Abstract
Background
The emergence of community-associated methicillin resistant Staphylococcus aureus (CA-MRSA) has caused a change in MRSA epidemiology worldwide. In the Middle East, the persistent spread of CA-MRSA isolates that were associated with multilocus sequence type (MLST) clonal complex 80 and with staphylococcal cassette chromosome mec (SCCmec) type IV (CC80-MRSA-IV), calls for novel approaches for infection control that would limit its spread.
Methodology/Principal Findings
In this study, the epidemiology of CC80-MRSA-IV was investigated in Jordan and Lebanon retrospectively covering the period from 2000 to 2011. Ninety-four S. aureus isolates, 63 (67%) collected from Lebanon and 31 (33%) collected from Jordan were included in this study. More than half of the isolates (56%) were associated with skin and soft tissue infections (SSTIs), and 73 (78%) were Panton-Valentine Leukocidin (PVL) positive. Majority of the isolates (84%) carried the gene for exofoliative toxin d (etd), 19% had the Toxic Shock Syndrome Toxin-1 gene (tst), and seven isolates from Jordan had a rare combination being positive for both tst and PVL genes. spa typing showed the prevalence of type t044 (85%) and pulsed-field gel electrophoresis (PFGE) recognized 21 different patterns. Antimicrobial susceptibility testing showed the prevalence (36%) of a unique resistant profile, which included resistance to streptomycin, kanamycin, and fusidic acid (SKF profile).
Conclusions
The genetic diversity among the CC80 isolates observed in this study poses an additional challenge to infection control of CA-MRSA epidemics. CA-MRSA related to ST80 in the Middle East was distinguished in this study from the ones described in other countries. Genetic diversity observed, which may be due to mutations and differences in the antibiotic regimens between countries may have led to the development of heterogeneous strains. Hence, it is difficult to maintain “the European CA-MRSA clone” as a uniform clone and it is better to designate as CC80-MRSA-IV isolates.
Introduction
Staphylococcus aureus, a highly adaptive and versatile gram-positive bacterium, is considered one of the most isolated human pathogens and the most common cause of skin and soft tissue infections (SSTIs) [1], [2]. Soon after the introduction of methicillin for the treatment of penicillin-resistant strains in 1959, methicillin-resistant S. aureus (MRSA) has emerged as an important hospital-associated (HA-MRSA) pathogen for its increased morbidity and mortality rates, healthcare costs, and length of hospital stay [3], [4]. HA-MRSA infections arise in individuals with predisposing risk factors, such as surgery or presence of an indwelling medical device. By contrast, many community-associated MRSA (CA-MRSA) infections arise in otherwise healthy individuals who do not have such risk factors. CA-MRSA infections are also known to be epidemic in some countries. These features suggest that CA-MRSA strains are more virulent and transmissible than are traditional HA-MRSA strains [5].
CA-MRSA lineages are genotypically and phenotypically unrelated to the former multi-drug resistant HA-MRSA, and recently have started replacing the once pandemic HA-MRSA clones (CC5, 8, 22, 36, and 45) in health care facilities [6], [7]. Continent-specific-PVL positive CA-MRSA clones were previously described: ST1-IV (USA400), ST8-IV (USA300), ST30-IV (Pacific/Oceania), ST59-IV/V (USA1000, Taiwan), and ST80-IV (European CA-MRSA) [8], which were also reported from other parts of the world [9], [10], [11], [12], [13].
Common to all lineages is that they are generally susceptible to non-β-lactam antibiotics, harbor the small-sized staphylococcal chromosomal cassette mec (SCCmec) types IV or V encoding methicillin resistance, and carry the Panton-Valentine Leukocidin (PVL) toxin genes [2], [14], [15]. European clone ST80-IV (allelic profile 1-3-1-14-11-51-10) was first identified in the early 1990s and today is found throughout Europe, the Middle East, and Northern Africa [10], [16], [17], [18], [19], [20]. This clonal lineage is PVL-positive, belongs to agr type III, has type 8 capsular polysaccharide, and is resistant to tetracycline, streptomycin, kanamycin, and fusidic acid with a pronounced susceptibility to gentamicin [18], [19], [20].
Compared to the CA-MRSA clone most common in the United States (USA300), the European CA-MRSA clone seems less well adapted to persist in hospital environments, where CC80-MRSA-IV has entered Danish hospitals but has not caused nosocomial infections [18], [21]. Several reports indicated the transmission of the ST80-IV clone to Europe from patients with a recent history of travel or family relation to the Mediterranean or Middle East [18], [22], [23], [24], [25].
In the Middle East, little information is available about the emergence and continuous spread of CC80 clone. Khalil et al. [12] performed molecular characterization of 103 S. aureus isolates (41 MRSA and 62 MSSA) recovered from stool and nose specimens collected from children admitted to the University of Jordan Hospital. Genotyping revealed 48 different spa types and identified distinct allelic profiles with the majority belonging to ST80. On the other hand molecular characterization of 130 S. aureus clinical isolates (93 MRSA and 37 MSSA) recovered from patients at the Clinical Microbiology Section of the American University of Beirut in Lebanon revealed the presence of 48 spa types that clustered into 30 different groups. MLST revealed 10 sequence types (STs) among the isolates, and the majority of the PVL-positive isolates (53%) were ST80-MRSA-IVc [10]. However, a similar more recent study was conducted on 132 S. aureus non-duplicate clinical isolates recovered in a period of six months at AUB-MC [11]. MRSA represented 30% of the isolates collected in this study, with the most common being: t021 (6%), t044 (5%), and t267 (5%). Clustering SCCmec with MLST identified seven MRSA and 20 MSSA clones, and confirmed that the PVL-positive ST80-MRSA-IV was the dominant clone in Lebanon. The present retrospective study provides data on the epidemiology and molecular characteristics of ninety-four CC80-MRSA-IV isolates collected from Lebanon and Jordan over an 11-year period (2000–2011).
