Abstract
OBJECTIVES
To characterize the epidemiology and clinical course of children with juvenile idiopathic arthritis-associated uveitis (JIA-U) in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry and explore differences between African American (AA) and Non-Hispanic White (NHW) children.
METHODS
There were 3,967 NHW and AA children with JIA enrolled in the CARRA Registry. Demographic and disease-related data were collected from time of diagnosis to enrollment. Children with JIA alone were compared to those with JIA-U. Children with JIA-U were then compared by race.
RESULTS
Mean age of children with JIA-U was 11.4 years (±4.5), 76.9% were female and 2.8% were AA. Children with JIA-U were younger at arthritis onset, female, required more medications, had <5 joints involved, had oligoarticular JIA, and ANA (+), RF (−) and anti-CCP (−). AA children with JIA-U had decreased uveitis frequency, were older at arthritis onset and more frequently diagnosed with enthesitis-related JIA. Predictors of uveitis development include female gender, early age of arthritis onset, and oligoarticular persistent and extended JIA classification, whereas polyarticular RF-positive JIA was protective.
CONCLUSIONS
The prevalence of JIA-U in AA and NHW children is 11.6% in the CARRA registry. Known risk markers (ANA, age at arthritis onset, and oligoarticular JIA) were more frequent in our JIA-U cohort. AA children had a lower frequency of JIA-U. There were significant differences in age of arthritis onset and JIA subtype between NHW and AA children, although the ANA, RF and HLA-B27 were similar. Exploration of race as a risk factor should be considered.
Keywords: juvenile idiopathic arthritis, uveitis, risk markers, outcomes
Introduction
Juvenile idiopathic arthritis (JIA) is the most common rheumatic disease of childhood with an incidence of 2 to 20 per 100,000 children, and a prevalence of 16 to 150 per 100,0001,2. It is a chronic arthritis of unknown etiology with 7 subtypes – oligoarticular persistent, oligoarticular extended polyarticular rheumatoid factor (RF)-positive, polyarticular RF-negative, systemic, psoriatic, enthesitis-related, and undifferentiated JIA. Although JIA is the most commonly used classification scheme, other categorizations include juvenile rheumatoid arthritis (JRA) consisting of 3 subtypes (pauciarticular, polyarticular, and systemic) and juvenile chronic arthritis (JCA) with 4 subtypes (pauciarticular, polyarticular, systemic and juvenile psoriatic).
JIA-associated uveitis (JIA-U), also known as iritis or iridocyclitis, is the most prevalent extra-articular manifestation of JIA in North America and occurs in 10–20% of children with JIA although reported in up to 38% of children3–12. It is a non-granulomatous chronic anterior eye inflammation that can cause vision loss and blindness and accounts for up to 80% of all pediatric anterior uveitis3.
Risk factors for uveitis
Uveitis is potentially blinding with a chronic and often relapsing course. Almost 80% of children have bilateral disease13,14. Risk factors associated with uveitis development include early age of arthritis onset, gender, short disease duration, arthritis subtype, and antinuclear antibody (ANA) seropositivity15–18. The American Academy of Pediatrics (AAP) Sections on Rheumatology and Ophthalmology have recommended screening guidelines for uveitis in children with JRA based on ANA seropositivity, JRA subtype, age at arthritis onset, and arthritis duration19,20.
Few studies on JIA-U focus on African American (AA) children21–23. In this population, the role of the ANA is unclear, predisposition to JIA subtype differs, and there appears to be a lower risk compared to non-Hispanic White (NHW) children. Hence, the risk for JIA-U may differ by race and should be explored.
Most studies in JIA-U are conducted in small cohorts. Our objective is to characterize the epidemiology and clinical course of children with JIA-U in a large cohort of AA and NHW with JIA in a multi-center registry, the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry.
Materials and Methods
This is a cross-sectional study based on a multi- center registry with 56 participating centers in the US which actively enrolls children with varied rheumatic diseases. Children with JIA alone and JIA-U were enrolled from May 2010 to June 2012. JIA was diagnosed by the treating physician based on the International League of Associations for Rheumatology (ILAR) JIA classification24. IRB approval was obtained. Demographic and disease-related data were collected from time of diagnosis to enrollment visit. Race and ethnicity were self-reported, and we only included children reported exclusively as NHW or AA.
Data Collection
Data collection was based on medical chart review, physician assessment, patient/parent recall, and physician/parent/patient completion of subjective measures. Disease related data included age at arthritis onset, JIA subtype, presence of uveitis, number of joints ever affected (< 5 or ≥ 5 joints that have had swelling, pain on movement, tenderness, and limitation of movement), radiographic evidence of joint damage, medications that have been used (non-steroidal anti-inflammatory medications (NSAIDs), glucocorticoids, non-biologic disease modifying anti-rheumatic drugs (DMARDs) and biologics), labs (ANA, RF, and cyclic citrullinated peptide antibody (anti-CCP) positivity or negativity) and HLA-B27 status.
Quality of life (QOL), function and disease activity were assessed. Children completed assessments if ≤10 years of age. Measures included: 1) physician global assessment of disease activity (0 – not active to 10 – very active), 2) health related QOL (“How do you rate your child’s health?” -excellent, very good, good, poor, very poor), and 3) parent/patient overall well-being score (“Considering all the ways that your child’s rheumatic condition affects your child, rate how your child is doing” - 0 – very well to 10 – very poor). A patient/parent pain scale score (“How much pain do you think your child had because of his/her rheumatic condition in the past week?” 0 – no pain to 10 – very severe pain) was also recorded. Children ≤9 years of age completed the Faces Pain Scale-Revised (FPS-R)25,26. The Childhood Health Assessment Questionnaire (CHAQ), a valid and reliable instrument that measures physical disability, was also completed. Twenty questions encompass 8 functional components: 1) dressing and grooming, 2) arising, 3) eating, 4) walking, 5) hygiene, 6) reach, 7) grip, and 8) activities. Each domain consists of 3 parameters: 1) difficulty in performing daily functions, 2) use of special aids or devices, and 3) activities that require assistance from another person. Scores range from 0 to 327. Greater scores indicate worse QOL, activity, pain or physical function.
