Abstract
The very low density lipoprotein (VLDL) receptor is a recently cloned member of the low density lipoprotein (LDL) receptor family that mediates the binding and uptake of VLDL when overexpressed in animal cells. Its sequence is 94% identical in humans and rabbits and 84% identical in humans and chickens, implying a conserved function. Its high level expression in muscle and adipose tissue suggests a role in VLDL triacylglycerol delivery. Mutations in the chicken homologue cause female sterility, owing to impaired VLDL and vitellogenin uptake during egg yolk formation. We used homologous recombination in mouse embryonic stem cells to produce homozygous knockout mice that lack immunodetectable VLDL receptors. Homozygous mice of both sexes were viable and normally fertile. Plasma levels of cholesterol, triacylglycerol, and lipoproteins were normal when the mice were fed normal, high-carbohydrate, or high-fat diets. The sole abnormality detected was a modest decrease in body weight, body mass index, and adipose tissue mass as determined by the weights of epididymal fat pads. We conclude that the VLDL receptor is not required for VLDL clearance from plasma or for ovulation in mice.
Full text
PDF
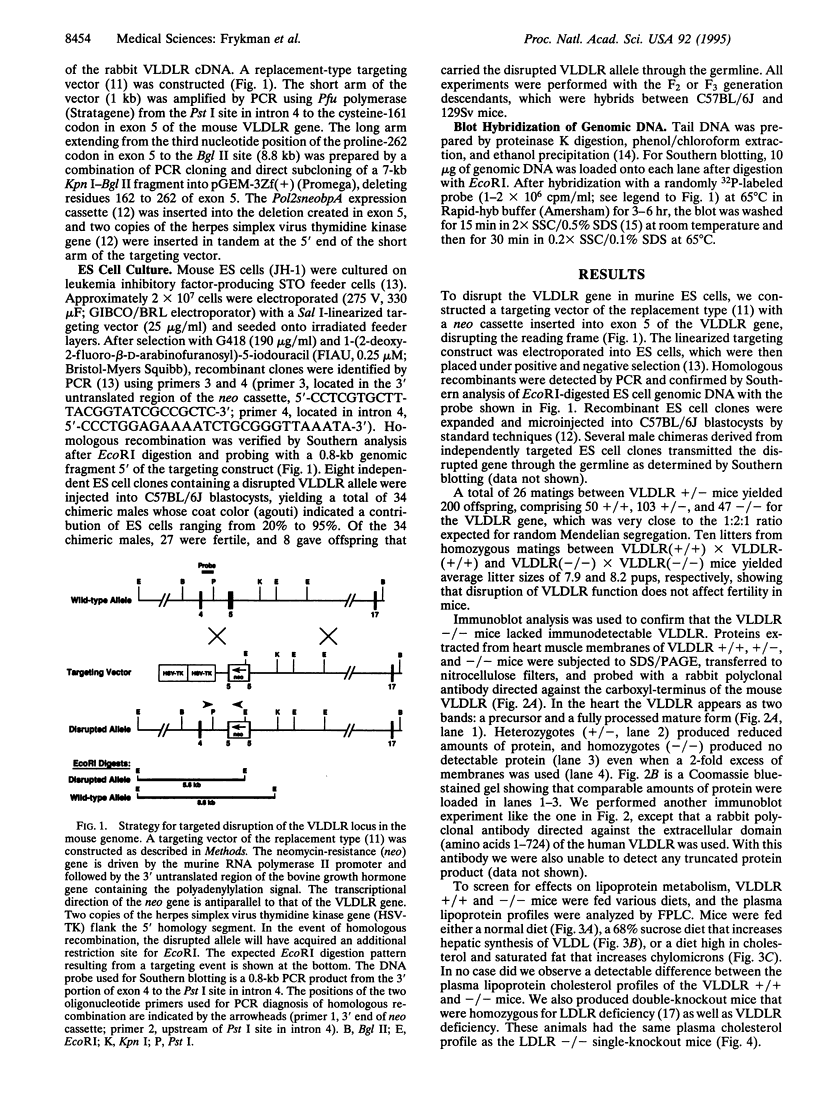
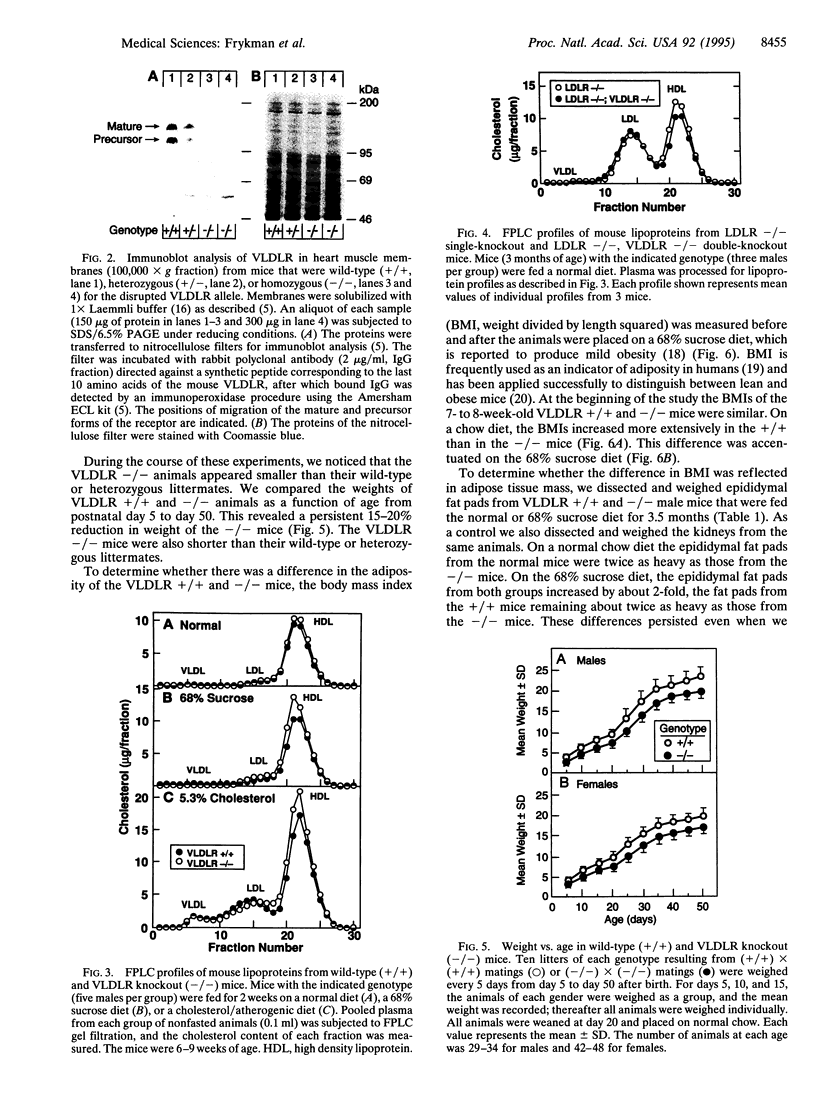


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahary N., Leibel R. L., Joseph L., Friedman J. M. Molecular mapping of the mouse db mutation. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8642–8646. doi: 10.1073/pnas.87.21.8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battey F. D., Gåfvels M. E., FitzGerald D. J., Argraves W. S., Chappell D. A., Strauss J. F., 3rd, Strickland D. K. The 39-kDa receptor-associated protein regulates ligand binding by the very low density lipoprotein receptor. J Biol Chem. 1994 Sep 16;269(37):23268–23273. [PubMed] [Google Scholar]