Materials and Methods
Ethical approval
Ethical approval was not required as clinical isolates were collected and stored as part of routine clinical care. Clinical isolates and patient records/information were anonymized and de-identified prior to analysis.
Hospital setting
Isolates from Jordan were obtained from the University of Jordan Hospital (UJH) in Amman, a governmental hospital that serves over 500,000 patients annually with a 547-inpatient bed capacity, while those from Lebanon were collected from the American University of Beirut Medical Center (AUB-MC) in Beirut, a private university hospital that provides tertiary services for over 300,000 patients annually with a 350-inpatient bed capacity.
Clinical Isolates
S. aureus isolates (n = 478) were collected from Lebanon and Jordan from 2000 to 2011. Isolates were confirmed as S. aureus by Gram staining, positive catalase reaction, and production of coagulase enzyme using SLIDEX Staph Plus agglutination kit (Biomérieux, France). All isolates identified to be spa type t044 and/or belonging to spa-clonal cluster 044 (spa-CC 044), were included in this study. In total 94 isolates (Jordan, n = 31; Lebanon, n = 63) were undertaken in this study. DNA was extracted using a Nucleospin Tissue kit (Macherey-Nagel, Germany) according to manufacturer’s instructions.
Clinical and demographic information
Clinical and demographic data were extracted from patients’ charts and lab discharge summaries and included: specimen origin (skin and soft tissue, respiratory, blood, stool, etc.), age, gender, time of isolation and hospitalization criteria (in or out-patient, surgery, etc.).
Antibiotic susceptibility testing
Antimicrobial susceptibility testing was performed using the Kirby-Baüer disk diffusion method according to Clinical and Laboratory Standards Institute (CLSI) recommendations [28] for streptomycin, kanamycin, tetracycline, gentamicin, fusidic acid, penicillin G, rifampicin, erythromycin, and clindamycin. Discs were purchased from Oxoid (Oxoids, UK) and Biorad (Bio-Rad, Marnes-la-Coquette, France) and samples were streaked on Muller-Hinton agar plates (Oxoids, UK) with an 18–20 hour incubation at 35±1°C. Resistance for fusidic acid (< 24 mm) was determined according to breakpoints defined by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) v3.0. S. aureus ATCC 29213 was used as a quality control strain to determine assay sensitivity.
Multiplex PCR (M-PCR) for detection of 16S rRNA, PVL, and mecA genes
Amplification of the 16S rRNA that served as an internal positive control, PVL (lukS-PV and lukF-PV), and mecA genes were done as described by McClure et al. [29]. PVL negative MRSA (N315) and PVL positive MSSA (ATCC 49775) were used as controls. The amplification reaction contained 1.5 µl of template DNA in a final volume of 25 µl containing 0.4, 0.8 and 0.8 µM for the primers specific for the 16S rRNA, lukS-PV, and mecA genes respectively with 2U of AmpliTaq (Fermentas), 1.5 mmol/l MgCl2, 1.6x Taq buffer, 0.2mM of each deoxynucleotide triphosphate (dNTP). The thermocycling conditions were set at 94°C for 5 min followed by 10 cycles of 94°C for 45 s, 55°C for 45 s, and 72°C for 75 s and 25 cycles of 94°C for 45 s, 50°C for 45 s, and 72°C for 75 s PCR products were resolved in a 1.8% (w/v) Metaphor (Lonza, Rockland, ME, USA) agarose gel in 0.5% Tris-borate-EDTA buffer (Bio-Rad, Hercules, CA) at 80 V/cm for 1 hour and were visualized with ethidium bromide.
Toxin gene profiling
Presence of exofoliative toxins a (eta), b (etb), and d (etd), and staphylococcal Toxic Shock Syndrome Toxin 1 (tst) genes was determined using previously described PCR primers [29], [30], [31] using a single M-PCR reaction. A Qiagen multiplex PCR kit was used where conditions were first optimized using the following reference strains: TC-142 (eta positive), TC-7 (etb positive), and NCTC11963 (tst positive). Reaction mixtures contained 1 µg of chromosomal template, 25 µl master mix with 3 mM MgCl2, 5 µl primer mix (2 mM in TE buffer for each primer) and RNase-free water to a final volume of 50 µl. The optimal cycling conditions were as follows: 95°C for 15 min; 30 cycles of 94°C for 30 s, 57°C for 1.5 min, and 72°C for 1.5 min; and a final extension at 72°C for 10 min.
MRSA SCCmec typing
SCCmec elements were typed using previously described PCR primers [32]. For multiplex PCR, a Qiagen multiplex PCR kit was used, and conditions were optimized using the following reference strains: MRSA NCTC 10442 (SCCmec I), MRSA N315 (SCCmec II), MRSA 85/2082 (SCCmec III), MRSA JCSC 4744 (SCCmec IVa), MRSA JCSC 2172 (SCCmec IVb), MRSA JCSC 47882 (SCCmec IVc), and MRSA WIS (SCCmec V) as previously described [32], [33]. Reaction mixtures contained 1 µg of chromosomal template, 25 µl master mix with 3 mM MgCl2, 5 µl primer mix (2 mM in TE buffer for each primer) and RNase-free water to a final volume of 50 µl. The optimal cycling conditions were as follows: 95°C for 15 min; 30 cycles of 94°C for 30 s, 57°C for 1.5 min, and 72°C for 1.5 min; and a final extension at 72°C for 10 min.
spa typing
The polymorphic X region of Staphylococcus protein A (spa) was amplified for all isolates as previously described [34], [35].
