | 73(96.1) | 130(95.6) | 330(86.6) | 0.002 |
| Insurance | 627 | ||||
| Medicare/Medicaid | 627 | 64(84.2) | 105(76.1) | 320(77.5) | 0.4 |
Values presented as Mean ± SD with ANOVA; or N (%) with Pearson's chi-square test unless otherwise stated.
There was appreciable disagreement between our predicted initiation strategy groups and observed, actual dialysis initiation (from USRDS data). In all there were 119 individuals for whom predicted initiation (early, later) and observed initiation from USRDS data were concordant, and an additional 47 individuals for whom the timing of dialysis initiation was not observed. Among 60 individuals for whom predicted initiation (early, later) and observed initiation from USRDS data were discordant, the large majority (n=54) were later initiators whom our model incorrectly predicted as early. Characteristics of patients who initiated dialysis by model-based and observed treatment assignments are described in S1.
Mortality
Median follow up for our study (N=652) was 4.3 years (interquartile range, 3.3 to 4.6 years). A total of 187 (28.7%) patients died, including 49 (33.6%) patients that were predicted to have an early initiation strategy, 18 (22.5%) patients that were predicted to have a later initiation strategy and 120 (28.2%) that remained undeclared throughout follow-up. Crude incidence rates for mortality were highest for those that remained ‘undeclared’, followed by those ultimately early and ultimately later, which did not differ statistically from one another (Table 3). Survival curves for the three groups are shown in Figure 2.
Table 3.
Incidence Rates for Mortality for Patients According to the Dialysis Initiation Strategy at the End of Follow up
| Treatment | Incidence Rate (deaths per 1000 person-years) | 95% CI | |
|---|---|---|---|
| Later | 98 | 59 | 150 |
