Abstract
Correlated inter-domain motions in proteins can mediate fundamental biochemical processes such as signal transduction and allostery. Here we characterize at structural level the inter-domain coupling in a multidomain enzyme, Adenylate Kinase (AK), using computational methods that exploit the shape information encoded in residual dipolar couplings (RDCs) measured under steric alignment by nuclear magnetic resonance (NMR). We find experimental evidence for a multi-state equilibrium distribution along the opening/closing pathway of Adenylate Kinase, previously proposed from computational work, in which inter-domain interactions disfavour states where only the AMP binding domain is closed. In summary, we provide a robust experimental technique for study of allosteric regulation in AK and other enzymes.
Author Summary
Most proteins contain several domains, and inter-domain motions play important roles in their biological functions. Describing the various inter-domain orientations that multi-domain proteins adopt at equilibrium is challenging, but key for understanding the relationship between protein structure and function. When more than two domains are present in a protein, correlated domain motions can be of fundamental importance for biological function. This type of behaviour is typical of molecular machines but is extremely challenging to characterize both from experimental and theoretical viewpoints. In this paper, we present a hybrid experimental/computational approach to address this problem by exploiting the information on molecular shape contained in nuclear magnetic resonance experiments to determine accurate conformation ensembles for the multi-domain enzyme adenylate kinase with help of advanced simulation methods.
Introduction
Conformational heterogeneity as a consequence of dynamics is an intrinsic feature of proteins linked to biological function.[1] An important aspect for our understanding of protein dynamics is a molecular characterization of the structural states that are populated and of how correlated conformational changes can mediate biological functions such as allostery and signal transduction.[2], [3] The characterization of correlated conformational changes requires a description of how local structural heterogeneity translates into global conformational changes through collective motions. Recent developments in the analysis of various NMR parameters at atomic resolution, such as spin relaxation rates[4] and residual dipolar couplings (RDCs),[5]–[7] have enabled the description of local structural heterogeneity. However, inferences of collective conformational changes from local correlated motions have relied on force fields or motional models.[7]–[9]
RDCs are NMR parameters that report on both the local and global structural properties of weakly aligned macromolecules and, under the assumption that alignment does not alter the properties of the protein, can be used to study the amplitude of dynamics, especially when combined with simulations.[5], [10] Alignment can be induced by steric and/or electrostatic interactions of the macromolecule with external media. Specifically, for a given conformation, RDCs depend on the geometrical properties of the environment of the nuclei in the molecular frame and on the direction and degree of alignment[11], [12] (Eq. 1, Fig. 1).
| (1) |
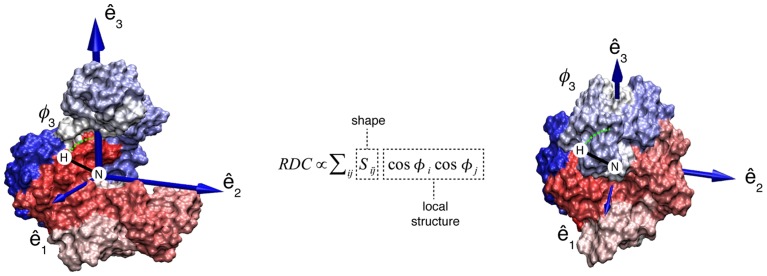
Figure 1. RDCs report on molecular shape.
RDCs depend on the geometry of the relevant nuclei (φ i) and on the degree and direction of alignment (S) of the molecule with respect to the laboratory frame. S is a 3×3 symmetric and traceless matrix, which contains information on the shape of the molecule. φ i is the angle between the inter-nuclear vector and axis i of the molecular reference frame where S is defined. The direction and magnitude of alignment are shown as eigenvectors (êi, i = 1, 2, 3) and eigenvalues (vector size, not in scale) for AKe in the open (left) and closed (right) states.
In Eq. 1, for two nuclei P and Q, φiPQ is the angle between the inter-nuclear vector and axis i of the molecular frame, Sij is an element of the alignment tensor (S), rPQ is the distance between P and Q, γX is the gyromagnetic ratio of nucleus X, µ0 is the magnetic susceptibility of vacuum and h is Planck's constant. The alignment tensor S is given by the alignment mechanism and, under steric alignment conditions, can be computed accurately and is closely related to the shape of the aligned macromolecule.[12]–[14] RDCs measured in steric alignment combine the local information contained in NMR parameters with the shape information contained in relaxation rates[15]–[17] and small angle X-ray scattering (SAXS).[18] Since inter-domain motions alter the shape of proteins RDCs measured in steric alignment have, as we will show, the potential to act as reporters of inter-domain structural heterogeneity.
RDCs can be used to study the structural heterogeneity of globular and disordered proteins by analysing the effect of (sub-ms) motional averaging on this NMR parameter. The effect of fast (sub-ns) local motions that do not directly alter the magnitude and main directions of alignment can be analysed in the molecular frame defined by the alignment tensor of the average structure.[19], [20] The analysis of motions that change the shape of the protein, that are typically slower than the timescale of alignment (0.5 to 5 ns) [21] needs on the contrary to explicitly consider that the various conformations in fast exchange that contribute to the measured RDC can have different alignment tensors.[20], [22] Here we show that for a molecule undergoing conformational changes in two distal sites the RDCs depend on the degree of correlation of such changes. We exploit this to characterize the degree of correlation of the inter-domain conformational changes occurring in the substrate-free state of E. coli Adenylate Kinase (AKe), an enzyme that undergoes conformational changes involving shape changes.