Multilocus sequence typing (MLST)
Twenty-four representative isolates were typed by MLST to confirm their relatedness to the CC80 clone. The isolates were selected based on variation of specimen origin, year of isolation and covering all different spa types within spa-CC 044. Amplification of the seven housekeeping genes (arcC, aroE, glpF, gmk, pta, tpi, and yqiL) by MLST was done as previously described [36].
PFGE fingerprinting
All isolates were subjected to PFGE typing using SmaI as previously described [37]. A bacteriophage lambda ladder PFG marker (New England BioLabs, UK) was included in each gel and NCTC 8325 was used as a quality control reference strain.
Data Analysis
spa types were assigned using Ridom Staph Type v2.2.1 database (Ridom GmbH, Würzburg, Germany) (www.ridom.de/spaserver/) and clustered into spa clonal clusters (spa-CCs) using the algorithm based upon repeat pattern (BURP) with clustering parameters excluding spa types with fewer than five repeats and grouping spa types to the same spa-CC if the cost was ≤4. CLC main workbench software v6.8.4 (CLC bio, Denmark) was used to assemble and align sequences of the seven housekeeping genes and sequence types (STs) were determined by submitting the allelic profile of representative alleles to the MLST database (http://saureus.mlst.net/) and eBURST v3.0 software was used to determine the clonal relationship of the isolates with the entire MLST database. PFGE fingerprints obtained were compared by means of Dice coefficient, and cluster analysis was performed by the unweighted pair group method with arithmetic means (UPGMA) using GelCompar and Bionumerics software v6.5 (Applied Maths, Sint-Martens-Latem, Belgium) with 1% band tolerance and 0.5% optimization settings. Groups were clustered according to the recommendations of Tenover et al. [26] and by applying a similarity coefficient of 80% to all dendrograms as suggested by Struelens et al. [27].
Categorical comparisons were performed using Chi-square test (X2). A P value of less than 0.05 was considered to be significant. The associations between resistance patterns with the sample origin, gender and site of infection were evaluated using the R statistical package (v. 3.0.2). The associations of the presence and absence of the toxins: eta, etb, etd, TSST-1 and PVL with the sample origin, gender and site of infection were also evaluated. The function used in R include “chisq.test()” from the package “stats”.
Results
Study Population
Ninety-four MRSA isolates identified to be spa type t044 and/or belonging to spa-clonal cluster 044 (spa-CC 044), and possibly related to ST80 were included in this study. Isolates were recovered from Lebanon (n = 63/94 isolates; 67%) and Jordan (n = 31/94; 33%) from 2000 to 2011 (Table 1 and Table 2). Around 56% of the isolates were associated with SSTIs, 15% with respiratory tract infections and 9% with bacteremia. Thirty-one of the isolates were from Jordan and 63 from Lebanon. Overall, 39% (n = 37/94) of the isolates were from females and 61% (n = 57/94) from males.
Table 1. Demographics and molecular characteristics of isolates collected from Lebanon.
| Site ofinfection | Gender | Age1 | spaType | MLST2 | ToxinProfiling3 | AntibioticProfile4 |
| Wound | F | 1 | t044 | 80 | etd, PVL | STR, KAN, TET, FUS, ERY, DA |
| Wound | M | 47 | t044 | 80 | eta, etd, PVL | STR, KAN |
| Pus | F | 11 | t044 | 80 | etd, PVL | STR, KAN, TET, FUS |
| Wound | M | 1M | t044 | 80 | etd, PVL | STR, KAN, FUS |
| Eye | F | 70 | t131 | 80 | PVL | STR, KAN, FUS |
| Biopsy | M | 71 | t4222 | 80 | etd, PVL | STR, KAN, TET, FUS |
| Wound | F | 18 | t044 | 80 | etd, PVL | STR, KAN, FUS, ERY, DA |
| DTA | M | 64 | t044 | 80 | etd | TET, FUS |
| Pus | M | 9 | t6438 | 80 | etd, PVL | STR, KAN, FUS |
| Bronchial Lavage | M | 47 | t044 | 80 | etd, PVL | STR, KAN, FUS |
| Others | F | 24 | t044 | 80 | etd, PVL | STR, KAN, TET, FUS |
| Abscess | M | 19 | t131 | 80 | etd, PVL | Sensitive to all tested antibiotics |
| Tracheal Aspirate | M | 6M | t021 | 80 | etd | STR, KAN, FUS |
| Wound | F | 36 | t9135 | 80 | etd, PVL | STR, KAN, FUS |
| Abscess | M | 41 | t044 | ND | etd, PVL | STR, KAN, FUS |
| Abscess | F | 34 | t044 | ND | etd, PVL | STR, KAN, FUS |
| DTA | M | 50 | t044 | ND | etd, PVL | STR, KAN, FUS |
| Abscess | M | 31 | t044 | ND | PVL | STR, KAN, FUS |
| Abscess | F | 29 | t044 | ND | etd, PVL | STR, KAN, FUS |
| Abscess | F | 62 | t044 | ND | etd, PVL | STR, KAN, FUS |
| Wound | M | 18 | t044 | ND | etd, PVL | FUS |
| DTA | M | 60 | t044 | ND | etd, PVL | Sensitive to all tested antibiotics |
| Wound | M | 4 | t044 | ND | etd, PVL | STR, KAN, TET, FUS |
| Wound | F | 57 | t044 | ND | etd, PVL | STR, KAN, FUS |