| Early | 148 | 110 | 193 |
| Undeclared | 280 | 233 | 333 |
Figure 2.
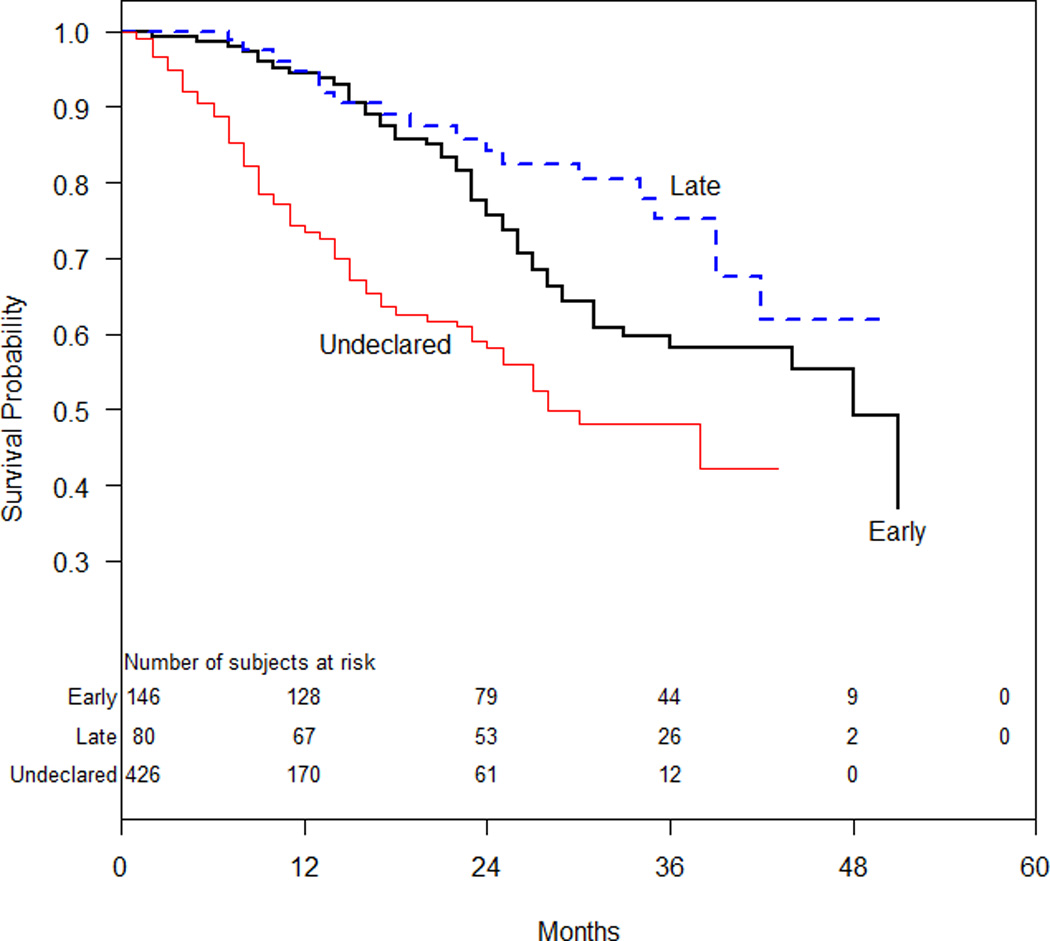
Kaplan-Meier Survival Curves of the Three Dialysis Initiation Strategies
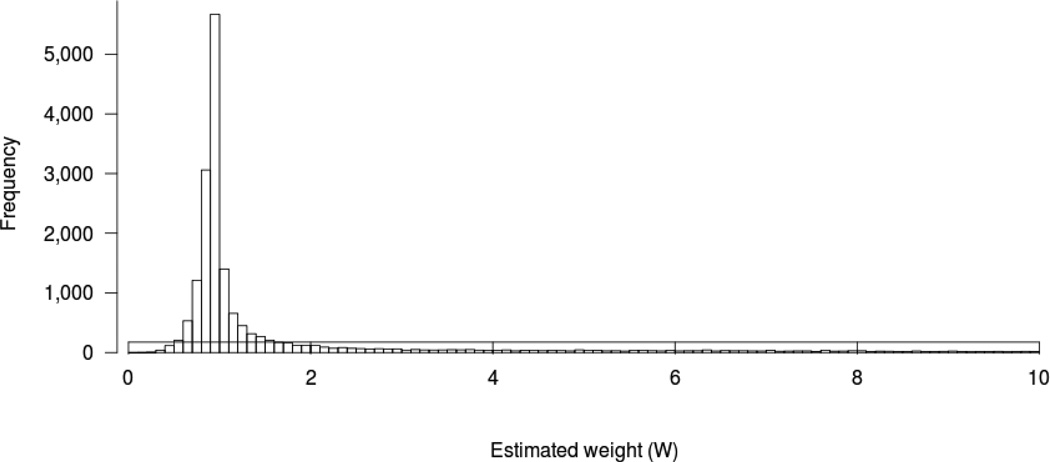
Our ‘from threshold’ Cox proportional hazard model analysis comparing early to later initiation strategies while adjusting for age, sex, race, comorbidities, and tobacco use demonstrated a considerable disadvantage for the early initiation strategy that was not statistically significant [hazard ratio (HR) 1.59, 95% confidence interval (CI) 0.89–2.84]. Our inverse probability weighted model, which accounted for both lead time and survivor bias, demonstrated a modest advantage for the early initiation strategy that was not statistically significant (OR 0.85, 95% CI 0.65–1.11). Figure 3 displays the distribution of the estimated weights.
Figure 3.
Distribution of the Estimated Weights (W) for the Inverse Probability Weighting Method
Sensitivity and Diagnostic Analyses
When we examined various thresholds for IPWs, we found that our point estimate comparing mortality of the early versus later initiation strategies moved closer to the null as weight truncation points were varied from 10 to 5 to 3 to 1 (e.g. OR 1.07 when weights were all set to 1, commensurate with a “naïve” un-weighted analysis). This suggested that individuals with high weights (i.e. individuals with rapidly declining eGFR who did not initiate dialysis) were influential in our primary analysis. Our analysis incorporating the USRDS record of the observed eGFR at dialysis initiation for patients initiating dialysis yielded an OR of 1.08 that did not approach statistical significance even before bootstrapping. Analyses including serum albumin and separately, year of study entry, yielded results similar to our primary analysis (data not shown).