Statistics
All statistical analyses were conducted in SAS 9.2 (Cary, NC). Statistical significance was assessed at the 0.05 level of significance. Differences in demographic and clinical characteristics between children with JIA alone and JIA-U were compared using Chi-square tests, two-sample tests or Wilcoxon rank-sum tests, as appropriate. Logistic regression was used to calculate odds ratios (OR) to estimate univariate associations between patient characteristics and the primary outcome, disease type (JIA or JIA-U). We analyzed the following characteristics as potential risk factors: gender, race, age at arthritis onset (years), JIA subtype, HLA-B27-positivity, ANA-positivity, RF-positivity and anti-CCP-positivity. For all analyses, the reference group was JIA alone.
Based on the results from the univariate analysis, we constructed multivariable logistic regression models using significant predictors (p < 0.1) from the univariate results to identify an optimal subset of risk markers that best predicted JIA-U. Prior to model construction, potential predictors were correlated to identify any potential sources of multicolinearity. If two or more predictors were significantly correlated with one another and the outcome (|ρ| > 0.4) then the most predictive characteristic was chosen. The final multivariate models were constructed using a modified backwards elimination procedure. In short, non-significant predictors (p > 0.05) were systematically removed until a significant increase in model fit (based on AIC and loglikelihood) could no longer be obtained or all of the predictors in the model were significant at the 0.05 level. The predictive power of the final model was assessed by calculating the area under the curve (AUC) for the receiver-operating characteristic (ROC) curve.
Results
Demographics
In our cohort of 3,967 children, the overall mean age ±SD at study enrollment was 11.4 years ±4.7. Females comprised 72% and 5.6% were AA (Table 1). This was similar to children with JIA alone and with JIA-U. Overall, 11.6% of our cohort was diagnosed with uveitis of which 2.8% were AA.
Table 1.
Comparison of JIA and JIA-U among African American and Non-Hispanic White Children
| All JIA (N = 3967) |
JIA-U (N = 459) |
JIA alone (N = 3508) |
p-value | |
|---|---|---|---|---|
| Demographic Characteristics1 | ||||
| Age at time of study, mean years ± SD | 11.4 ± 4.7 | 11.1 ± 4.5 | 11.5 ± 4.7 | 0.096 |
| Gender, female | 2857 (72.0%) | 353 (76.9%) | 2504 (71.4%) | 0.013* |
| Race | 0.010* | |||
| Non-Hispanic White | 3747 (94.5%) | 446 (97.2%) | 3301 (94.1%) | |
| African American | 220 (5.6%) | 13 (2.8%) | 207 (5.9%) | |
| Disease Characteristics1 | ||||
| Age at arthritis onset, years ± SD | 6.4 ± 4.4 | 4.2 ± 3.6 | 6.7 ± 4.5 | < 0.001* |
| JIA, subtype, N (%) | < 0.001* | |||
| Systemic | 295 (7.5%) | 2 (0.4%) | 293 (8.4%) | < 0.001* |
| Polyarticular RF-negative | 1188 (30.3%) | 109 (24.2%) | 1079 (31.1%) | 0.003* |
| Polyarticular RF-positive | 214 (5.5%) | 3 (0.7%) | 211 (6.1%) | < 0.001* |
| Oligoarticular persistent | 1140 (29.1%) | 191 (42.4%) | 949 (27.4%) | < 0.001* |
| Oligoarticular extended | 321 (8.2%) | 66 (14.7%) | 255 (7.4%) | < 0.001* |
| Psoriatic | 251 (6.4%) | 31 (6.9%) | 220 (6.3%) | 0.655 |
| Enthesitis-related | 416 (10.6%) | 34 (7.6%) | 382 (11.0%) | 0.025* |
| Undifferentiated | 95 (2.4%) | 14 (3.1%) | 81 (2.3%) | 0.313 |
| Labs1 | ||||
| ANA-positive | 1736 (49.3%) | 270 (65.4%) | 1466 (47.1%) | < 0.001* |
| RF-positive | 129 (9.1%) | 2 (1.4%) | 127 (10.0%) | 0.001* |
| HLA-B27-positive | 324 (15.0%) | 47 (19.0%) | 277 (14.4%) | 0.057 |
| Anti-CCP-positive | 145 (9.5%) | 4 (2.9%) | 141 (10.2%) | 0.006* |
| Quality of Life Measures2, a | ||||
| Physician global assessment | 1.6 ± 1.9 | 1.3 ± 1.7 | 1.6 ± 2.0 | < 0.001* |
| CHAQb | 0.36 ± 0.60 | 0.26 ± 0.50 | 0.37 ± 0.61 | < 0.001* |
| Patient/parent pain scale score | 2.6 ± 2.6 | 2.0 ± 2.5 | 2.7 ± 2.7 | < 0.001* |