| Wound | M | 35 | t044 | ND | etd, PVL | STR, KAN, TET, FUS, ERY |
| Wound | F | 52 | t044 | ND | etd, PVL | STR, KAN, TET, FUS, ERY |
| Wound | M | 35 | t044 | ND | etd, PVL | STR, KAN, TET, FUS |
| Wound | M | 21 | t044 | ND | etd, PVL | STR, KAN, TET, FUS |
| Wound | M | 74 | t044 | ND | etd | STR, KAN, FUS |
| Wound | F | 27 | t131 | ND | etd, PVL | STR, KAN, FUS |
| Blood | M | 72 | t044 | ND | etd, PVL | STR, KAN, TET, FUS |
| Wound | M | 53 | t044 | ND | PVL | STR, KAN, FUS |
| Wound | M | 30 | t044 | ND | etd | STR, KAN, TET, GEN, FUS, ERY, DA |
| Catheter | F | 89 | t044 | ND | etd, PVL | STR, KAN, FUS |
| Wound | F | 41 | t044 | ND | etd, PVL | STR, KAN, FUS, ERY |
| Wound | M | 82 | t044 | ND | PVL | STR, KAN, FUS |
| DTA | M | 72 | t044 | ND | etd, PVL | STR, KAN, FUS, ERY |
| Wound | M | 78 | t044 | ND | etd, PVL | STR, KAN, TET, FUS, ERY |
| Wound | F | 42 | t044 | ND | etd, PVL | STR, KAN, TET, FUS |
| Wound | F | 38 | t044 | ND | etd, PVL | STR, KAN, TET, FUS |
| Wound | F | 63 | t044 | ND | PVL | STR, KAN, FUS |
| DTA | M | 74 | t044 | ND | etd, PVL | STR, KAN, FUS |
| Wound | M | 21 | t044 | ND | etd, PVL | STR, KAN, TET, FUS, ERY |
| Pus | F | 27 | t044 | ND | etd, PVL | STR, KAN, FUS, ERY |
| Wound | M | 1 | t044 | ND | etd, PVL | STR, KAN, FUS |
| Pus | M | 46 | t044 | ND | etd, PVL | STR, KAN, FUS, ERY |
| Wound | F | 24 | t044 | ND | etd, PVL | STR, KAN, FUS, ERY |
| Blood | M | 60 | t044 | ND | etd, PVL | STR, KAN, FUS |
| Cyst | M | 20 | t044 | ND | etd, PVL | FUS |
| Wound | M | 58 | t044 | ND | etd, PVL | FUS |
| Wound | F | 68 | t044 | ND | etd, PVL | STR, KAN, FUS |
| Pus | F | 59 | t044 | ND | etd, PVL | STR, KAN, TET, FUS |
| Abscess | F | 20 | t044 | ND | etd, PVL | STR, KAN, FUS, ERY |
| Biopsy | M | 72 | t044 | ND | PVL | STR, KAN, FUS |
| Pus | M | 47 | t044 | ND | etd | STR, KAN, FUS |
| Ear | F | 45 | t044 | ND | etd, PVL | TET, FUS, ERY |
| Abscess | M | 28 | t044 | ND | PVL | STR, KAN, TET, FUS |
| Pus | F | 89 | t044 | ND | PVL | STR, KAN, FUS |
| Eye | F | 4W | t131 | ND | PVL | TET, FUS |
| Eye | M | 4W | t131 | ND | PVL | TET, FUS |
| Abdominal Fluid | M | 24 | t044 | ND | etd, PVL | STR, KAN |
| Pus | F | 18 | t044 | ND | etd, PVL | STR, KAN, TET, FUS |
| Abscess | M | 24 | t044 | ND | etd, PVL | STR, KAN |
W: weeks; M: months.
ND: non-determinant.
eta: exofoliative toxin a gene; etd: exofoliative toxin d gene; PVL: Panton-Valentine Leukocidin gene.
STR: streptomycin; KAN: kanamycin; TET: tetracycline; GEN: gentamicin; FUS: fusidic acid; ERY: erythromycin; DA: clindamycin.
Table 2. Demographics and molecular characteristics of isolates collected from Jordan.
| Site ofinfection | Gender | Age1 | SpaType | MLST2 | ToxinProfile3 | AntibioticProfile4 |
| Nose | F | 4M | t044 | 80 | etd, tst | STR, KAN, FUS, ERY |
| Stool | M | 12M | t044 | 80 | etd, tst | STR, KAN, FUS |
| Nose | M | 16D | t044 | 80 | etd, tst, PVL | STR, KAN, TET, FUS, ERY |
| Nose | M | 12M | t131 | 80 | etd, tst | STR, KAN, FUS |
| Nose | M | 1M | t5849 | 80 | etd, tst | STR, KAN, FUS, ERY |
| Wound | F | 31 | t044 | 80 | etd, PVL | STR, KAN, TET, FUS |
| Wound | M | 22 | t044 | 80 | etd, tst, PVL | STR, KAN, FUS, ERY |
| Ear | M | 17 | t5802 | 80 | PVL | STR, KAN, FUS, ERY |
| Pus | F | 12 | t5849 | 80 | etd, PVL | STR, KAN, FUS, ERY |
| Gall Bladder | M | 25 | t044 | 997 | etd, tst, PVL | STR, KAN, TET, FUS, ERY, DA |
| Stool | M | 26D | t044 | ND | - | STR, KAN, FUS, ERY |
| Stool | F | 4M | t044 | ND | etd, tst | STR, KAN, FUS, ERY |
| Nose | M | 12M | t044 | ND | etd, tst | STR, KAN, FUS, ERY |
| Stool | M | 1M | t044 | ND | etd, tst | STR, KAN, FUS |
| Stool | M | 2M | t044 | ND | etd, tst | STR, KAN, FUS, ERY |
| Nose | F | 15D | t044 | ND | etd, tst | STR, KAN, FUS |
| Stool | F | 15D | t044 | ND | etd, tst | STR, KAN, FUS |
| Nose | M | 15D | t044 | ND | etd, tst | STR, KAN |
| Abdominal Fluid | F | 89 | t044 | ND | etd, tst, PVL | STR, KAN, TET, FUS |
| Gall Bladder | F | 37 | t044 | ND | etd, PVL | STR, KAN, FUS, ERY |
| Blood | M | 33 | t044 | ND | etd, PVL | STR, KAN, TET, FUS, ERY |
| Blood | M | 29 | t044 | ND | - | TET, FUS |
| Blood | F | 43 | t044 | ND | etd, PVL | STR, KAN, FUS |
| Peritoneal Fluid | M | 45 | t044 | ND | - | ERY |
| Blood | M | 66 | t044 | ND | - | FUS, ERY |
| Pus | M | 1D | t044 | ND | etd, tst, PVL | STR, KAN, FUS, ERY |
| Wound | F | 35 | t044 | ND | etd, PVL | STR, KAN, FUS, ERY, DA |
| Blood | M | 52 | t044 | ND | etd, tst, PVL | STR, KAN, FUS |
| Nose | M | 40 | t044 | ND | etd, PVL | STR, KAN, FUS |
| Wound | F | 35 | t044 | ND | etd, tst, PVL | STR, KAN, FUS, ERY, DA |
| Blood | M | 64 | t6438 | ND | etd | ERY, DA |
D: days; W: weeks; M: months.