In testing our Cox model, we found evidence of non-proportionality (Wald chi-square for absence of interaction between time > 18 months and any treatment=12.0, 2 df; p-value=0.0025). This appeared to hinge largely on the undeclared group, as the test for modification of the early versus late pairwise comparison did not approach significance (p=0.466). Moreover the post-18 months point estimate from the interaction model (early versus late HR of 1.60) was comparable to our original, overall estimate (HR=1.59). Consequently we consider the appearance of post-18-month divergence in survival between early and late initiation groups interesting (Figure 2) but found nothing to contradict the non-significance of the early-versus- late comparison found through our primary Cox modeling.
DISCUSSION
In this comparative effectiveness study of the timing of dialysis initiation, we employed data providing lead time preceding dialysis initiation and novel statistical strategies to overcome potential biases of previous observational studies. Using data from a cohort of patients with advanced CKD, we found that the majority of patients did not initiate dialysis during follow up, and many died without ever receiving dialysis. When we anchored our analysis at a common eGFR, and accounted for those patients who died prior to dialysis initiation, we observed no statistically significant difference in survival for early and later initiation strategies. This finding is consistent with clinical trial results3, but contrasts with several observational studies largely reporting worse survival for patients initiating dialysis early30.
There were several differences in the characteristics of those patients who did or did not initiate dialysis in our study, which may shed light on our outcome results. While those who initiated dialysis had less favorable laboratory profiles and similar comorbid disease burden to those who did not initiate, the initiators had other characteristics associated with better survival on dialysis. These included younger age, greater proportion of individuals of African American or other race and greater body weight. Notably, our undeclared group had a higher mortality rate than has been observed in other studies of patients with advanced CKD31, perhaps due to their older age (mean 71.9 years).
Both our ‘from threshold’ analysis and inverse probability weighted models demonstrated no statistically significant difference in mortality among early and later initiation strategies, and moreover, estimates from these two methods were in opposite directions of harm/benefit. These findings suggest that observational studies not accounting for these weaknesses may produce biased findings. Our results were more consistent with that of the IDEAL trial which randomized patients from a ‘threshold’ of eGFR between 10 and 15 ml/min per 1.73m2 to either early or late initiation, and found no difference in mortality between treatment groups (HR 1.04, 95% CI 0.83–1.30). As in our study, the IDEAL trial also accounted for the 32 patients (3.9%) who died after randomization, but prior to initiating dialysis.3
There are several possible explanations for our findings. First, aside from age, gender and laboratory measures, the initiation strategy groups had few differences in our study. This is in contrast to studies reporting greater comorbidities among early compared to later initiators in the US5,11, and may have been related to our study population originating from the same health system which may subscribe to a certain practice pattern regarding dialysis initiation. Second, our analysis included only 80 individuals with the later initiation strategy, which limited our power to discern differences. Finally, the IDEAL investigators recently reported that echocardiographic findings at baseline and one year did not differ for early and later initiators in their trial.32 A lack of effect of timing of dialysis initiation on cardiac function would support our finding of no survival difference, despite a few reports of the potential effect of dialysis on left ventricular hypertrophy33–35.
