Abstract
Lipid vesicles carrying the purified membrane C5b-9 complex [C5b-9(m)] of complement were analyzed immunochemically and in the electron microscope after treatment with a combination of trypsin and alpha-chymotrypsin. Under reducing conditions, the externally oriented annulus was removed. The remaining part of the C5b-9(m), representing approximately half of the total mass of the macromolecular complex, was visualized in the electron microscope as a hollow cylindrical structure with walls of 1-nm thickness. This structure remained tenaciously attached to the lipid bilayer, projecting 8-9 nm from the external membrane surface into the aqueous environment. Cleavage of C5b-9(m) by proteolysis and reduction resulted in a sharp reduction of tis antigenic determinants. One hydrophilic protease-resistant C5 derivative was released from the membrane and recovered in the fluid phase. The membrane-bound residue almost totally lacked antigens precipitable with antisera to C5, C6, C9, and C5b-9(m).
Full text
PDF

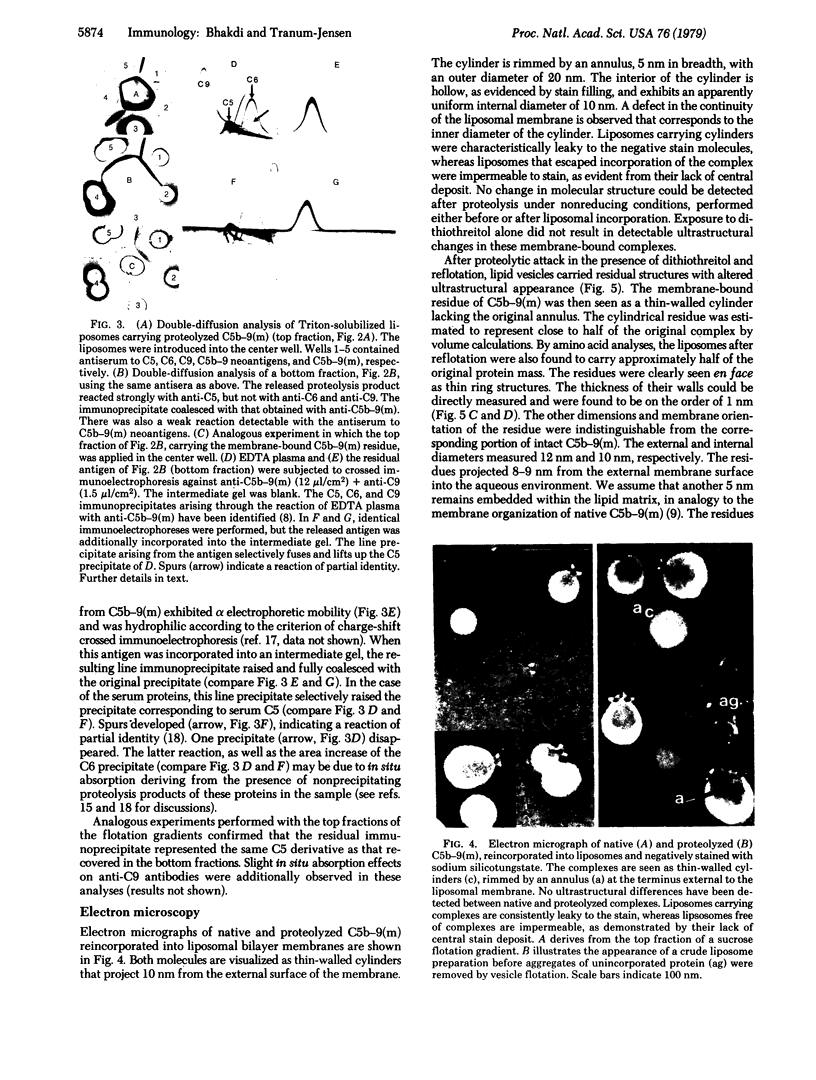
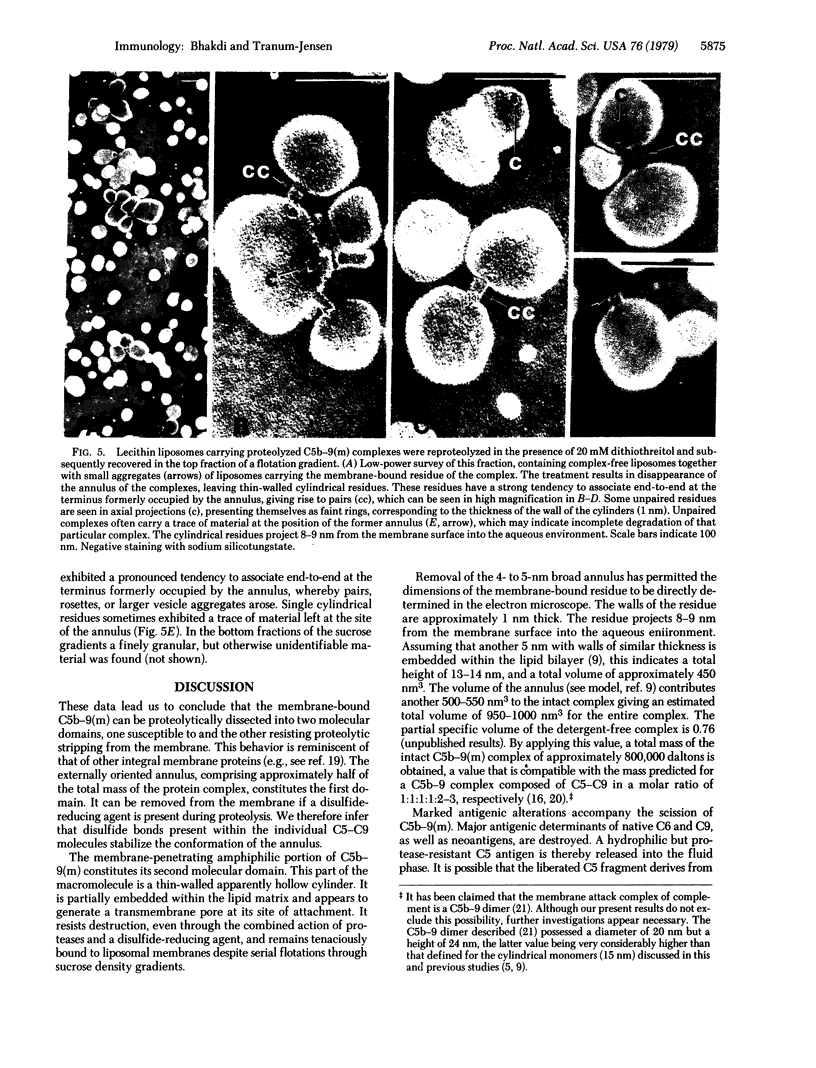

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelsen N. H., Bock E., Kroll J. Comparison of antisera. Scand J Immunol Suppl. 1973;1:101–103. [PubMed] [Google Scholar]
- BORSOS T., DOURMASHKIN R. R., HUMPHREY J. H. LESIONS IN ERYTHROCYTE MEMBRANES CAUSED BY IMMUNE HAEMOLYSIS. Nature. 1964 Apr 18;202:251–252. doi: 10.1038/202251a0. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Bhakdi-Lehnen B., Bjerrum O. J. Detection of amphiphilic proteins and peptides in complex mixtures. Charge-shift crossed immunoelectrophoresis and two-dimensional charge-shift electrophoresis. Biochim Biophys Acta. 1977 Oct 3;470(1):35–44. doi: 10.1016/0005-2736(77)90059-1. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Bjerrum O. J., Bhakdi-Lehnen B., Tranum-Jensen J. Complement lysis: evidence for an amphiphilic nature of the terminal membrane C5b-9 complex of human complement. J Immunol. 1978 Dec;121(6):2526–2532. [PubMed] [Google Scholar]