ND: non-determinant.
etd: exofoliative toxin d gene; tst: toxic shock syndrome toxin 1 gene; PVL: Panton-Valentine Leukocidin gene.
STR: streptomycin; KAN: kanamycin; TET: tetracycline; FUS: fusidic acid; ERY: erythromycin; DA: clindamycin.
Characteristics of the MRSA clones
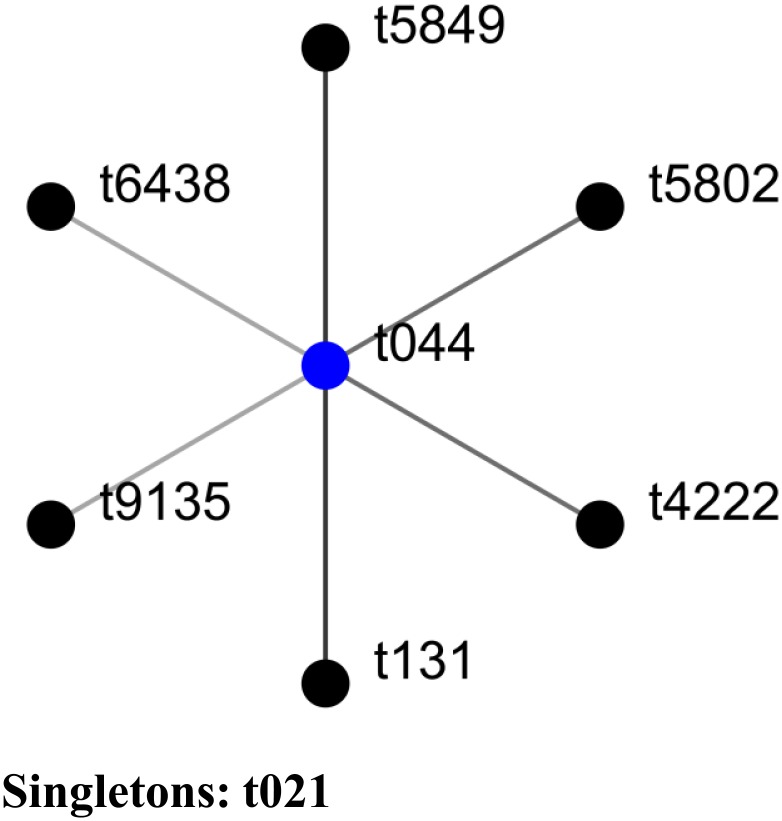
spa typing of all 94 isolates revealed that the majority (85%) were of spa type t044 (Jordan n = 26/32; Lebanon n = 54/63) followed by single locus variants (SLV) of type t044 (t131, t5802, t5849, and t4222) or t131 (t5802), double locus variants (DLV) of type t044 (t6438 and t9135) and a singleton (t021) (Figure 1).
Figure 1. Population snapshot based on BURP analysis of all recovered spa types.
Each dot represents a unique spa type. The diameter of the a dot is proportional to the quantity of the corresponding spa type. Blue dot represents group founder, defined as the spa type with the highest founder score within a CC.
The isolates selected for MLST typing were chosen based on variation of specimen origin, year of isolation and covering all different spa types within spa-CC 044 (Table 1 and Table 2). MLST typing of these isolates showed that all belonged to ST80 except for one from Jordan, which was ST997. ST997 however, is also within the CC80 and is a SLV from ST80 (http://saureus.mlst.net/eburst/database.asp). This isolate, which was recovered in 2009 from Jordan, was positive for the PVL, etd, and tst genes and resistant to streptomycin, kanamycin, fusidic acid, erythromycin, and clindamycin (Table 2).
Antibiotic Susceptibility
In this study the common resistance pattern observed was that of SKF (n = 34/96; 36%) (Table 3). Resistance against streptomycin, kanamycin and fusidic acid was comparable regardless of the source (Jordan or Lebanon) being 86, 86 and 91%, respectively. A significant difference was detected between Jordan and Lebanon with respect to resistance to tertracycline (p = 0.0017), with the ones from Lebanon showing a higher resistance rate (n = 22/63; 35% Lebanon vs. n = 6/31; 19% Jordan). In contrast, the resistance rate against erythromycin within isolates recovered from Jordan (n = 19/31; 61%) was higher compared to those from Lebanon (n = 14/63; 22%) and the difference was also significant (p = 0.000565).