| Patient/parent overall well-being score | 2.3 ± 2.3 | 1.9 ± 2.1 | 2.4 ± 2.3 | < 0.001* |
| Health Related quality of life | 0.271 | |||
| Excellent | 957 (24.4%) | 124 (27.4%) | 833 (24.0%) | |
| Very Good | 1642 (41.9%) | 193 (42.7%) | 1449 (41.8%) | |
| Good | 1196 (30.5%) | 119 (26.3%) | 1077 (31.1%) | |
| Poor | 116 (3.0%) | 15 (3.3%) | 101 (2.9%) | |
| Very Poor | 10 (0.3%) | 1 (0.2%) | 9 (0.3%) | |
| Parent/subject assessment of disease activity | 2.6 ± 2.7 | 2.1 ± 2.6 | 2.6 ± 2.7 | < 0.001* |
| Medications ever used1 | ||||
| Daily NSAIDs | 2018 (51.4%) | 154 (34.4%) | 1864 (53.6%) | < 0.001* |
| Glucocorticoids | 2576 (65.5%) | 325 (71.9%) | 2251 (64.7%) | 0.002* |
| Non-biologics immune modulator or DMARDs | 2945 (74.5%) | 409 (89.3%) | 2536 (72.5%) | < 0.001* |
| Methotrexate subcutaneous | 1922 (65.7%) | 311 (76.8%) | 1611 (63.9%) | < 0.001* |
| Methotrexate oral | 1867 (64.1%) | 268 (67.2%) | 1599 (63.6%) | 0.171 |
| Cyclosporine A | 56 (1.9%) | 17 (4.2%) | 39 (1.6%) | < 0.001* |
| Azathioprine | 18 (0.6%) | 2 (0.5%) | 16 (0.6%) | 1.000 |
| Biologics | 1744 (44.1%) | 255 (55.7%) | 1489 (42.6%) | < 0.001* |
| Infliximab | 330 (19.0%) | 126 (49.4%) | 204 (13.7%) | < 0.001* |
| Etanercept | 1330 (76.3%) | 122 (48.4%) | 1208 (81.0%) | < 0.001* |
| Adalimumab | 514 (29.5%) | 140 (55.1%) | 374 (25.2%) | < 0.001* |
| Abatacept | 101 (5.8%) | 11 (4.3%) | 90 (6.1%) | 0.312 |
| Steroid Eye Injections | 1 (0.3%) | 1 (0.4%) | 0 (0.0%) | 1.000 |
| Steroid Eye Drops | 56 (17.3%) | 51 (94.4%) | 5 (1.9%) |
p = <0.05.; Chi-square, two-sample or Wilcoxon rank-sum tests
Higher scores indicate worse disease;
Childhood Health Assessment Questionnaire
N (%) unless otherwise indicated;
Mean ± SD
Disease Characteristics
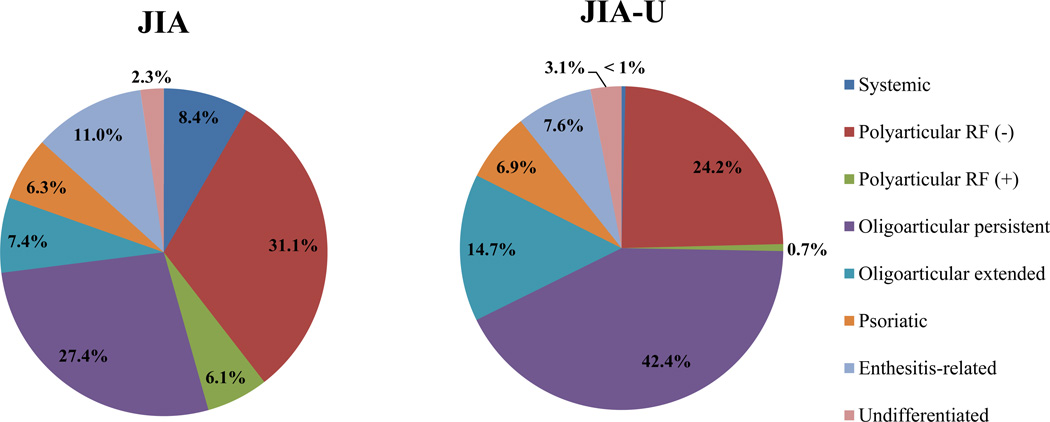
Children with JIA-U had a mean age (SD) of 4.2 years (±3.6) at time of arthritis diagnosis and were significantly younger compared to children with JIA alone with a mean age of 6.7 years (±4.5) (p < 0.001). They were more frequently of the oligoarticular persistent (42.4% vs. 27.4%; p < 0.001) and extended (14.7% vs. 7.4%; p < 0.001) JIA subtypes, and more likely to have <5 joints ever involved compared to JIA alone (54.8% vs. 43.3%; p < 0.001) (Figure 1). There was no significant difference in joint damage as evidenced by radiographic exam. They were also more frequently ANA-positive (65.4% vs. 47.1%; p < 0.001).
Figure 1.
Frequency of JIA subtypes in children with and without JIA-associated uveitis
Children with JIA alone were more frequently of the systemic (8.4%, p<0.001), polyarticular RF-negative (31.1%, p=0.003), polyarticular RF-positive (6.1%, <0.001), and enthesitis-related JIA subtypes (11%, p=0.025) (Figure 1). They were more frequently RFpositive (10%, p=0.001) and anti-CCP antibody-positive (10.2%, p=0.006).
Medication use
Children with JIA alone were more frequently on daily NSAIDs therapy (34.4% vs. 53.6%; p < 0.001), but children with JIA-U were more likely to have used glucocorticoids (71.9% vs. 64.7%; p = 0.002), biologic therapies (55.7% vs. 42.6%; p < 0.001), and non-biologic immune modulators or DMARDs (89.3% vs.72.5%; p < 0.001) (Table 1). Hence, they were more commonly on mycophenolate, subcutaneous methotrexate, cyclosporine A, infliximab and adalimumab whereas children with JIA alone were more frequently on etanercept. Of 54 children with JIA-U with data, 51 (94.4%) required steroid ocular drops and 1(0.4%) required steroid ocular injections.
Measures of quality of life and function
Children with JIA alone had worse physician global assessment scores (1.6 ± 2.0 vs. 1.3 ±1.7, p < 0.001), parent/patient overall well-being scores (2.4 ±2.3 vs.1.9 ± 2, p < 0.001) and parent/patient assessment of disease activity (2.6 ± 2.7 vs. 2.1 ± 2.6, p < 0.001) compared to JIA-U. As expected, they also had worse CHAQ scores (0.37 ±0.61 vs. 0.26 ± 0.50; p < 0.001), and reported higher pain scores (2.7 ± 2.7 vs. 2.0 ± 2.5; p < 0.001). However, there were no significant differences in health related QOL scores.