- Bhakdi S., Bjerrum O. J., Rother U., Knüfermann H., Wallach D. F. Immunochemical analyses of membrane-bound complement. Detection of the terminal complement complex and its similarity to "intrinsic" erythrocyte membrane proteins. Biochim Biophys Acta. 1975 Sep 16;406(1):21–35. doi: 10.1016/0005-2736(75)90039-5. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Ey P., Bhakdi-Lehnen B. Isolation of the terminal complement complex from target sheep erythrocyte membranes. Biochim Biophys Acta. 1976 Feb 6;419(3):445–457. doi: 10.1016/0005-2736(76)90258-3. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Molecular nature of the complement lesion. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5655–5659. doi: 10.1073/pnas.75.11.5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesecker G., Podack E. R., Halverson C. A., Müller-Eberhard H. J. C5b-9 dimer: isolation from complement lysed cells and ultrastructural identification with complement-dependent membrane lesions. J Exp Med. 1979 Feb 1;149(2):448–458. doi: 10.1084/jem.149.2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giavedoni E. B., Chow Y. M., Dalmasso A. P. The functional size of the primary complement lesion in resealed erythrocyte membrane ghosts. J Immunol. 1979 Jan;122(1):240–245. [PubMed] [Google Scholar]
- Hammer C. H., Nicholson A., Mayer M. M. On the mechanism of cytolysis by complement: evidence on insertion of C5b and C7 subunits of the C5b,6,7 complex into phospholipid bilayers of erythrocyte membranes. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5076–5080. doi: 10.1073/pnas.72.12.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer C. H., Shin M. L., Abramovitz A. S., Mayer M. M. On the mechanism of cell membrane damage by complement: evidence on insertion of polypeptide chains from C8 and C9 into the lipid bilayer of erythrocytes. J Immunol. 1977 Jul;119(1):1–8. [PubMed] [Google Scholar]
- Humphrey J. H., Dourmashkin R. R. The lesions in cell membranes caused by complement. Adv Immunol. 1969;11:75–115. doi: 10.1016/s0065-2776(08)60478-2. [DOI] [PubMed] [Google Scholar]