Results and Discussion
Our approach is based on the determination of ensembles that collectively agree with RDCs. The generation of the ensemble is divided in two steps (see Methods and Supporting Information). First, an enumeration (ca. 105) of inter-domain orientations is performed using unrestrained simulations. Secondly, the ensemble of minimal size that best fits the RDCs is identified by a genetic algorithm (see Figs. S1 and S2).[23] To maximize the coverage of conformational space in the first step we used PELE, an all atom Monte Carlo simulation algorithm with a move set designed to explore normal modes (see Methods and Supporting Information).[24] The RDCs of each conformer are calculated via Eq. 1 using two independent methods to compute the S from knowledge of the structure.[12], [13] In this case we used only the steric tensor because it is related to shape but it is principle possible to use also the electrostatic tensor, particularly when conformational changes modulate the charge distribution.
AKe is an essential enzyme that catalyses the reversible conversion of ATP and AMP into two ADP molecules. The role of AKe is to maintain the energy balance in the cell, which is essential. AKe is a modular enzyme composed of three sub-domains: a CORE domain responsible for thermal stability[25], [26] and two flexible substrate-binding domains referred to as LID and AMPbd (Fig 2a). Crystallographic structures of AKe have been solved for the open (inactive) and closed (active) states.[27] Substrate-free AKe has been shown to sample a closed-like state using single-molecule Föster resonance energy transfer (smFRET) experiments.[28], [29] We previously reported an analysis of AKe using RDCs in the substrate-free (open) and substrate-bound (closed) states where we fit the RDCs to the corresponding crystallographic structures.[30] We found that the fit of the closed state was better than that of the free state, which we interpreted as evidence for inter-domain dynamics in the substrate-free enzyme.
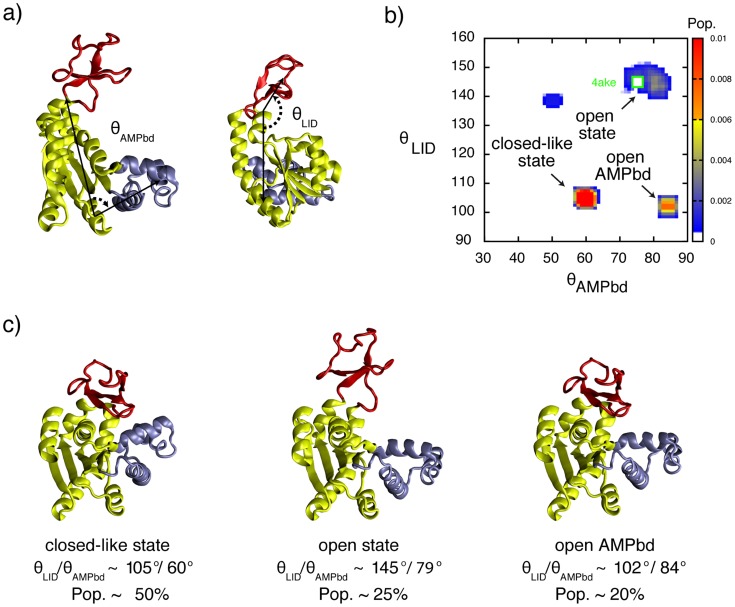
Figure 2. Determination of correlated inter-domain conformational heterogeneity in AKe from RDCs.
(A) X-ray structure of substrate free open AKe (pdb code 4ake). The CORE (yellow), LID (red) and AMPbd (cyan) domains are shown. The angles used to describe inter-domain conformational changes (see methods) are shown. (B) Inter-domain orientation distribution for AKe. The robustness of the distributions against various sources of error is discussed in the Supporting Information. (C) Representative conformations for each basin are shown together with angles and their estimated populations; little structural heterogeneity occurs within basins. The uncertainties in the populations due to estimated experimental in the measurements of the RDCs and computational errors are estimated to 10%.
To identify major conformational states sampled by substrate-free AKe we used backbone NH RDCs measured in steric alignment to select ensembles of conformations from a pool derived from simulations started from the open and closed X-ray structures[27],[31] (see Methods). The ensembles collectively agree with the RDCs (Q≈0.26) even though the structures they contain do not (Q>0.6) when considered individually. An ensemble size of 4 to 64 accounted for the data and equivalent distributions were observed regardless of ensemble size and method used to calculate the RDCs (Figs S3 to S5). It is worth noting that the LID domain of AKe is in equilibrium with a locally unfolded state with a population of ca. 5% at 37°C;[32] under the conditions used in this study (25°C) this state has a low population (<1%). Similarly, cracking of the AMPbd along the closing mechanism may play a role.[26], [33] However, cracking occurs at the transition state, and therefore has a very low population. The states obtained for AKe are shown in Fig. 2b. An analysis of the results indicated that substrate free AKe samples three major states corresponding to i) a closed-like state (θLID ∼105°, θAMPbd ∼60°, population ∼0.5±0.1), where the LID is closed and the AMPbd slightly opened ii) the open state (θLID ∼146°, θAMPbd ∼74°, population ∼0.25±0.1), where both nucleotide binding domains are open, and iii) conformations in which the degree of domain opening is intermediate between that of the fully closed and fully open. The estimate of the fraction closed AKe from our analysis is 0.5, which is in agreement with numbers from single molecule FRET studies where fractions of 0.6[29] and 0.3[28] has been enumerated.
We performed additional control simulations to assess the robustness of the distributions obtained. Scrambling the RDCs lead to a list of restraints that could not be fit to any distribution (Fig. S6), showing that the distribution obtained is encoded in the data and not biased by the population of the conformers in the pool. The distribution shown in Fig. 2a was found to be robust to various sources of error, including errors in the experimental RDCs (Fig. S7) and in the prediction of the alignment tensor (Fig. S8). We also performed computational experiments to ensure that a single set of RDCs suffices to distinguish between ensembles with a distinct number of states and identify correlated structural changes (see Figs. S9 to S13).