Racial differences in uveitis
Of 459 children with JIA-U (11.6% of cohort), 446 were NHW and 13 were AA. NHW children had a higher prevalence of uveitis compared to AA children (11.9% vs. 5.9%, p = 0.007). AA children were more frequently older at age of arthritis onset (8.0 ±5.1 vs. 4.2 ± 3.5; p = 0.035) and were more likely to be diagnosed with enthesitis-related JIA (30.8% vs. 6.9%; p = 0.001). There was no significant difference in gender, joint involvement, lab status (ANA, RF, HLA-B27, and anti-CCP) or medication use.
AA children with JIA-U also had worse physician global assessment scores which approached significance (2.2 ± 1.9, p = 0.050). There were no significant differences in the CHAQ or the pain scale, overall well-being, heath related QOL, or parent/subject disease activity assessment scores. Likewise, the use of glucocorticoids, biologics and non-biologics immune modulators or DMARDs was similar in both groups except for Azathioprine in AA children (10% vs. 0.3%, p = 0.0048).
Risk Factors for JIA-U
The results of the univariate and multivariate analyses are provided in tables 2 and 3. Of the 15 risk factors examined, 12 were significantly associated with an increase or decrease in the odds of having JIA-U. Prior to multivariate analysis, potential risk factors were correlated to identify potential sources of multicolinearity. Polyarticular RF-negative and oligoarticular persistent JIA were found to be significantly negatively correlated (r = −0.42, p < 0.001) since these two subtypes accounted for approximately 67% (300 / 450) of all JIA-U cases. Thus, if a patient did not have the oligoarticular persistent subtype then they were highly likely to have the polyarticular RF-negative subtype or vice versa. Since JIA-U patients had a higher prevalence of the oligoarticular persistent subtype, the polyarticular RF-negative subtype was eliminated from model consideration.
Table 2.
Patient risk Factors associated with JIA-U
| Risk Factor | Univariate Odds Ratio (95% Confidence Interval) |
|---|---|
| Gender (Female) | 1.34 (1.00 – 1.68)* |
| Race (AA) | 0.47 (0.26 – 0.82)** |
| Age at arthritis onset | 0.86 (0.83 – 0.88)*** |
| JIA subtype | |
| Systemic | 0.05 (0.01 – 0.19)*** |
| Polyarticular RF-negative | 0.70 (0.56 – 0.88)** |
| Polyarticular RF-positive | 0.10 (0.03 – 0.32)*** |
| Oligoarticular persistent | 1.93 (1.58 – 2.36)*** |
| Oligoarticular extended | 2.15 (1.61 – 2.87)*** |
| Psoriatic | 1.08 (0.74 – 1.60) |
| Enthesitis related | 0.66 (0.46 – 0.94)* |
| Undifferentiated | 0.75 (0.42 – 1.33) |
| Labs | |
| ANA positive | 2.12 (1.71 – 2.63)*** |
| RF positive | 0.13 (0.03 – 0.54)** |
p< 0.05,
p < 0.01,
p < 0.001
Table 3.
Odds ratios and 95% confidence intervals for significant predictors of JIA-U.
| Predictor | Odds Ratio | 95% CI |
|---|---|---|
| Gender (Female vs. Male) | 1.36 | (0.86 – 2.11) |
| Age of Onset | 0.92 | (0.87 – 0.98) |
| Gender*Age of Onset (Female Only) | 0.93 | (0.87 – 0.999) |
| ANA-positive (Yes vs. No) | 1.61 | (1.27 – 2.05) |
| Polyarticular RF-positive (Yes vs. No) | 0.26 | (0.08 – 0.82) |
| Oligoarticular Persistent (Yes vs. No) | 1.61 | (1.27 – 2.05) |
| Oligoarticular Extended (Yes vs. No) | 1.89 | (1.34 – 2.67) |
After multivariate logistic modeling, significant predictors of JIA-U were female gender, younger age of onset, ANA-positivity, polyarticular RF-positive JIA, oligoarticular persistent JIA and oligoarticular extended JIA. Parameter estimates, representing the log-odds, and p-values for the final model are provided in Table 2 and the exponentiated regression coefficients and associated 95% confidence intervals are provided in Table 3. A significant interaction between gender and age of onset was detected (p = 0.049). Regardless of disease (JIA or JIA-U), females had earlier onset of arthritis than males; however, the difference in age of onset between males and females with JIA was not as pronounced as in JIA-U. For JIA alone, the average age of onset of symptoms in males was 7.3 [95% CI: 6.9 – 7.5] years and in females was 6.5 years [95% CI: 6.3 – 6.7]. In contrast, in JIA-U patients, the average age of onset of symptoms in males was 5.9 years [95% CI: 5.0 – 6.7] and in females was 3.8 years [95% CI 3.4 – 4.1]. The area under the receiver operating curve for the final model was 0.714 indicating fair accuracy of the model.
Discussion
There is an 11% prevalence of JIA-U in AA and NWH children in the CARRA registry which comprises the largest database of children with JIA and JIA-U to date. The reported prevalence of uveitis in a juvenile arthritis population has ranged widely from 10–38% which can be attributed to the use of different juvenile arthritis classification schemes and varied follow up5–8,10,11.
Risk factors for uveitis development
Common risk markers for uveitis development include ANA-positivity, young age at arthritis onset, JIA subtypes such as oligoarticular, psoriatic, enthesitis- related and undifferentiated JIA, and female gender11,14,16,28–33. The AAP guidelines recommend uveitis screening every 3 months for a child who is ANA-positive, of oligoarticular or polyarticular JRA subtypes, have arthritis onset ≤ 6 years of age, and < 4 years arthritis duration19,20. Our results are consistent with the AAP guidelines and also confirm past studies wherein we noted the significance of the ANA, age at arthritis onset, JIA subtypes, female gender, RF, and HLA-B2734,35. Only two reports have noted an association between anti-CCP-positivity and uveitis36,37.