Table 3. Percentage distribution of resistance patterns.
| Profilenumber | Antibiogram1 | Lebanon (%) | Jordan (%) | Total (%) |
| 1 | STR, KAN, FUS | 26 (41) | 8 (26) | 34 (36) |
| 2 | STR, KAN, FUS, ERY | 6 (10) | 11 (35) | 17 (18) |
| 3 | STR, KAN, TET, FUS | 12 (19) | 2 (6) | 14 (15) |
| 4 | STR, KAN, TET, FUS, ERY | 4 (6) | 2 (6) | 6 (6) |
| 5 | TET, FUS | 3 (5) | 1 (3) | 4 (4) |
| 6 | STR, KAN | 3 (5) | 1 (3) | 4 (4) |
| 7 | STR, KAN, FUS, ERY, DA | 1 (2) | 2 (6) | 3 (3) |
| 8 | FUS | 3 (5) | - | 3 (3) |
| 9 | STR, KAN, TET, FUS, ERY, DA | 1 (2) | 1 (3) | 2 (2) |
| 10 | TET, FUS, ERY | 1 (2) | - | 1 (1) |
| 11 | STR, KAN, TET, GEN, FUS, ERY, DA | 1 (2) | - | 1 (1) |
| 12 | FUS, ERY | - | 1 (3) | 1 (1) |
| 13 | ERY, DA | - | 1 (3) | 1 (1) |
| 14 | ERY | - | 1 (3) | 1 (1) |
STR: streptomycin; KAN: kanamycin; TET: tetracycline; GEN: gentamicin; FUS: fusidic acid; ERY: erythromycin; DA: clindamycin.
Toxins
PVL genes were detected in 78% (n = 73/94) of the isolates, with 52% (n = 16/31) of the isolates from Jordan and 8% (n = 5/63) from Lebanon being PVL-negative (Table 1 and Table 2). eta toxin gene was only detected in one isolate recovered from wound in Lebanon, which was additionally PVL positive, while none was positive for etb. Genetic diversity was additionally observed between the two set of isolates (Jordan vs. Lebanon) with the etd and the tst genes, where 64% of the isolates from Lebanon were positive for etd gene and none for tst compared to 28% for etd gene and 19% for tst in isolates from Jordan. PVL and TSST-1 were both found to be significantly associated to the sample origin (PVL: p = 6.295e-06 and TSST-1: p = 1.136e-10), PVL mainly detected in isolates from Lebanon and TSST-1 only in isolates from Jordan, and with the site of infection (PVL: p = 8.726e-05 and TSST-1: p = 0.0009773); being higher in isolates from wound, pus, and abscess versus all other sites of infection. Both toxin genes were not significantly associated to gender and none of the remaining toxins was significantly associated to the origin, gender, or site of infection. Seven of the isolates, all collected from Jordan, were positive for PVL, tst and etd genes. Six of the isolates were recovered in 2008 and only one in 2009. There was no correlation between the isolates’ resistance and PFGE patterns.
One PVL-negative isolate from Lebanon had the common European antibiotic resistance pattern (TSKF) with additional resistance to gentamicin, clindamycin, and erythromycin. tst positive isolates on the other hand, in addition to being resistant to the β -lactam drugs were resistant to streptomycin, kanamycin, and fusidic acid. spa typing of all the seven tst positive and MLST typing of three revealed that all were spa type t044, two were ST80 and one ST977, with all belonging to the CC80 lineage. Finally, a clear heterogeneity was detected within the other studied toxin genes too, with 64% of the isolates from Lebanon being positive for etd gene compared to only 28% for those from Jordan (Table 1 and Table 2).
Overall, 16% (n = 15/94) of the isolates had the same genetic characteristics as that of the European ST80 (etd positive, PVL positive, SCCmec-IV and TSKF resistance pattern). It is noteworthy that all 15 isolates were recovered from Lebanon (Table 1).
PFGE
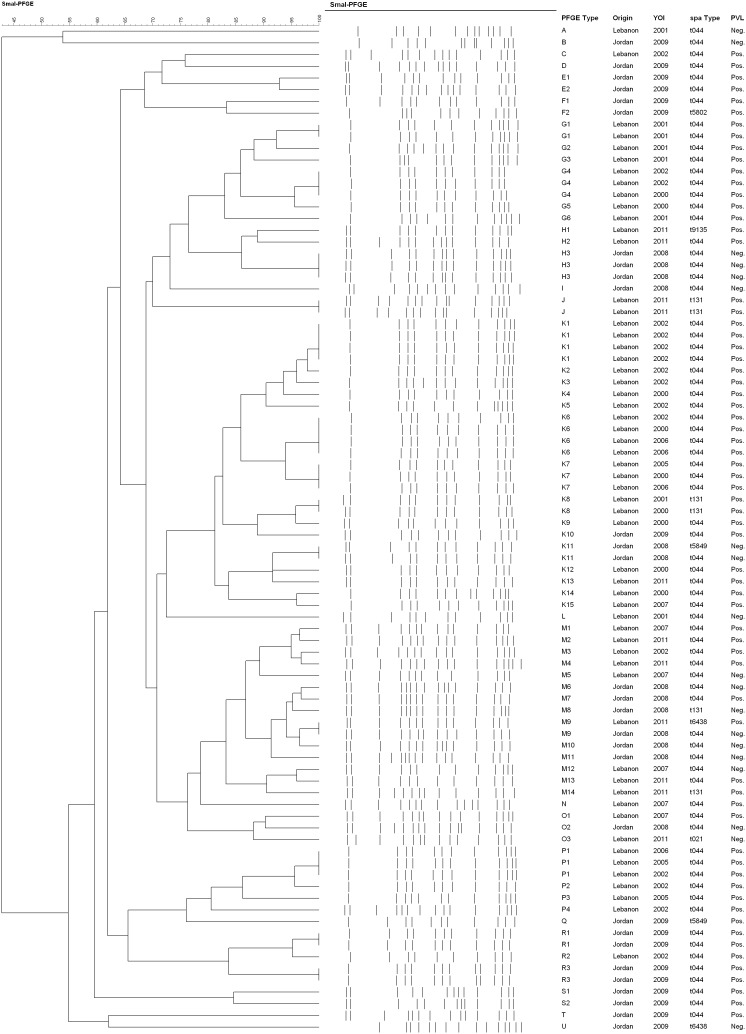
PFGE-based analysis clustered the 94 isolates in 21 different clonal groups when employing 80% as a similarity cutoff value, with 26% of the isolates clustering in one group designated as clonal group K (Figure 2, Table 1 and Table 2). This pulsotype had isolates from both countries, and all except for two isolates from Jordan were PVL positive, and were of spa types: t044, t131, and t5849. The genetic diversity occurred during the whole study period with isolates from both countries showing diversification and at times coexistence. The diversity between the isolates recovered from both countries however, was again clearly seen with the lack of any common pulsotype. Finally, different spa types, resistance profiles, and toxin genes did not correlate with specific PFGE subtypes.