Our study had limitations. Although the CCF CKD registry itself is quite large, our study sample size was small because the registry included few patients with predictable eGFR declines. Thus, our findings are most generalizable to patients with predictable declines in kidney function for whom a nephrologist has a ‘choice’ in timing of dialysis initiation. Further, many patients only had eGFR measured at a few time points, at irregular intervals, and/or with significant variation over time. Thus, we interpolated eGFR trajectories, and defined dialysis initiation groups by their predicted eGFR rather than observed eGFR among actual initiators. While we believe this approach was the least susceptible to systematic bias, it did misclassify many observed “later” patients as “early”, perhaps due to patient differences in eGFR trajectories. Li et al. recently reported that eGFR often followed a nonlinear trajectory or prolonged period of nonprogression among African Americans with hypertensive kidney disease36. Considering the predominant pattern of our mis-prediction and our fitted weights model, our data are consistent with a frequent substantial acceleration in disease severity shortly predating dialysis initiation. Further study of the impact of eGFR trajectory on dialysis outcomes is warranted. Additionally, we lacked comorbidity assessment close to study entry, time-varying covariates during follow-up and data on the circumstances surrounding patient deaths. It is also possible that patients underwent eGFR assessments outside of the CCF system which we were unable to ascertain.
As an analytic limitation, we conditioned on our first-stage estimates (of study entry time) in our second stage analysis (of time between study entry and mortality). While this is common practice, so doing overlooks inter-individual correlation the mixed modeling procedure induces between study entry time estimates, which in turn stands to anti-conservatively bias our bootstrapped standard error estimates when considered unconditionally. This issue merits further consideration in methodologically oriented work. In the current case, most BLUP trajectories fitted persons’ observed data so closely that any induced dependency in study entry times should be minimal. Additionally, our findings were not statistically significant and stood only to become less so by eliminating dependency effects, hence we believe that the issue has not substantively affected our conclusions.
Nonetheless, to our knowledge, this is the first US-based study accounting for both lead time bias and survivor bias in an analysis of outcomes related to dialysis timing. We provided descriptive data on patients who did or did not initiate dialysis which may serve to generate hypotheses for future studies of eGFR trajectories and appropriate care plans for patients with advanced CKD. The key implication of our study is that later initiation may not have a mortality benefit when lead time bias is accounted for and survival is ascertained in the setting of advanced CKD. Ultimately a larger study is needed to confirm our findings, and other outcomes, such as quality of life, functional status and withdrawal from dialysis37 deserve further investigation to determine if they are influenced by dialysis timing. We recommend future studies evaluating patients prior to their reaching a disease severity at which dialysis initiation is commonly considered and following them for some years thereafter.
Supplementary Material
Acknowledgements
The Developing Evidence to Inform Decisions about Effectiveness (DEcIDE) Network Patient Outcomes in ESRD Study was supported by the Agency for Healthcare Research and Quality (AHRQ) contract HHSA290200500341I, Task Order #6.
The creation of the Cleveland Clinic Foundation CKD registry was funded by an unrestricted grant from Amgen, Inc. to the Department of Nephrology and Hypertension Research and Education Fund.
Dr. Crews was supported by the Harold Amos Medical Faculty Development Program of the Robert Wood Johnson Foundation, Princeton NJ.
Dr. Scialla was supported by K23 DK095949 from the National Institute for Diabetes, Digestive and Kidney Diseases (NIDDK), Bethesda MD.
Dr. Michels was supported by Postdoctoral Full Fellowship Abroad Grant (KFB 11.005) of the Dutch Kidney Foundation (Nierstichting).
Dr. Jolly was supported by K23 DK091363 from NIDDK.
Dr. Shafi was supported by K23 DK083514 from NIDDK.
The authors wish to acknowledge Dr. Arvid Sjölander for helpful conversations and his sharing of code relating to our statistical methodology.
Dr. Miskulin receives salary support from Dialysis Clinic Inc, a not-for-profit dialysis provider.