An analysis of the conformational ensemble allowed us to assess the presence and degree of inter-domain correlation, an important property of AKe. As shown in Fig. 2b, the ATP and AMP domain movements are correlated, disfavouring conformations where the LID is open and the AMPbd is closed. Our findings agree with free energy calculations[34], molecular dynamics simulations[35] as well as with normal mode analysis.[33], [36] Although it is impossible to derive the closure pathway from equilibrium data, the conformational states observed favour a step-wise mechanism in which LID closure takes place before AMPbd closure. Such a mechanism would be beneficial because the initial closure of the LID, together with the substantially higher affinity of this domain for its substrate (ATP) as compared to AMP (50 vs 1700 µM),[30] would reduce the probability of non-productive binding of AMP to the LID.[37]
Single-molecule FRET[28], [29] and NMR[28] have shown that the open and closed states of AKe are in equilibrium and that nucleotide binding shifts it towards the closed state.[30] Of particular interest are smFRET experiments, as these can resolve conformational states and provide a qualitative validation of the ensemble. Two different reaction coordinates, corresponding to distances between residues in the LID and the AMPbd (Aquifex AK)[28] and between residues in the LID and the CORE domain (AKe),[29] have been studied. In the former the authors resolved two states in which open conformations were populated to ca. 70%, whereas in the latter the authors found that the open conformation was disfavoured, with a population of ca. 40%. These results can be rationalized based on the ensemble. Following the approach of Beckstein et al.,[38] where distances corresponding to the open and closed states are mapped in domain angle space, we estimated open populations along the LID-AMPbd and LID-CORE smFRET reaction coordinates >50% and ∼30%, respectively (for LID-AMPbd large uncertainties are found due to difficulties in assigning open and closed states as quantified by smFRET). Overall, the ensemble is able to qualitatively reconcile two apparently contradictory smFRET studies, which also suggests that steric alignment does not significantly alter the structural heterogeneity of AKe.
Here we have used the dependence of RDCs on global molecular shape via S to determine ensembles reporting on the amplitude and degree of correlation of inter-domain motions. Although RDCs are uniquely suited for this, this is a challenging endeavour due to their intrinsic degeneracies.[39] It is therefore relevant to discuss the factors that allowed the approach used in this work to alleviate them. The first factor is that our approach accounts for how shape changes alter the value of the RDC by computing S for each inter-domain orientation i.e. two inter-domain orientations that would have degenerate RDCs when the tensor is assumed to be constant can be differentiated if they have sufficiently large differences in shape (Fig. S14).[39] A second factor is the presence of structural constraints due to the covalent linkage, where steric clashes between domains restrict the available conformational space;[40], [41] AKe is a well-structured proteins where this effect is particularly important but for multi-domain proteins with flexible linker sequences RDCs measured in multiple alignment media will be required. Alternatively, RDCs could be complemented with structural restraints derived from smFRET or SAXS data.
Correlated motions in proteins are thought to mediate biochemical functionality.[42] Recently, we have identified weak long-range correlated motions in a surface patch of ubiquitin involved in molecular recognition.[7] Here, we move a step forward and find correlated domain movements in a representative multi-domain enzyme. The observed inter-domain correlation suggests functional roles in allowing ligand access, in adopting the inter-domain orientation necessary for catalysis as well as in binding allostery. Our results, therefore, reinforce that correlated inter-domain motions in proteins can mediate important biochemical processes.
Methods
RDC calculation
NH RDCs used in this work for free AKe were measured in stretched polyacrylamide gels.[30] Two independent methods, PALES [12] and ALMOND[13], [13]were used to calculate the alignment tensor using Eq. 1. They gave equivalent distributions as shown in Figs. S3–S5. In Figs. 2 we provide the distributions that lead the best agreement between calculated and experimental RDCs [43] (Tab. S1). For AKe it was obtained using ALMOND. The agreement between calculated and experimental (or reference) RDCs was assessed by the quality factor Q [43] defined in Eq. 2.
| (2) |
The calculated RDCs were scaled, after averaging across the ensemble, to minimize Q to account for the difficulty of predicting the absolute concentration of alignment medium. We used RDCs corresponding to structured regions in the protein (see Fig. S15). For AKe, we found that RDCs in loop regions were more difficult to fit and therefore we decided to exclude them. However, we note that the ensemble derived including RDCs in the loop regions remains unaffected. We also evaluated the impact of the alignment media by monitoring changes in chemical shifts induced by the presence of the stretched polyacrylamide gel. As shown in the Supporting Figure S16, no significant chemical shift perturbations were observed when apo AKe was immersed into the anisotropic phase. As a reference the chemical shift perturbations induced by binding of the inhibitor Ap5A to AKe are also displayed. A thorough analysis of the robustness of the procedure to various source of error is provided as Supporting Information. To select the optimum ensemble size we monitored the agreement with RDCs used to guide the genetic algorithm and to RDCs left out of this process or free RDCs. Ten sets of randomly removed RDCs (20%) or free RDCs sets were used and 20 independent calculations were run for each set of randomly removed RDCs. The ensemble was qualitatively validated against two independent smFRET experiments (see results and discussion).
Reference pool of conformations
An extensive set of inter-domain conformations (ca. 105) was generated for AKe. The X-ray structures 1ake [31] and 4ake [27] were used as seeds. Trajectories were generated where either the AMP or ATP binding domain open (or closed). From each state along the trajectory a second trajectory was generated where the other domain was forced to open (or to close). These simulations were performed with the molecular simulation package CHARMM c35.[44] A time step of 1.0 fs was used. Simulations were performed at 300K. A shape-term (biasing) potential on the backbone atoms was used. The CHARMM commands "CONStraint HARmonic BEStfit COMParison" and the IC table tool (CONStraint IC BOND ANGL IMPR DIHE) were used to sample inter-domain orientations between open and closed conformations. The initial force was set to 0.1 and exponentially increased by a factor of 1.05 during 200 cycles of 100 steps each. The degree of opening ranged between ∼30–90° and ∼90–160° for the AMP- and ATP-binding domains (see angle definitions for AKe). The angles sampled covered the range observed by known X-ray structures of AKe and related proteins. [38] An advanced sampling strategy based on the PELE [24] method was used (see Protein Energy Landscape Exploration, see Supporting Information). This allowed identifying high-energy conformations which are unlikely to be of sufficiently low energy to be present in the crystalline state or sampled by conventional MD.