Oligoarticular and polyarticular RF-negative JIA tend to have a greater uveitis risk although the significance of extended vs. persistent subtypes differs11,12,14,16,28,32,38. In our cohort, children with JIA-U were more frequently diagnosed with oligoarticular persistent (42.4%) and extended JIA (14.7%) (Figure 1). JIA alone were more commonly diagnosed with systemic, polyarticular RF-positive, and enthesitis-related JIA. This is similar to BenEzra’s study wherein children with polyarticular and systemic JRA were at lower risk for uveitis10. We confirm that children with oligoarticular JIA are at an increased uveitis risk12,29.
Interestingly, medication use differed since children with uveitis required more DMARDs and biologic therapies. This may be secondary to the need for more aggressive treatment for ocular inflammation, especially since the uveitis group had milder forms of JIA. Etanercept was the exception since there have been reports of decreased effectiveness compared to other tumor necrosis factor alpha inhibitors for uveitis treatment39–44.
Predictors of uveitis development
On initial analysis, female gender, younger age at arthritis onset, oligoarticular persistent and extended JIA, and ANA-positivity appeared to be associated with uveitis development. AA race, systemic JIA, polyarticular RF-negative JIA, enthesitis-related JIA and RF-positivity appeared to be significantly negatively associated with JIA-U. However, after performing multivariate logistic regression, only female gender, early age of arthritis onset, oligoarticular persistent and extended JIA subtypes remained significant risks for uveitis susceptibility whereas polyarticular RF-positive JIA was protective. The effects of AA race, systemic JIA, polyarticular RF-negative JIA, enthesitis related JIA, and anti-CCP-positivity were no longer protective which may be secondary to the small size of AA patients with uveitis, and decreased number of patients in the less common JIA subtypes.
Not all JIA subtypes are included in the AAP guidelines for uveitis screening. Heilinghaus et. al created guidelines specific for JIA and recommend 3 month assessments for children with oligoarticular, RF-negative polyarticular, psoriatic and undifferentiated JIA who are ANA-positive, ≤6 years at JIA onset, and have had arthritis for ≤4 years16. Our study also demonstrated that children with psoriatic JIA have a greater frequency of uveitis, but this only approached significance. Likewise, many children with JIA-U were diagnosed with polyarticular RF-negative JIA, but significantly less than in the JIA alone group. Hence, the importance of other JIA subtypes requires further exploration since risk for uveitis development and need for screening may differ and are not all included in the current AAP guidelines.
Studies have noted an increase in uveitis susceptibility in females and an increase in ocular complications in males33,45. Interestingly, we observed an interaction between female gender and young age of arthritis onset. Hence, in our cohort, the impact of arthritis age of onset on uveitis development is affected by female gender. We could hypothesize that children who are diagnosed with JIA at a very young age have an increased risk for JIA-U if they are female.
The role of race in uveitis
Little is known about the impact of race on uveitis. Race plays a significant role in other conditions such as sarcoidosis and systemic lupus erythematosus. Likewise, there appears to be an association between JIA subtype and race wherein children who are AA or native North American are more likely to develop polyarticular RF-positive JIA, NHW children develop extended oligoarticular JIA and psoriatic JIA, and Asians develop enthesitis-related JIA23,46. Since different races have varied risk for JIA subtypes, and the JIA subtypes vary in their risk for uveitis, race may influence uveitis susceptibility.
Most studies of JIA-U have been in children of European ancestry with only a few focused on children of AA descent (n=30–42)21–23. In three studies, JIA-U prevalence ranged from 4–8% in AA children, with absence of ANA-positivity. In a 1984 study, none of the 8.3% of 42 South African children diagnosed with JIA-U were ANA-positive47. Similarly, in 1997, 20% of 172 children with JRA were AA, 8.7% of these had uveitis, and none were ANA-positive22. In 2007, a study of 758 children with JIA consisting of 4% blacks demonstrated that black children had a lower risk of developing JIA-U compared to children of European ancestry23. In a cohort of 859 children with JIA, there was a relative risk (RR) of 1.27 (p = 0.036) for developing uveitis in European children and a RR of 0.36 (p < 0.0001) in Non-European children23.
In concurrence with the literature, our cohort consisted of 3% AA children with a lower frequency of JIA-U compared to NHW children (97%). As expected in children with uveitis, there were more children of the oligoarticular persistent subtype in both groups. They were similar in RF-negative status and percentage of females. However, there were significant differences in JIA subtype wherein AA children were more frequently of the enthesitis-related JIA subtype. There was increased HLA-B27 positivity (40% vs. 18.1%; p = 0.100), but this was not statistically significant. AA children with uveitis were older at time of arthritis onset (8.0 ± 5.1 vs. 4.2 ± 3.5, p = 0.035). Since young age at arthritis onset is a risk factor for uveitis, this may explain why AA children have decreased uveitis11.
Interestingly, there was no difference in ANA status. Previous studies have shown that the ANA is usually absent in AA children with uveitis hence further investigation in a larger cohort of AA children is important since the ANA is taken into consideration in determining the schedule for uveitis screening in the AAP guidelines22,47. Risk factors for JIA-U may differ between races. To our knowledge, no studies have examined the role of JIA subtype and race in uveitis susceptibility or severity.
Both groups were similar in their use of DMARDs and biologic therapies. Since, AA children had a lower frequency of uveitis, it is possible that the indication for therapy differed since AA children had worse arthritis and NHW children had more uveitis. Therefore, it is important to determine whether medications are used specifically for arthritis or uveitis in future studies.
Quality of life and function
Children with JIA alone had worse disease activity, overall well-being, physical function and pain as evidenced by their scores in subjective measures. Since they were more frequently diagnosed with the JIA subtypes with greater joint involvement, they may have increased functional disability. However, most children had approximately 5 years of arthritis prior to enrollment (age at arthritis onset 6.4 ± 4.4 years and age at study enrollment 11.4 ± 4.7 years), their arthritis may have been better controlled or the children had adapted to their disease. Hence, scores may have differed if measured earlier in the disease course.