- Bjornson L. K., Kayden H. J., Miller E., Moshell A. N. The transport of alpha-tocopherol and beta-carotene in human blood. J Lipid Res. 1976 Jul;17(4):343–352. [PubMed] [Google Scholar]
- Bronson S. K., Smithies O. Altering mice by homologous recombination using embryonic stem cells. J Biol Chem. 1994 Nov 4;269(44):27155–27158. [PubMed] [Google Scholar]
- Bujo H., Hermann M., Kaderli M. O., Jacobsen L., Sugawara S., Nimpf J., Yamamoto T., Schneider W. J. Chicken oocyte growth is mediated by an eight ligand binding repeat member of the LDL receptor family. EMBO J. 1994 Nov 1;13(21):5165–5175. doi: 10.1002/j.1460-2075.1994.tb06847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi M. R. Altering the genome by homologous recombination. Science. 1989 Jun 16;244(4910):1288–1292. doi: 10.1126/science.2660260. [DOI] [PubMed] [Google Scholar]
- Ishibashi S., Brown M. S., Goldstein J. L., Gerard R. D., Hammer R. E., Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest. 1993 Aug;92(2):883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokinen E. V., Landschulz K. T., Wyne K. L., Ho Y. K., Frykman P. K., Hobbs H. H. Regulation of the very low density lipoprotein receptor by thyroid hormone in rat skeletal muscle. J Biol Chem. 1994 Oct 21;269(42):26411–26418. [PubMed] [Google Scholar]
- Keys A., Fidanza F., Karvonen M. J., Kimura N., Taylor H. L. Indices of relative weight and obesity. J Chronic Dis. 1972 Jul 1;25(6):329–343. doi: 10.1016/0021-9681(72)90027-6. [DOI] [PubMed] [Google Scholar]
- Krieger M., Herz J. Structures and functions of multiligand lipoprotein receptors: macrophage scavenger receptors and LDL receptor-related protein (LRP). Annu Rev Biochem. 1994;63:601–637. doi: 10.1146/annurev.bi.63.070194.003125. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- Sakai J., Hoshino A., Takahashi S., Miura Y., Ishii H., Suzuki H., Kawarabayasi Y., Yamamoto T. Structure, chromosome location, and expression of the human very low density lipoprotein receptor gene. J Biol Chem. 1994 Jan 21;269(3):2173–2182. [PubMed] [Google Scholar]