Searching algorithm
An in-house genetic algorithm GA was developed to efficiently search within the pool of structures used to determine the experimental inter-domain orientation distributions of T4L and AKe. Initially 1000 ensembles were generated with structures randomly selected from a pool of ca. 105 conformations (see reference pool of conformations above). Ensembles of size N = 1, 2, 4, 8, 16, 32 and 64 were used. The 103 ensembles of size N were submitted to evolution through 1000 steps. At each step two new sets of 103 ensembles were generated by mutation and crossing operations, leaving 3×103 ensembles. At each step the best 103 ensembles, based on the value of Q (see below), were retained. After 1000 iterations the best ensemble was saved and the complete procedure was repeated 200 times. Because too high mutation and recombination rates may lead to loss of good solutions and to premature convergence of the GA, these parameters evolved during the calculations. Initially, the mutation and crossing rates were set to 100% and 2%, respectively. At each step new mutation and crossing rates were determined by dividing/multiplying them by a factor of 1.001, respectively. In Figs. S1 and S2 a scheme illustrating the GA and the convergence of the method are shown. The error in the population of the states observed were determined from the influence of RDC experimental uncertainty by performing 200 calculations with random Gaussian error added to each experimental RDC. We used a standard deviation of 1.0 Hz for AKe, three times the estimated experimental error.
Inter-domain angle definitions
The definition used by Beckstein et al. [38] was adopted in this work. Briefly, the angle formed between the AMP-binding domain (AMPbd) and the core domain (CORE) was determined as the angle formed by two vectors: Vector 1 connects the centers of mass (Cα atoms) of CORE residues 90–100 with residues 115–125 (“hinge region” between the CORE and LID domains). Vector 2 connects the centers of mass of CORE residues 90–100 and AMPbd residues 35–55. The angle formed between the ATP-binding domain (LID) and the CORE was defined equivalently using the angle formed between two vectors: Vector 1 connects the centers of mass of residues 115–125 (“hinge region” CORE–LID domains) with CORE residues 179–185. Vector 2 connects the centers of mass for residues 115–125 (“hinge region” CORE–LID) with LID residues 125–153.
Supporting Information
Schematic flow-chart showing the steps involved in the calculation of inter-domain conformational heterogeneity from RDCs. In step 1 a pool of conformations is generated. In step 2 an algorithm for searching ensembles of structures that match experimental data is used (see Methods in the main text). The algorithm randomly selects 103 ensembles of size N from a pool of ca. 105 conformations generated in the first step. For each value of N the agreement of the ensemble with the experimental RDCs is optimized by substituting ensemble members with structures of the pool as well as by swapping structures between ensembles. The best 103 ensembles generated in the second step are retained and the process is repeated until agreement between calculated and experimental RDCs is reached. The optimal value of N is determined by monitoring the agreement with RDCs used to guide the genetic algorithm (Qwork) and RDCs left out of the calculation (free RDCs, Qfree).
(TIF)
Genetic algorithm. a, Convergence of the algorithm developed in this work to select ensembles of structures that match RDCs. Both data sets, restrained (Qwork) and unrestrained RDCs (Qfree), reached a plateau region after 400 iterations. b, Mutation and crossing operation rates applied at each step of the ensemble refinement.
(TIF)
Fitting of NH RDCs for AKe and determination of the optimum ensemble size by monitoring the agreement with RDCs used to guide the genetic algorithm and RDCs left out of the calculation (20%). The results obtained using two independent methods to calculate the alignment tensor, ALMOND and PALES, are shown. The Qfactor for working (Qwork; restrained) and free (Qfree; unrestrained) RDCs are shown (see Supporting Text S1). For each ensemble size 200 independent calculations were performed and the results pooled.
(TIF)
Experimental AKe inter-domain orientation distributions obtained for ensembles of several sizes (see Supporting Text S1). The distributions were obtained using the ALMOND method to calculate the alignment tensor (see Fig. S5 for distributions obtained using PALES). For each ensemble size 200 independent calculations were performed and the results pooled. The x and y axis correspond to the θLID and θAMPbd angles (in degrees) shown in Fig. 3a.
(TIF)
Experimental AKe inter-domain orientation distributions obtained for ensembles of several sizes (see Supporting Text S1). The distributions were obtained using PALES to calculate the alignment tensor (see Fig. S4 for distributions obtained using the ALMOND method). For each ensemble size 200 independent calculations were performed and the results pooled. The x and y axis correspond to the θAMPbd and θLID angles (in degrees) shown in Fig. 2a.
(TIF)
Distributions obtained for AKe after randomly scrambling the experimental RDCs to obtain incorrect lists of restraints (see Supporting Text S1). The Q factor obtained in all cases was >0.9, indicating that it was not possible to fit the incorrect data to any physically possible distribution of inter-domain orientations; for each ensemble size a different list of randomly scrambled RDCs was used. For AKe, the x and y axis correspond to the θAMPbd and θLID angles (in degrees) shown in Fig. 2a.
(TIF)
Impact of error in the experimentally measured RDCs of AKe. The fitting of RDCs (Qwork) as well as the distributions obtained are shown. Random Gaussian error was added to the RDCs prior to ensemble calculations. The results shown were obtained using the ALMOND method to calculate the alignment tensor (see Supporting Text S1). For AKe, The x and y axis correspond to the θAMPbd and θLID angles (in degrees) shown in Fig. 2a.
(TIF)
Experimental distributions of AKe as a function of error in the prediction of the alignment magnitude (see Supporting Text S1). The RDCs of each conformation were scaled by a number which depended on its position along the RC(s) used in this work. The error was increased exponentially along the RC(s) reaching at the end points of the RC a value of 2, 3, 10, 20 and 50 fold. The x and y axis correspond to the θAMPbd and θLID angles (in degrees) shown in Fig. 2a.