- Kolb W. P., Haxby J. A., Arroyave C. M., Müller-Eberhard H. J. Molecular analysis of the membrane attack mechanism of complement. J Exp Med. 1972 Mar 1;135(3):549–566. doi: 10.1084/jem.135.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb W. P., Muller-Eberhard H. J. The membrane attack mechanism of complement. Isolation and subunit composition of the C5b-9 complex. J Exp Med. 1975 Apr 1;141(4):724–735. [PMC free article] [PubMed] [Google Scholar]
- Mayer M. M., Hammer C. H., Michaels D. W., Shin M. L. Immunologically mediated membrane damage: the mechanism of complement action and the similarity of lymphocyte-mediated cytoxicity. Immunochemistry. 1978 Nov;15(10-11):813–831. doi: 10.1016/0161-5890(78)90115-3. [DOI] [PubMed] [Google Scholar]
- Mayer M. M. Mechanism of cytolysis by complement. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2954–2958. doi: 10.1073/pnas.69.10.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels D. W., Abramovitz A. S., Hammer C. H., Mayer M. M. Increased ion permeability of planar lipid bilayer membranes after treatment with the C5b-9 cytolytic attack mechanism of complement. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2852–2856. doi: 10.1073/pnas.73.8.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Eberhard H. J. Complement. Annu Rev Biochem. 1975;44:697–724. doi: 10.1146/annurev.bi.44.070175.003405. [DOI] [PubMed] [Google Scholar]
- Nilsson U. R., Mandle R. J., Jr, McConnell-Mapes J. A. Human C3 and C5: subunit structure and modifications by trypsin and C42-C423. J Immunol. 1975 Feb;114(2 Pt 2):815–822. [PubMed] [Google Scholar]
- Tanford C., Reynolds J. A. Characterization of membrane proteins in detergent solutions. Biochim Biophys Acta. 1976 Oct 26;457(2):133–170. doi: 10.1016/0304-4157(76)90009-5. [DOI] [PubMed] [Google Scholar]
- Tranum-Jensen J., Bhakdi S., Bhakdi-Lehnen B., Bjerrum O. J., Speth V. Complement lysis: the ultrastructure and orientation of the C5b-9 complex on target sheep erythrocyte membranes. Scand J Immunol. 1978;7(1):45–46. doi: 10.1111/j.1365-3083.1978.tb00425.x. [DOI] [PubMed] [Google Scholar]