Figure 2. Dendrogram of PFGE clusters of CC80 isolates.
SmaI macrorestriction patterns were analyzed using the Dice coefficient and visualized by un-weighted pair group method (UPGMA), using average linkages with 1% tolerance and 1% optimization settings. PFGE groups determined by cluster analysis are numbered from A-U. Origin, year of isolation (YOI), spa type, and PVL status of each isolate is also included.
Discussion
In Europe, most CA-MRSA isolates were associated with CC80-IV with the first report detecting an ST80-IV isolate being in 1998 in Greece [38]. Since then, sporadic ST80-IV cases have been reported in many European countries, which argued the possibility of the clone being introduced from the Middle East [18], [21], [25], [39]. Geographically, the Middle East is a heterogeneous region composed of 17 countries that vary substantially in terms of size, population and culture. Several reports from the Middle East have previously detected and reported the circulation of ST80-IV clone [10], [12], [40], [41], [42], [43], [44], [45]. Understanding the ST80-IV epidemic in the Middle East and its potential successful transmission to Europe was an important endeavor towards better control. Accordingly this study was conducted, which included a collection of projected CC80 related MRSA recovered from Lebanon (n = 63/94 isolates; 67%) and Jordan (n = 31/94; 33%) from 2000 to 2011, in an attempt to determine the relatedness, if it exists, between the European ST80-IV and the ones prevalent in the Middle East.
CA-MRSA have been associated primarily with community acquired infections, predominantly SSTIs, in young people [2]. Having 56% of the isolates in this study associated with SSTIs agrees with the notion of ST80-IV isolates being primarily associated with SSTIs in patients outside hospitals [46]. However, isolates causing invasive infections, including bacteremia (9%) and respiratory tract infections (15%) were also detected and included.
The most common antimicrobial resistance pattern observed within ST80 isolates circulating in Europe is the one against tetracycline, streptomycin, kanamycin and fusidic acid (TSKF pattern) [18], [47]. A different common pattern however, was detected among the isolates examined in this study, being mainly that of SKF (n = 34/96; 36%). Resistance against streptomycin, kanamycin and fusidic acid was comparable regardless of the source (Jordan or Lebanon) being 86, 86 and 91%, respectively. Compared to the European ST80 isolates, we detected in general a higher susceptibility to tetracycline especially with the ones recovered from Jordan (Lebanon n = 22/63; 35% resistant vs. n = 6/31; 19% for Jordan). On the other hand, higher resistance to erythromycin was detected within the isolates from Jordan (n = 19/31; 61%) as compared to those from Lebanon (n = 14/63; 22%). Similarly, Udo and Srakhoo [45] also showed the presence of variations in the resistance patterns between isolates recovered from Kuwait when compared to the European ST80 clone. This diversity reflects differences in the treatment regimens that exist between those countries.
PVL, a prophage-encoded bi-component pore-forming protein, is encoded by two genes: lukS-PV and lukF-PV residing in genomes of some bacteriophages (e.g.: ΦSa2958, ΦSa2MW, ΦPVL) and these are readily transferrable following selective bacterial infection [48]. At elevated concentrations, PVL causes host cell lysis; however at lower concentrations, PVL primes neutrophils to release inflammatory mediators such as leukotriene B4, IL-8, granule contents and reactive oxygen species [49]. Although its role in pathogenicity remains controversial, many murine-conducted studies show the role of PVL in mitochondrial inactivation and apoptosis as well as its association with certain established diseases such as necrotizing pneumonia and SSTIs [2], [50], [51]. PVL genes were detected in 78% (n = 73/94) of the isolates. Contrary to the European PVL-positive ST80, 52% (n = 16/31) of the isolates from Jordan and 8% (n = 5/63) from Lebanon were PVL-negative. PVL-negative ST80 was previously detected in Kuwait [45], Algeria [52], Switzerland and France [53]. Whether these isolates arose from PVL positive ones due to the loss of the PVL phage or represent native ST80 backgrounds that had not previously acquired the PVL phage is something that needs to be further clarified. It is however noteworthy, that a PVL-negative isolate from Lebanon had the common European antibiotic resistance pattern (TSKF) with additional resistance to gentamicin, clindamycin, and erythromycin. This finding was in line with the study conducted by Ramdani-Bouguessa et al. [54] from Algeria, thus posing a problem of having a possibility of ST80 invading hospital settings that adds an additional significant threat to public health. Finally, two out of the four isolates that were both PVL and etd negative were recovered from blood and were of spa type t044 and SCCmec-IV. Previously Adler et al. [40] demonstrated that PVL negative and SCCmec-IV S. aureus isolates were associated with pediatric HA-MRSA bloodstream infections.