(TIF)
Reconstruction of a synthetic (computer designed) unimodal distribution represented by a magenta box with an increasing number of conformations, N = 1, 8 and 32. Ensembles with 8 or more members fit equally well the synthetic RDCs and best predicted RDCs (20%) left out of the calculations (see Supporting Text S1). The x and y axis correspond to the θAMPbd and θLID angles (in degrees) shown in Fig. 2a.
(TIF)
Reconstruction of a synthetic (computer designed) four state distribution represented by magenta boxes of equal population with N = 1, 2, 4, 8, 16 and 32. Ensembles with 16 or more members fit equally well the synthetic RDCs and best predicted RDCs (20%) left out of the calculations (see Supporting Text S1). The x and y axis correspond to the θAMPbd and θLID angles (in degrees) shown in Fig. 2a.
(TIF)
Reconstruction of synthetic unimodal inter-domain orientation distributions of AKe represented by a magenta box from NH RDCs (see Supporting Text S1). Distributions rebuilt with added random Gaussian are shown (standard deviations of 1%, 3% and 6% with respect to the maximum coupling). The best ensemble size was determined by monitoring the agreement with RDCs used to guide the genetic algorithm (Qwork) and RDCs left out of the calculation (free RDCS, Qfree) (Fig. S13). The x and y axis correspond to the θAMPbd and θLID angles (in degrees) shown in Fig. 2a.
(TIF)
Reconstruction of synthetic multi-modal inter-domain orientation distributions of AKe represented by magenta boxes of equal population from NH RDCs (see Supporting Text S1). Distributions rebuilt with added random Gaussian error with standard deviations corresponding to 1%, 3% and 6% of error respect the maximum coupling are shown. The best ensemble size was determined by monitoring the agreement with RDCs used to guide the genetic algorithm (Qwork) and RDCs left out of the calculation (free RDCS, Qfree) (Fig. S13). The x and y axis correspond to the θAMPbd and θLID angles (in degrees) shown in Fig. 2a.
(TIF)
Agreement with RDCs left out of the calculations (20%) for AKe protein for the synthetic distributions shown in Figs. S11 and S12 (see Supporting Text S1). The Qfactor for working (Qwork; restrained) and free (Qfree; unrestrained) RDCs is shown. Distributions rebuilt with added random Gaussian error with standard deviations of 1% (first row), 3% (second row) and 6% (third row) of error with respect the maximum coupling are shown.
(TIF)
Maximum deviation in the fitted populations as a function of the alignment tensor prediction error for AKe protein for computer designed distributions (see Supporting Text S1). The target distributions covered closed/open states whose populations varied between 0% to 100% in steps of 1 percent. For each degree of random error added 100 independent runs were performed.
(TIF)
Sequence (first raw) and secondary structure of AKe in the open (pdb code 4ake; second raw) and closed (pdb code 1ake; third raw) states. $ indicates that the RDC was used in the calculations. Residues labeled with * were excluded. The label – indicates that the RDC was not available. The AMPbd (residues 28–72) and LID (113–176) domains are highlighted in red and yellow, respectively. Residues 1–27, 73–112 and 176–214 correspond to the CORE domain.
(TIF)
Alignment of AKe does not induce significant chemical shift perturbations. Shown are chemical shift perturbations to apo AKe induced by the inhibitor Ap5A (red) and by introduction of the enzyme into the anisotropic stretched polyacrylamide gel (black). Chemical shift perturbations were calculated according to: δω = 0.2|Δ15N|+| Δ1H |.
(TIF)
Agreement between the calculated and experimental RDCs for AKe. S calc refers to the result obtained when S is predicted based on the atomic coordinates of each protein conformation, whereas S fit refers to the result obtained when S was fit using single value decomposition. The Q values are those of the final ensemble.
(DOCX)
Average structural root-mean-squared deviation (RMSD) between the calculated ensemble members and reference X-ray structures for AKe.
(DOCX)
NH RDCs measured for E. coli AKe. RDCs used in ensemble refinement are shown in Fig. S15 (average experimental error <0.3 Hz).
(DOCX)
Supporting methods.