- Soriano P., Montgomery C., Geske R., Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991 Feb 22;64(4):693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- Stifani S., Barber D. L., Nimpf J., Schneider W. J. A single chicken oocyte plasma membrane protein mediates uptake of very low density lipoprotein and vitellogenin. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1955–1959. doi: 10.1073/pnas.87.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki J., Takahashi S., Oida K., Shimada A., Kohno M., Tamai T., Miyabo S., Yamamoto T., Nakai T. Lipid accumulation and foam cell formation in Chinese hamster ovary cells overexpressing very low density lipoprotein receptor. Biochem Biophys Res Commun. 1995 Jan 26;206(3):835–842. doi: 10.1006/bbrc.1995.1119. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Kawarabayasi Y., Nakai T., Sakai J., Yamamoto T. Rabbit very low density lipoprotein receptor: a low density lipoprotein receptor-like protein with distinct ligand specificity. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9252–9256. doi: 10.1073/pnas.89.19.9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willnow T. E., Herz J. Homologous recombination for gene replacement in mouse cell lines. Methods Cell Biol. 1994;43(Pt A):305–334. doi: 10.1016/s0091-679x(08)60610-x. [DOI] [PubMed] [Google Scholar]
- Willnow T. E., Sheng Z., Ishibashi S., Herz J. Inhibition of hepatic chylomicron remnant uptake by gene transfer of a receptor antagonist. Science. 1994 Jun 3;264(5164):1471–1474. doi: 10.1126/science.7515194. [DOI] [PubMed] [Google Scholar]