Appendix
The DEcIDE Network Patient Outcomes in End-Stage Renal Disease Study Team consists of members from the Johns Hopkins University, Baltimore, Maryland (L. Ebony Boulware, Karen Bandeen-Roche, Courtney Cook, Josef Coresh, Deidra Crews, Patti Ephraim, Bernard Jaar, Jeonyong Kim, Yang Liu, Jason Luly, Aidan McDermott, Wieneke Michels, Paul Scheel, Tariq Shafi, Stephen Sozio, Albert Wu, Jing Zhou); University of California, San Francisco, California (Neil Powe); Chronic Disease Research Group, Minneapolis, Minnesota (Allan Collins, Robert Foley, David Gilbertson, Haifeng Guo, Joseph Grill, Charles Herzog, Jiannong Liu, Wendy St. Peter); Cleveland Clinic Foundation, Cleveland, Ohio (Joseph Nally, Susana Arrigain, Stacey Jolly, Vicky Konig, Xiaobo Liu, Sankar Navaneethan, Jesse Schold,); Dialysis Clinic, Incorporated, Nashville, Tennessee (Karen Majchrzak, Philip Zager); Tufts University, Boston, Massachusetts (Dana Miskulin, Klemens Meyer); University of Miami, Miami, Florida (Julia Scialla); University of Manitoba, Winnipeg, Manitoba (Navdeep Tangri); and Academic Medical Center, Amsterdam, The Netherlands (Wieneke Michels).
Footnotes
Publisher's Disclaimer: Disclaimers
Identifiable information, on which this report, presentation, or other form of disclosure is based, is confidential and protected by federal law, Section 903(c) of the Public Health Service Act, 42 USC 299a-1(c). Any identifiable information that is knowingly disclosed is disclosed solely for the purpose for which it has been supplied. No identifiable information about any individual supplying the information or described in it will be knowingly disclosed except with the prior consent of that individual.
The data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the U.S. government.
Potential Conflicts of Interest
All other authors report no relevant conflicts of interest.
REFERENCES
- 1.O'Hare AM, Choi AI, Boscardin WJ, et al. Trends in timing of initiation of chronic dialysis in the United States. Arch Intern Med. 2011 Oct 10;171(18):1663–1669. doi: 10.1001/archinternmed.2011.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosansky SJ, Clark WF, Eggers P, Glassock RJ. Initiation of dialysis at higher GFRs: is the apparent rising tide of early dialysis harmful or helpful? Kidney Int. 2009 Aug;76(3):257–261. doi: 10.1038/ki.2009.161. [DOI] [PubMed] [Google Scholar]
- 3.Cooper BA, Branley P, Bulfone L, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010 Aug 12;363(7):609–619. doi: 10.1056/NEJMoa1000552. [DOI] [PubMed] [Google Scholar]
- 4.Beddhu S, Samore MH, Roberts MS, et al. Impact of timing of initiation of dialysis on mortality. Journal of the American Society of Nephrology : JASN. 2003 Sep;14(9):2305–2312. doi: 10.1097/01.asn.0000080184.67406.11. [DOI] [PubMed] [Google Scholar]
- 5.Kazmi WH, Gilbertson DT, Obrador GT, et al. Effect of comorbidity on the increased mortality associated with early initiation of dialysis. Am J Kidney Dis. 2005 Nov;46(5):887–896. doi: 10.1053/j.ajkd.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Shiao CC, Huang JW, Chien KL, Chuang HF, Chen YM, Wu KD. Early initiation of dialysis and late implantation of catheters adversely affect outcomes of patients on chronic peritoneal dialysis. Peritoneal dialysis international : journal of the International Society for Peritoneal Dialysis. 2008 Jan-Feb;28(1):73–81. [PubMed] [Google Scholar]
- 7.Sawhney S, Djurdjev O, Simpson K, Macleod A, Levin A. Survival and dialysis initiation: comparing British Columbia and Scotland registries. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2009 Oct;24(10):3186–3192. doi: 10.1093/ndt/gfp189. [DOI] [PubMed] [Google Scholar]
- 8.Stel VS, Dekker FW, Ansell D, et al. Residual renal function at the start of dialysis and clinical outcomes. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2009 Oct;24(10):3175–3182. doi: 10.1093/ndt/gfp264. [DOI] [PubMed] [Google Scholar]