(DOCX)
Funding Statement
This work was supported by IRB (RBF, CWB, XS), the BSC (SEM, BC, VG), the Swedish Research Council (621-2010-5247 to MWW), “Umeå University Young researcher award” (MWW), ICREA (XS) MICINN (CTQ2009-08850-BQU to XS), MINECO (FIS2008-00114 to VG and BIO2012-31043 to XS), Marató de TV3 (102030 to XS) and the Juan de la Cierva (SEM) and Ramón y Cajal (CWB) programs. The authors acknowledge the use of computational resources of the Red Española de Supercomputación (RES). Spanish ministry of education and science MICINN CTQ2009-08850, MINECO FIS2008-00114, MINECO BIO2012-31043 (http://www.mecd.gob.es) Marató de TV3 102030. Swedish Research Council (http://www.vr.se/) Grant Number 621-2010-5247. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Eisenmesser EZ, Millet O, Labeikovsky W, Korzhnev DM, Wolf-Watz M, et al. (2005) Intrinsic dynamics of an enzyme underlies catalysis. Nature 438: 117–121. [DOI] [PubMed] [Google Scholar]
- 2. Kern D, Zuiderweg ERP (2003) The role of dynamics in allosteric regulation. Curr Opin Struct Biol 13: 748–757. [DOI] [PubMed] [Google Scholar]
- 3. del Sol A, Tsai C-J, Ma B, Nussinov R (2009) The Origin of Allosteric Functional Modulation: Multiple Pre-existing Pathways. Structure 17: 1042–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maragakis P, Lindorff-Larsen K, Eastwood MP, Dror R, Klepeis J, et al. (2008) Microsecond Molecular Dynamics Simulation Shows Effect of Slow Loop Dynamics on Backbone Amide Order Parameters of Proteins. J Phys Chem B 112(19): 6155–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clore GM, Schwieters CD (2004) How much backbone motion in ubiquitin is required to account for dipolar coupling data measured in multiple alignment media as assessed by independent cross-validation? J Am Chem Soc 126: 2923–2938. [DOI] [PubMed] [Google Scholar]
- 6. Lange OF, Lakomek N-A, Farès C, Schröder GF, Walter KFA, et al. (2008) Recognition dynamics up to microseconds revealed from an RDC-derived ubiquitin ensemble in solution. Science 320: 1471–1475. [DOI] [PubMed] [Google Scholar]
- 7. Fenwick RB, Esteban-Martín S, Richter B, Lee D, Walter KFA, et al. (2011) Weak Long-Range Correlated Motions in a Surface Patch of Ubiquitin Involved in Molecular Recognition. J Am Chem Soc 133: 10336–10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clore GM, Schwieters CD (2004) Amplitudes of protein backbone dynamics and correlated motions in a small alpha/beta protein: correspondence of dipolar coupling and heteronuclear relaxation measurements. Biochemistry 43: 10678–10691. [DOI] [PubMed] [Google Scholar]
- 9. Bouvignies G, Bernadó P, Meier S, Cho K-, Grzesiek S, et al. (2005) Identification of slow correlated motions in proteins using residual dipolar and hydrogen-bond scalar couplings. Proc Natl Acad Sci U S A 102: 13885–13890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Simone A, Richter B, Salvatella X, Vendruscolo M (2009) Toward an accurate determination of free energy landscapes in solution states of proteins. J Am Chem Soc 131: 3810–3811. [DOI] [PubMed] [Google Scholar]
- 11. Tjandra N, Bax A (1997) Direct measurement of distances and angles in biomolecules by NMR in a dilute liquid crystalline medium. Science 278: 1111–1114. [DOI] [PubMed] [Google Scholar]
- 12. Zweckstetter M, Bax A (2000) Prediction of Sterically Induced Alignment in a Dilute Liquid Crystalline Phase: Aid to Protein …. J Am Chem Soc 122: 3791–3792. [Google Scholar]
- 13. Almond A, Axelsen JB (2002) Physical interpretation of residual dipolar couplings in neutral aligned media. J Am Chem Soc 124: 9986–9987. [DOI] [PubMed] [Google Scholar]
- 14. Berlin K, O'Leary DP, Fushman D (2010) Structural assembly of molecular complexes based on residual dipolar couplings. J Am Chem Soc 132: 8961–8972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bruschweiler R, Liao X, Wright PE (1995) Long-range motional restrictions in a multidomain zinc-finger protein from anisotropic tumbling. Science 268: 886–889. [DOI] [PubMed] [Google Scholar]
- 16. Tjandra N, Garrett DS, Gronenborn AM, Bax A, Clore GM (1997) Defining long range order in NMR structure determination from the dependence of heteronuclear relaxation times on rotational diffusion anisotropy. Nat Struct Biol 4: 443–449. [DOI] [PubMed] [Google Scholar]
- 17. Ryabov Y, Suh J-Y, Grishaev A, Clore GM, Schwieters CD (2009) Using the experimentally determined components of the overall rotational diffusion tensor to restrain molecular shape and size in NMR structure determination of globular proteins and protein-protein complexes. J Am Chem Soc 131: 9522–9531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Putnam CD, Hammel M, Hura GL, Tainer JA (2007) X-ray solution scattering (SAXS) combined with crystallography and computation: defining accurate macromolecular structures, conformations and assemblies in solution. Q Rev Biophys 40: 191–285. [DOI] [PubMed] [Google Scholar]
- 19. Meiler J, Prompers JJ, Peti W, Griesinger C, Brüschweiler R (2001) Model-free approach to the dynamic interpretation of residual dipolar couplings in globular proteins. J Am Chem Soc 123: 6098–6107. [DOI] [PubMed] [Google Scholar]
- 20. Salvatella X, Richter B, Vendruscolo M (2008) Influence of the fluctuations of the alignment tensor on the analysis of the structure and dynamics of proteins using residual dipolar couplings. J Biomol NMR 40: 71–81. [DOI] [PubMed] [Google Scholar]
- 21. Meier S, Blackledge M, Grzesiek S (2008) Conformational distributions of unfolded polypeptides from novel NMR techniques. J Chem Phys 128: 052204. [DOI] [PubMed] [Google Scholar]
- 22. De Simone A, Montalvao RW, Vendruscolo M (2011) Determination of Conformational Equilibria in Proteins Using Residual Dipolar Couplings. J Chem Theory Comput 7: 4189–4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nodet G, Salmon L, Ozenne V, Meier S, Jensen MR, et al. (2009) Quantitative description of backbone conformational sampling of unfolded proteins at amino acid resolution from NMR residual dipolar couplings. J Am Chem Soc 131: 17908–17918. [DOI] [PubMed] [Google Scholar]
- 24. Borrelli KW, Vitalis A, Alcantara R, Guallar V (2005) PELE: Protein Energy Landscape Exploration. A Novel Monte Carlo Based Technique. J Chem Theory Comput 1: 1304–1311. [DOI] [PubMed] [Google Scholar]