We expected children with JIA-U to score worse since they had both ocular and musculoskeletal involvement. However, there was no data on the status of their uveitis and disease may have been quiescent. Likewise, uveitis can be asymptomatic.
Strengths and Limitations
Our cohort consisted of the largest population of children with JIA and JIA-U from diverse geographic regions which may be a true representation of JIA and uveitis in a U.S. population. Although our cohort of AA children with uveitis was small, this was similar to the literature.
Due to the large scale of this registry and limitation in data collection, not all details of uveitis are known (i.e. date of uveitis onset, visual symptoms, uveitis complications such as cataracts, glaucoma, vision loss or blindness or reason for medications). Likewise, uveitis in some patients may be undiagnosed since the disease is often asymptomatic.
Only joint counts and the CHAQ were used to measure physical disability. Additionally, joint counts were measured as < or ≥5 joints and not as total number of joints. The clinical ocular exam (i.e. slit lamp exam or visual acuity), and other validated subjected measures such as the Pediatric Quality of Life Inventory (PedsQL) to measure overall QOL, or the Effects of Youngsters’ Eyesight on Quality of Life (EYE-Q) to measure visual function and vision related QOL could be considered but may be difficult to implement in such a large cohort since they are time consuming to administer48,49.
Conclusion
JIA-associated uveitis was found in 11% of children with JIA in the CARRA registry which consists of the largest cohort of JIA-U to date. Although we confirm known risk markers of uveitis, our findings suggest that consideration should be given to other factors such as race and JIA subtypes as this may affect screening guidelines. Their role in the development of ocular complications should also be further explored.
Acknowledgement
The authors would like to thank Matthew Kent and Joseph Young for their assistance with medical chart review and data entry, and the following CARRA Registry site principal investigators: L. Abramson, B. Adams, J. Birmingham, P. Blier, S. Bowyer, E. Chalom, F. Dedeoglu, P. Ferguson, B. Gottlieb, T. Graham, M. Klein-Gitelman, D. Goldsmith, G. Higgins, J.R. Hollister, J. Hsu, A. Huttenlocher, N. Ilowite, L. Imundo, C. Inman, T. Jerath, L. Jung, P. Kahn, D. Kingsbury, A. Lasky, T. Lehman, C. Lindsley, J. Lopez-Benitez, D. McCurdy, N. Moorthy, B. Myones, K. Nanda, J. Olson, K. O’Neil, K. Onel, K. Peterson, S. Prahalad, M. Punaro, A. Quintero, C. Rabinovich, A. Reed, S. Ringold, D. Rothman, N. Ruth, C. Sandborg, E. von Scheven, K. Schikler, D. Sherry, N. Singer, S. Spalding, R. Syed, K. Torok, R.
Grant Support: The work was done with the support of NIH funding through CARRA. CARRA receives support and funding from Friends of CARRA, the Arthritis Foundation, and the NIH. Dr. Angeles-Han was supported by Award Number K23EY021760 from the National Eye Institute and also by a grant from the American College of Rheumatology Research and Education Foundation and the Arthritis Foundation Career Development Bridge Funding Award. Dr. Prahalad is supported by The National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01-AR060893), The Marcus Foundation Inc. and The Arthritis Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Eye Institute, the National Institute of Arthritis and Musculoskeletal and Skin Diseases or the National Institutes of Health.
References
- 1.Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet. 2007 Mar 3;369(9563):767–778. doi: 10.1016/S0140-6736(07)60363-8. [DOI] [PubMed] [Google Scholar]
- 2.Cassidy JP, RE, Laxer RM, Lindsley CB. Textbook of Pediatric Rheumatology. Philadelphia: Elsevier Saunders; 2005. [Google Scholar]
- 3.Foster CS. Diagnosis and treatment of juvenile idiopathic arthritis-associated uveitis. Curr Opin Ophthalmol. 2003 Dec;14(6):395–398. doi: 10.1097/00055735-200312000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Nordal EB, Songstad NT, Berntson L, Moen T, Straume B, Rygg M. Biomarkers of chronic uveitis in juvenile idiopathic arthritis: predictive value of antihistone antibodies and antinuclear antibodies. The Journal of rheumatology. 2009 Aug;36(8):1737–1743. doi: 10.3899/jrheum.081318. [DOI] [PubMed] [Google Scholar]
- 5.Kanski JJ. Juvenile arthritis and uveitis. Survey of ophthalmology. 1990 Jan-Feb;34(4):253–267. doi: 10.1016/0039-6257(90)90026-r. [DOI] [PubMed] [Google Scholar]
- 6.Kesen MR, Setlur V, Goldstein DA. Juvenile idiopathic arthritis-related uveitis. Int Ophthalmol Clin. 2008 Summer;48(3):21–38. doi: 10.1097/IIO.0b013e31817d998f. [DOI] [PubMed] [Google Scholar]
- 7.Kotaniemi K, Savolainen A, Karma A, Aho K. Recent advances in uveitis of juvenile idiopathic arthritis. Survey of ophthalmology. 2003 Sep-Oct;48(5):489–502. doi: 10.1016/s0039-6257(03)00084-5. [DOI] [PubMed] [Google Scholar]
- 8.Zierhut M, Michels H, Stubiger N, Besch D, Deuter C, Heiligenhaus A. Uveitis in children. Int Ophthalmol Clin. 2005 Spring;45(2):135–156. doi: 10.1097/01.iio.0000155903.87679.c2. [DOI] [PubMed] [Google Scholar]