- 9.Lassalle M, Labeeuw M, Frimat L, et al. Age and comorbidity may explain the paradoxical association of an early dialysis start with poor survival. Kidney Int. 2010 Apr;77(8):700–707. doi: 10.1038/ki.2010.14. [DOI] [PubMed] [Google Scholar]
- 10.Hwang SJ, Yang WC, Lin MY, Mau LW, Chen HC. Impact of the clinical conditions at dialysis initiation on mortality in incident haemodialysis patients: a national cohort study in Taiwan. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2010 Aug;25(8):2616–2624. doi: 10.1093/ndt/gfq308. [DOI] [PubMed] [Google Scholar]
- 11.Wright S, Klausner D, Baird B, et al. Timing of dialysis initiation and survival in ESRD. Clinical journal of the American Society of Nephrology : CJASN. 2010 Oct;5(10):1828–1835. doi: 10.2215/CJN.06230909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosansky SJ, Eggers P, Jackson K, Glassock R, Clark WF. Early start of hemodialysis may be harmful. Arch Intern Med. 2011 Mar 14;171(5):396–403. doi: 10.1001/archinternmed.2010.415. [DOI] [PubMed] [Google Scholar]
- 13.Clark WF, Na Y, Rosansky SJ, et al. Association between estimated glomerular filtration rate at initiation of dialysis and mortality. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2011 Jan 11;183(1):47–53. doi: 10.1503/cmaj.100349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans M, Tettamanti G, Nyren O, Bellocco R, Fored CM, Elinder CG. No survival benefit from early-start dialysis in a population-based, inception cohort study of Swedish patients with chronic kidney disease. Journal of internal medicine. 2011 Mar;269(3):289–298. doi: 10.1111/j.1365-2796.2010.02280.x. [DOI] [PubMed] [Google Scholar]
- 15.Bao Y, Dalrymple L, Chertow GM, Kaysen GA, Johansen KL. Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch Intern Med. 2012 Jul 23;172(14):1071–1077. doi: 10.1001/archinternmed.2012.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson B, Harwood L, Locking-Cusolito H, et al. Optimal timing of initiation of chronic hemodialysis? Hemodial Int. 2007 Apr;11(2):263–269. doi: 10.1111/j.1542-4758.2007.00178.x. [DOI] [PubMed] [Google Scholar]
- 17.Molnar MZ, Streja E, Kovesdy CP, et al. Estimated glomerular filtration rate at reinitiation of dialysis and mortality in failed kidney transplant recipients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2012 Jul;27(7):2913–2921. doi: 10.1093/ndt/gfs004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Traynor JP, Simpson K, Geddes CC, Deighan CJ, Fox JG. Early initiation of dialysis fails to prolong survival in patients with end-stage renal failure. Journal of the American Society of Nephrology : JASN. 2002 Aug;13(8):2125–2132. doi: 10.1097/01.asn.0000025294.40179.e8. [DOI] [PubMed] [Google Scholar]
- 19.Sjolander A, Nyren O, Bellocco R, Evans M. Comparing different strategies for timing of dialysis initiation through inverse probability weighting. American journal of epidemiology. 2011 Nov 15;174(10):1204–1210. doi: 10.1093/aje/kwr249. [DOI] [PubMed] [Google Scholar]
- 20.Korevaar JC, Jansen MA, Dekker FW, et al. When to initiate dialysis: effect of proposed US guidelines on survival. Lancet. 2001 Sep 29;358(9287):1046–1050. doi: 10.1016/S0140-6736(01)06180-3. [DOI] [PubMed] [Google Scholar]
- 21.Tang SC, Ho YW, Tang AW, et al. Delaying initiation of dialysis till symptomatic uraemia--is it too late? Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2007 Jul;22(7):1926–1932. doi: 10.1093/ndt/gfm109. [DOI] [PubMed] [Google Scholar]
- 22.Coronel F, Cigarran S, Herrero JA. Early initiation of peritoneal dialysis in diabetic patients. Scandinavian journal of urology and nephrology. 2009;43(2):148–153. doi: 10.1080/00365590802602903. [DOI] [PubMed] [Google Scholar]