- 25. Rundqvist L, Adén J, Sparrman T, Wallgren M, Olsson U, et al. (2009) Noncooperative folding of subdomains in adenylate kinase. Biochemistry 48: 1911–1927. [DOI] [PubMed] [Google Scholar]
- 26. Olsson U, Wolf-Watz M (2010) Overlap between folding and functional energy landscapes for adenylate kinase conformational change. Nat Commun 1: 111. [DOI] [PubMed] [Google Scholar]
- 27. Müller CW, Schlauderer GJ, Reinstein J, Schulz GE (1996) Adenylate kinase motions during catalysis: an energetic counterweight balancing substrate binding. Structure 4: 147–156. [DOI] [PubMed] [Google Scholar]
- 28. Henzler-Wildman KA, Thai V, Lei M, Ott M, Wolf-Watz M, et al. (2007) Intrinsic motions along an enzymatic reaction trajectory. Nature 450: 838–844. [DOI] [PubMed] [Google Scholar]
- 29. Hanson JA, Duderstadt K, Watkins LP, Bhattacharyya S, Brokaw J, et al. (2007) Illuminating the mechanistic roles of enzyme conformational dynamics. Proc Natl Acad Sci U S A 104: 18055–18060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Adén J, Wolf-Watz M (2007) NMR identification of transient complexes critical to adenylate kinase catalysis. J Am Chem Soc 129: 14003–14012. [DOI] [PubMed] [Google Scholar]
- 31. Müller CW, Schulz GE (1992) Structure of the complex between adenylate kinase from Escherichia coli and the inhibitor Ap5A refined at 1.9 A resolution. A model for a catalytic transition state. J Mol Biol 224: 159–177. [DOI] [PubMed] [Google Scholar]
- 32. Schrank TP, Bolen DW, Hilser VJ (2009) Rational modulation of conformational fluctuations in adenylate kinase reveals a local unfolding mechanism for allostery and functional adaptation in proteins. Proc Natl Acad Sci U S A 106: 16984–16989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Whitford PC, Gosavi S, Onuchic JN (2008) Conformational transitions in adenylate kinase. Allosteric communication reduces misligation. J Biol Chem 283: 2042–2048. [DOI] [PubMed] [Google Scholar]
- 34. Whitford PC, Miyashita O, Levy Y, Onuchic JN (2007) Conformational transitions of adenylate kinase: switching by cracking. J Mol Biol 366: 1661–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kubitzki MB, de Groot BL (2008) The atomistic mechanism of conformational transition in adenylate kinase: a TEE-REX molecular dynamics study. Structure 16: 1175–1182. [DOI] [PubMed] [Google Scholar]
- 36. Arora K, Brooks CL (2007) Large-scale allosteric conformational transitions of adenylate kinase appear to involve a population-shift mechanism. Proc Natl Acad Sci U S A 104: 18496–18501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sinev MA, Sineva EV, V I, E H (1996) Towards a mechanism of AMP-substrate inhibition in adenylate kinase from Escherichia coli. FEBS Lett 397: 273–276. [DOI] [PubMed] [Google Scholar]
- 38. Beckstein O, Denning EJ, Perilla JR, Woolf TB (2009) Zipping and unzipping of adenylate kinase: atomistic insights into the ensemble of open/closed transitions. J Mol Biol 394: 160–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fischer MW, Losonczi JA, Weaver JL, Prestegard JH (1999) Domain orientation and dynamics in multidomain proteins from residual dipolar couplings. Biochemistry 38: 9013–9022. [DOI] [PubMed] [Google Scholar]
- 40. Wüthrich K (2003) NMR studies of structure and function of biological macromolecules (Nobel Lecture). J Biomol NMR 27: 13–39. [DOI] [PubMed] [Google Scholar]
- 41. Hus J-C, Salmon L, Bouvignies G, Lotze J, Blackledge M, et al. (2008) 16-fold degeneracy of peptide plane orientations from residual dipolar couplings: analytical treatment and implications for protein structure determination. J Am Chem Soc 130: 15927–15937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brüschweiler R (2011) Protein dynamics: whispering within. Nat Chem 3: 665–666. [DOI] [PubMed] [Google Scholar]
- 43. Cornilescu G, Marquardt J, Ottiger M, Bax A (1998) Validation of protein structure from anisotropic carbonyl chemical shifts in a dilute liquid …. J Am Chem Soc 120: 6836–6837. [Google Scholar]
- 44. Brooks BR, Brooks CL, Mackerell AD, Nilsson L, Petrella RJ, et al. (2009) CHARMM: the biomolecular simulation program. J Comput Chem 30: 1545–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Schematic flow-chart showing the steps involved in the calculation of inter-domain conformational heterogeneity from RDCs. In step 1 a pool of conformations is generated. In step 2 an algorithm for searching ensembles of structures that match experimental data is used (see Methods in the main text). The algorithm randomly selects 103 ensembles of size N from a pool of ca. 105 conformations generated in the first step. For each value of N the agreement of the ensemble with the experimental RDCs is optimized by substituting ensemble members with structures of the pool as well as by swapping structures between ensembles. The best 103 ensembles generated in the second step are retained and the process is repeated until agreement between calculated and experimental RDCs is reached. The optimal value of N is determined by monitoring the agreement with RDCs used to guide the genetic algorithm (Qwork) and RDCs left out of the calculation (free RDCs, Qfree).
(TIF)
Genetic algorithm. a, Convergence of the algorithm developed in this work to select ensembles of structures that match RDCs. Both data sets, restrained (Qwork) and unrestrained RDCs (Qfree), reached a plateau region after 400 iterations. b, Mutation and crossing operation rates applied at each step of the ensemble refinement.
(TIF)
Fitting of NH RDCs for AKe and determination of the optimum ensemble size by monitoring the agreement with RDCs used to guide the genetic algorithm and RDCs left out of the calculation (20%). The results obtained using two independent methods to calculate the alignment tensor, ALMOND and PALES, are shown. The Qfactor for working (Qwork; restrained) and free (Qfree; unrestrained) RDCs are shown (see Supporting Text S1). For each ensemble size 200 independent calculations were performed and the results pooled.
(TIF)
Experimental AKe inter-domain orientation distributions obtained for ensembles of several sizes (see Supporting Text S1). The distributions were obtained using the ALMOND method to calculate the alignment tensor (see Fig. S5 for distributions obtained using PALES). For each ensemble size 200 independent calculations were performed and the results pooled. The x and y axis correspond to the θLID and θAMPbd angles (in degrees) shown in Fig. 3a.