- 9.Kanski JJ. Uveitis in juvenile chronic arthritis. Clinical and experimental rheumatology. 1990 Sep-Oct;8(5):499–503. [PubMed] [Google Scholar]
- 10.BenEzra D, Cohen E, Behar-Cohen F. Uveitis and juvenile idiopathic arthritis: A cohort study. Clin Ophthalmol. 2007 Dec;1(4):513–518. [PMC free article] [PubMed] [Google Scholar]
- 11.Kotaniemi K, Kautiainen H, Karma A, Aho K. Occurrence of uveitis in recently diagnosed juvenile chronic arthritis: a prospective study. Ophthalmology. 2001 Nov;108(11):2071–2075. doi: 10.1016/s0161-6420(01)00773-4. [DOI] [PubMed] [Google Scholar]
- 12.Sabri K, Saurenmann RK, Silverman ED, Levin AV. Course, complications, and outcome of juvenile arthritis-related uveitis. J Aapos. 2008 Dec;12(6):539–545. doi: 10.1016/j.jaapos.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Dana MR, Merayo-Lloves J, Schaumberg DA, Foster CS. Visual outcomes prognosticators in juvenile rheumatoid arthritis-associated uveitis. Ophthalmology. 1997 Feb;104(2):236–244. doi: 10.1016/s0161-6420(97)30329-7. [DOI] [PubMed] [Google Scholar]
- 14.Paroli MP, Speranza S, Marino M, Pirraglia MP, Pivetti-Pezzi P. Prognosis of juvenile rheumatoid arthritis-associated uveitis. European journal of ophthalmology. 2003 Aug-Sep;13(7):616–621. doi: 10.1177/112067210301300704. [DOI] [PubMed] [Google Scholar]
- 15.Raymaekers A, Foets B, Wouters C, Casteels I. Visual outcome in children with juvenile idiopathic arthritis related uveitis. Bull Soc Belge Ophtalmol. 2006;(300):67–72. [PubMed] [Google Scholar]
- 16.Heiligenhaus A, Niewerth M, Ganser G, Heinz C, Minden K. Prevalence and complications of uveitis in juvenile idiopathic arthritis in a population-based nation-wide study in Germany: suggested modification of the current screening guidelines. Rheumatology (Oxford, England) 2007 Jun;46(6):1015–1019. doi: 10.1093/rheumatology/kem053. [DOI] [PubMed] [Google Scholar]
- 17.Thorne JE, Woreta F, Kedhar SR, Dunn JP, Jabs DA. Juvenile idiopathic arthritis-associated uveitis: incidence of ocular complications and visual acuity loss. American journal of ophthalmology. 2007 May;143(5):840–846. doi: 10.1016/j.ajo.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 18.Angeles-Han STYS, Vogler LB. Updates on the risk markers and outcomes of severe juvenile idiopathic arthritis-associated uveitis. International Journal of Clinical Rheumatology. 2013;8(1) doi: 10.2217/ijr.12.83. IN PRESS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cassidy J, Kivlin J, Lindsley C, Nocton J. Ophthalmologic examinations in children with juvenile rheumatoid arthritis. Pediatrics. 2006 May;117(5):1843–1845. doi: 10.1542/peds.2006-0421. [DOI] [PubMed] [Google Scholar]
- 20.American Academy of Pediatrics Section on Rheumatology and Section on Ophthalmology: Guidelines for ophthalmologic examinations in children with juvenile rheumatoid arthritis. Pediatrics. 1993 Aug;92(2):295–296. [PubMed] [Google Scholar]
- 21.Greenwood BM. Polyarthritis in Western Nigeria. II. Still's disease. Annals of the rheumatic diseases. 1969 Nov;28(6):617–623. doi: 10.1136/ard.28.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz MM, Simpson P, Kerr KL, Jarvis JN. Juvenile rheumatoid arthritis in African Americans. The Journal of rheumatology. 1997 Sep;24(9):1826–1829. [PubMed] [Google Scholar]
- 23.Saurenmann RK, Rose JB, Tyrrell P, et al. Epidemiology of juvenile idiopathic arthritis in a multiethnic cohort: ethnicity as a risk factor. Arthritis and rheumatism. 2007 Jun;56(6):1974–1984. doi: 10.1002/art.22709. [DOI] [PubMed] [Google Scholar]
- 24.Petty RE, Southwood TR, Manners P, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. The Journal of rheumatology. 2004 Feb;31(2):390–392. [PubMed] [Google Scholar]
- 25.Hicks CL, von Baeyer CL, Spafford PA, van Korlaar I, Goodenough B. The Faces Pain Scale-Revised: toward a common metric in pediatric pain measurement. Pain. 2001 Aug;93(2):173–183. doi: 10.1016/S0304-3959(01)00314-1. [DOI] [PubMed] [Google Scholar]
- 26.Bieri D, Reeve RA, Champion GD, Addicoat L, Ziegler JB. The Faces Pain Scale for the selfassessment of the severity of pain experienced by children: development, initial validation, and preliminary investigation for ratio scale properties. Pain. 1990 May;41(2):139–150. doi: 10.1016/0304-3959(90)90018-9. [DOI] [PubMed] [Google Scholar]
- 27.Singh G, Athreya BH, Fries JF, Goldsmith DP. Measurement of health status in children with juvenile rheumatoid arthritis. Arthritis and rheumatism. 1994 Dec;37(12):1761–1769. doi: 10.1002/art.1780371209. [DOI] [PubMed] [Google Scholar]
- 28.Chen CS, Roberton D, Hammerton ME. Juvenile arthritis-associated uveitis: visual outcomes and prognosis. Can J Ophthalmol. 2004 Oct;39(6):614–620. doi: 10.1016/s0008-4182(04)80026-7. [DOI] [PubMed] [Google Scholar]
- 29.Saurenmann RK, Levin AV, Feldman BM, et al. Prevalence, risk factors, and outcome of uveitis in juvenile idiopathic arthritis: a long-term followup study. Arthritis and rheumatism. 2007 Feb;56(2):647–657. doi: 10.1002/art.22381. [DOI] [PubMed] [Google Scholar]