- 23.Boulware EL, Tangri N, Ephraim PL, et al. Comparative effectiveness studies to improve clinical outcomes in end stage renal disease: the DEcIDE patient outcomes in end stage renal disease study. BMC nephrology. 2012 Dec 6;13(1):167. doi: 10.1186/1471-2369-13-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Navaneethan SD, Jolly SE, Schold JD, et al. Development and validation of an electronic health record-based chronic kidney disease registry. Clinical journal of the American Society of Nephrology : CJASN. 2011 Jan;6(1):40–49. doi: 10.2215/CJN.04230510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clinical chemistry. 2007 Apr;53(4):766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 26.Wegman EJ. Hyperdimensional Data-Analysis Using Parallel Coordinates. J Am Stat Assoc. 1990 Sep;85(411):664–675. [Google Scholar]
- 27.van de Luijtgaarden MW, Noordzij M, Tomson C, et al. Factors influencing the decision to start renal replacement therapy: results of a survey among European nephrologists. Am J Kidney Dis. 2012 Dec;60(6):940–948. doi: 10.1053/j.ajkd.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 28.Cain LE, Robins JM, Lanoy E, Logan R, Costagliola D, Hernan MA. When to start treatment? A systematic approach to the comparison of dynamic regimes using observational data. The international journal of biostatistics. 2010;6(2) doi: 10.2202/1557-4679.1212. Article 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D'Agostino RB, Lee ML, Belanger AJ, Cupples LA, Anderson K, Kannel WB. Relation of pooled logistic regression to time dependent Cox regression analysis: the Framingham Heart Study. Statistics in medicine. 1990 Dec;9(12):1501–1515. doi: 10.1002/sim.4780091214. [DOI] [PubMed] [Google Scholar]
- 30.Susantitaphong P, Altamimi S, Ashkar M, et al. GFR at Initiation of Dialysis and Mortality in CKD: A Meta-analysis. Am J Kidney Dis. 2012 Jun;59(6):829–840. doi: 10.1053/j.ajkd.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. The New England journal of medicine. 2004 Sep 23;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 32.Whalley GA, Marwick TH, Doughty RN, et al. Effect of Early Initiation of Dialysis on Cardiac Structure and Function: Results From the Echo Substudy of the IDEAL Trial. Am J Kidney Dis. 2013 Feb;61(2):262–270. doi: 10.1053/j.ajkd.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 33.Hernandez D, Gonzalez A, Rufino M, et al. Time-dependent changes in cardiac growth after kidney transplantation: the impact of pre-dialysis ventricular mass. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2007 Sep;22(9):2678–2685. doi: 10.1093/ndt/gfm247. [DOI] [PubMed] [Google Scholar]
- 34.Gunal AI, Kirciman E, Guler M, Yavuzkir M, Celiker H. Should the preservation of residual renal function cost volume overload and its consequence left ventricular hypertrophy in new hemodialysis patients? Renal Failure. 2004;26(4):405–409. doi: 10.1081/jdi-120039825. [DOI] [PubMed] [Google Scholar]
- 35.Selim G, Stojceva-Taneva O, Polenakovic M, et al. Effect of nephrology referral on the initiation of haemodyalisis and mortality in ESRD patients. Prilozi / Makedonska akademija na naukite i umetnostite, Oddelenie za bioloski i medicinski nauki = Contributions / Macedonian Academy of Sciences and Arts, Section of Biological and Medical Sciences. 2007 Dec;28(2):111–126. [PubMed] [Google Scholar]
- 36.Li L, Astor BC, Lewis J, et al. Longitudinal progression trajectory of GFR among patients with CKD. Am J Kidney Dis. 2012 Apr;59(4):504–512. doi: 10.1053/j.ajkd.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ellwood AD, Jassal SV, Suri RS, Clark WF, Na Y, Moist LM. Early dialysis initiation and rates and timing of withdrawal from dialysis in Canada. Clin J Am Soc Nephrol. Feb;8(2):265–270. doi: 10.2215/CJN.01000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.