(TIF)
Experimental AKe inter-domain orientation distributions obtained for ensembles of several sizes (see Supporting Text S1). The distributions were obtained using PALES to calculate the alignment tensor (see Fig. S4 for distributions obtained using the ALMOND method). For each ensemble size 200 independent calculations were performed and the results pooled. The x and y axis correspond to the θAMPbd and θLID angles (in degrees) shown in Fig. 2a.
(TIF)
Distributions obtained for AKe after randomly scrambling the experimental RDCs to obtain incorrect lists of restraints (see Supporting Text S1). The Q factor obtained in all cases was >0.9, indicating that it was not possible to fit the incorrect data to any physically possible distribution of inter-domain orientations; for each ensemble size a different list of randomly scrambled RDCs was used. For AKe, the x and y axis correspond to the θAMPbd and θLID angles (in degrees) shown in Fig. 2a.
(TIF)
Impact of error in the experimentally measured RDCs of AKe. The fitting of RDCs (Qwork) as well as the distributions obtained are shown. Random Gaussian error was added to the RDCs prior to ensemble calculations. The results shown were obtained using the ALMOND method to calculate the alignment tensor (see Supporting Text S1). For AKe, The x and y axis correspond to the θAMPbd and θLID angles (in degrees) shown in Fig. 2a.
(TIF)
Experimental distributions of AKe as a function of error in the prediction of the alignment magnitude (see Supporting Text S1). The RDCs of each conformation were scaled by a number which depended on its position along the RC(s) used in this work. The error was increased exponentially along the RC(s) reaching at the end points of the RC a value of 2, 3, 10, 20 and 50 fold. The x and y axis correspond to the θAMPbd and θLID angles (in degrees) shown in Fig. 2a.
(TIF)
Reconstruction of a synthetic (computer designed) unimodal distribution represented by a magenta box with an increasing number of conformations, N = 1, 8 and 32. Ensembles with 8 or more members fit equally well the synthetic RDCs and best predicted RDCs (20%) left out of the calculations (see Supporting Text S1). The x and y axis correspond to the θAMPbd and θLID angles (in degrees) shown in Fig. 2a.
(TIF)
Reconstruction of a synthetic (computer designed) four state distribution represented by magenta boxes of equal population with N = 1, 2, 4, 8, 16 and 32. Ensembles with 16 or more members fit equally well the synthetic RDCs and best predicted RDCs (20%) left out of the calculations (see Supporting Text S1). The x and y axis correspond to the θAMPbd and θLID angles (in degrees) shown in Fig. 2a.
(TIF)
Reconstruction of synthetic unimodal inter-domain orientation distributions of AKe represented by a magenta box from NH RDCs (see Supporting Text S1). Distributions rebuilt with added random Gaussian are shown (standard deviations of 1%, 3% and 6% with respect to the maximum coupling). The best ensemble size was determined by monitoring the agreement with RDCs used to guide the genetic algorithm (Qwork) and RDCs left out of the calculation (free RDCS, Qfree) (Fig. S13). The x and y axis correspond to the θAMPbd and θLID angles (in degrees) shown in Fig. 2a.
(TIF)
Reconstruction of synthetic multi-modal inter-domain orientation distributions of AKe represented by magenta boxes of equal population from NH RDCs (see Supporting Text S1). Distributions rebuilt with added random Gaussian error with standard deviations corresponding to 1%, 3% and 6% of error respect the maximum coupling are shown. The best ensemble size was determined by monitoring the agreement with RDCs used to guide the genetic algorithm (Qwork) and RDCs left out of the calculation (free RDCS, Qfree) (Fig. S13). The x and y axis correspond to the θAMPbd and θLID angles (in degrees) shown in Fig. 2a.
(TIF)
Agreement with RDCs left out of the calculations (20%) for AKe protein for the synthetic distributions shown in Figs. S11 and S12 (see Supporting Text S1). The Qfactor for working (Qwork; restrained) and free (Qfree; unrestrained) RDCs is shown. Distributions rebuilt with added random Gaussian error with standard deviations of 1% (first row), 3% (second row) and 6% (third row) of error with respect the maximum coupling are shown.
(TIF)
Maximum deviation in the fitted populations as a function of the alignment tensor prediction error for AKe protein for computer designed distributions (see Supporting Text S1). The target distributions covered closed/open states whose populations varied between 0% to 100% in steps of 1 percent. For each degree of random error added 100 independent runs were performed.
(TIF)
Sequence (first raw) and secondary structure of AKe in the open (pdb code 4ake; second raw) and closed (pdb code 1ake; third raw) states. $ indicates that the RDC was used in the calculations. Residues labeled with * were excluded. The label – indicates that the RDC was not available. The AMPbd (residues 28–72) and LID (113–176) domains are highlighted in red and yellow, respectively. Residues 1–27, 73–112 and 176–214 correspond to the CORE domain.
(TIF)
Alignment of AKe does not induce significant chemical shift perturbations. Shown are chemical shift perturbations to apo AKe induced by the inhibitor Ap5A (red) and by introduction of the enzyme into the anisotropic stretched polyacrylamide gel (black). Chemical shift perturbations were calculated according to: δω = 0.2|Δ15N|+| Δ1H |.
(TIF)
Agreement between the calculated and experimental RDCs for AKe. S calc refers to the result obtained when S is predicted based on the atomic coordinates of each protein conformation, whereas S fit refers to the result obtained when S was fit using single value decomposition. The Q values are those of the final ensemble.
(DOCX)
Average structural root-mean-squared deviation (RMSD) between the calculated ensemble members and reference X-ray structures for AKe.
(DOCX)
NH RDCs measured for E. coli AKe. RDCs used in ensemble refinement are shown in Fig. S15 (average experimental error <0.3 Hz).
(DOCX)
Supporting methods.
(DOCX)