- 30.Bolt IB, Cannizzaro E, Seger R, Saurenmann RK. Risk factors and longterm outcome of juvenile idiopathic arthritis-associated uveitis in Switzerland. The Journal of rheumatology. 2008 Apr;35(4):703–706. [PubMed] [Google Scholar]
- 31.Cassidy JT, Sullivan DB, Petty RE. Clinical patterns of chronic iridocyclitis in children with juvenile rheumatoid arthritis. Arthritis and rheumatism. 1977 Mar;20(2 Suppl):224–227. [PubMed] [Google Scholar]
- 32.Sim KT, Venning HE, Barrett S, Gregson RM, Amoaku WM. Extended oligoarthritis and other risk factors for developing JIA-associated uveitis under ILAR classification and its implication for current screening guideline. Ocular immunology and inflammation. 2006 Dec;14(6):353–357. doi: 10.1080/09273940600977233. [DOI] [PubMed] [Google Scholar]
- 33.Saurenmann RK, Levin AV, Feldman BM, Laxer RM, Schneider R, Silverman ED. Risk factors for development of uveitis differ between girls and boys with juvenile idiopathic arthritis. Arthritis and rheumatism. 2010 Jun;62(6):1824–1828. doi: 10.1002/art.27416. [DOI] [PubMed] [Google Scholar]
- 34.Wakefield D, Chang JH, Amjadi S, Maconochie Z, Abu El-Asrar A, McCluskey P. What is new HLAB27 acute anterior uveitis? Ocular immunology and inflammation. 2011 Apr;19(2):139–144. doi: 10.3109/09273948.2010.542269. [DOI] [PubMed] [Google Scholar]
- 35.Brewerton DA, Caffrey M, Nicholls A, Walters D, James DC. Acute anterior uveitis and HL-A 27. Lancet. 1973 Nov 3;302(7836):994–996. doi: 10.1016/s0140-6736(73)91090-8. [DOI] [PubMed] [Google Scholar]
- 36.Wladis EJ, Pappa C, Cavaliere LF. Anticyclic-citrullinated protein antibodies in the diagnosis of ophthalmic inflammatory disease. Ophthalmic plastic and reconstructive surgery. 2011 Jan-Feb;27(1):e1–e2. doi: 10.1097/IOP.0b013e3181c70c2e. [DOI] [PubMed] [Google Scholar]
- 37.Itty S, Pulido JS, Bakri SJ, Baratz KH, Matteson EL, Hodge DO. Anti-cyclic citrullinated peptide, rheumatoid factor, and ocular symptoms typical of rheumatoid arthritis. Transactions of the American Ophthalmological Society. 2008;106:75–81. discussion 81–73. [PMC free article] [PubMed] [Google Scholar]
- 38.Ozdal PC, Vianna RN, Deschenes J. Visual outcome of juvenile rheumatoid arthritis-associated uveitis in adults. Ocular immunology and inflammation. 2005 Feb;13(1):33–38. doi: 10.1080/09273940590909220. [DOI] [PubMed] [Google Scholar]
- 39.Smith JA, Thompson DJ, Whitcup SM, et al. A randomized, placebo-controlled, double-masked clinical trial of etanercept for the treatment of uveitis associated with juvenile idiopathic arthritis. Arthritis and rheumatism. 2005 Feb 15;53(1):18–23. doi: 10.1002/art.20904. [DOI] [PubMed] [Google Scholar]
- 40.Galor A, Perez VL, Hammel JP, Lowder CY. Differential effectiveness of etanercept and infliximab in the treatment of ocular inflammation. Ophthalmology. 2006 Dec;113(12):2317–2323. doi: 10.1016/j.ophtha.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 41.Lim LL, Fraunfelder FW, Rosenbaum JT. Do tumor necrosis factor inhibitors cause uveitis? A registry-based study. Arthritis and rheumatism. 2007 Oct;56(10):3248–3252. doi: 10.1002/art.22918. [DOI] [PubMed] [Google Scholar]
- 42.Tynjala P, Lindahl P, Honkanen V, Lahdenne P, Kotaniemi K. Infliximab and etanercept in the treatment of chronic uveitis associated with refractory juvenile idiopathic arthritis. Annals of the rheumatic diseases. 2007 Apr;66(4):548–550. doi: 10.1136/ard.2006.058248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Foeldvari I, Nielsen S, Kummerle-Deschner J, et al. Tumor necrosis factor-alpha blocker in treatment of juvenile idiopathic arthritis-associated uveitis refractory to second-line agents: results of a multinational survey. The Journal of rheumatology. 2007 May;34(5):1146–1150. [PubMed] [Google Scholar]
- 44.Saurenmann RK, Levin AV, Rose JB, et al. Tumour necrosis factor alpha inhibitors in the treatment of childhood uveitis. Rheumatology (Oxford, England) 2006 Aug;45(8):982–989. doi: 10.1093/rheumatology/kel030. [DOI] [PubMed] [Google Scholar]
- 45.Ayuso VK, Ten Cate HA, van der Does P, Rothova A, de Boer JH. Male gender and poor visual outcome in uveitis associated with juvenile idiopathic arthritis. American journal of ophthalmology. 2010 Jun;149(6):987–993. doi: 10.1016/j.ajo.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 46.Graham TB, Glass DN. Juvenile rheumatoid arthritis: ethnic differences in diagnostic types. The Journal of rheumatology. 1997 Sep;24(9):1677–1679. [PubMed] [Google Scholar]
- 47.Haffejee IE, Raga J, Coovadia HM. Juvenile chronic arthritis in black and Indian South African children. S Afr Med J. 1984 Mar 31;65(13):510–514. [PubMed] [Google Scholar]
- 48.Angeles-Han ST, Griffin KW, Harrison MJ, et al. Development of a vision-related quality of life instrument for children ages 8–18 years for use in juvenile idiopathic arthritis-associated uveitis. Arthritis Care Res (Hoboken) 2011 Sep;63(9):1254–1261. doi: 10.1002/acr.20524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Angeles-Han ST, Griffin KW, Lehman TJ, et al. The importance of visual function in the quality of life of children with uveitis. J AAPOS. 2010 Mar 15; doi: 10.1016/j.jaapos.2009.12.160. [DOI] [PMC free article] [PubMed] [Google Scholar]