Another significant finding adding up to the observed genetic diversity within the isolates undertaken in this study at one hand and the European ST80-IV on the other, was the detection of both tst and PVL genes in seven of the isolates that were recovered from Jordan. TSST-1 is a superantigen that stimulates the release of large amounts of proinflammatory factors in human infection, has been associated with human toxic shock syndrome [55], and causes sepsis by uncontrolled stimulation of T lymphocytes triggering a cytokine storm [56]. TSST-1 element is carried on a pathogenicity island known now as Sapl1, carrying the tst and other virulence factors [57]. A single strain of S. aureus rarely produces both PVL and TSST-1 [58]. However, Holmes et al. [59] previously documented the tst genes in four of 30 PVL-positive isolates. All the isolates were typed and belonged to lineages CC30, CC5 and CC22 some of which were multi-drug resistant. Similarly, tst positive isolates in this study, in addition to being resistant to the β-lactam drugs were resistant to streptomycin, kanamycin, and fusidic acid. spa typing of all the seven and MLST typing of three revealed that all were spa t044, two were ST80 and one ST997, with all belonging to the CC80 lineage. Our finding was in line with a recent study in Jordan in which two putative ST80-IV isolates belonging to spa type t044 harbored both the PVL and tst genes [41]. Zhi et al. [58] showed that a PVL-carrying phage from strain MSSA 68111, which was positive for both PVL and tst genes, was a variant of icosahedral-head type phage FPVL. These findings suggested that FPVL and FPVLv68111 might have evolved from a common ancestor and that genetic drift may have occurred in one or both. Features unique to FPVLv68111 may have permitted MSSA68111 to acquire the genes for TSST-1 production. Whether a similar genetic drift led to having isolates positive for both toxins in those isolates from Jordan needs to be further investigated, specially that it was a significant deviation from the norm and that it indicated the emergence of hypervirulent S. aureus strains. Finally, a clear heterogeneity was additionally detected within the other studied toxin genes, with 64% of the isolates from Lebanon being positive for etd gene compared to only 28% for those from Jordan; detecting etd toxin gene is a common finding within the European ST80 isolates [18], [53], [60], which emphasizes again that ST80-IV should be considered as a clonal lineage. Yamaguchi et al. [61], showed that the etd gene was carried on a pathogenicity island and hypothesized that ETD may play a pathogenic role in a variety of infections by destroying epithelial barriers, helping bacteria to spread or invade tissues. This could partly explain the success of the isolates within some of the ST80 isolates, which usually carried the gene for ETD in combination with the gene for PVL [59].
The most common spa type so far detected within the CC80-MRSA-IV isolates has been type t044 (spa repeat pattern r07 r23 r12 r34 r34 r33 r34) in Europe [53], [62], [63], [64], [65], Africa [17], [20], [43], [66], and Asia [10], [12], [44]. spa typing of the isolates in this study revealed the following types: t044, single locus variants (SLV) of type t044 (t131, t5802, t5849, and t4222) or t131 (t5802), double locus variants (DLV) of type t044 (t6438 and t9135) and a singleton (t021 annotated to CC30). spa type t131 was frequently reported among CC80-MRSA-IV isolates from Europe [18], [21], [64], [65]. It is noteworthy however, that one isolate within the spa type t131, which also belonged to the ST80-IV and was positive for PVL and etd genes, showed no resistance to any of the tested antibiotics. This was in contrast to what was recently reported by Hadjihannas et al. [65], with a similar isolate recovered from Greece belonging to the ST80-IVc and spa type (t131), but expressing an increase in resistance repertoire to include five different classes of non–β-lactam antibiotics, namely, quinolones, macrolides, clindamycin, fusidic acid, and tetracyclines again re-emphasizing the existing genetic diversity within ST80-IV clonal lineage.
All isolates chosen for MLST typing, based on variation of specimen origin, year of isolation and covering all different spa types within spa-CC 044, were ST80 except for one from Jordan, which was ST997. ST997 however, is also within the CC80 and is a SLV from ST80 (http://saureus.mlst.net/eburst/database.asp).
PFGE-based analysis clustered the 94 isolates in 21 different clonal groups when employing 80% as a similarity cutoff value, with 26% of the isolates clustering in one group designated as clonal group K. This pulsotype had isolates from both countries, all except for two isolates from Jordan were PVL positive, and isolates were of spa types: t044, t131, and t5849. Different spa types, resistance profiles, and toxin genes did not correlate with specific PFGE subtypes. The diversity observed within the isolates recovered from both countries along with the lack of any common pulsotype, diminishes the possibility of cross transmission.
European CA-MRSA has previously been described as a rather uniform clone. However, the high degree of molecular diversity observed in recent years, and being additionally supported by the diversity observed in this study, makes it difficult to maintain “the European CA-MRSA clone” as a uniform clone, and it is better to refer to them, and as suggested previously, as CC80-MRSA-IV isolates [18]. Close surveillance of these strains is essential to monitor their spread, antimicrobial resistance profiles, and association with disease. Finally, the successful expansion of ST80 and the heterogeneity observed in this study calls for novel approaches in infection control measures to monitor their spread.
Acknowledgments
Part of this work has been presented at the 113th American society for microbiology general meeting held in Denver, California, May 2013 and at the 15th International Symposium on Staphylococci and Staphylococcal infections held in Lyon, France, August 2012.
Funding Statement
This work was supported by the Lebanese American University Research Council. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. McCarthy AJ, Lindsay JA (2010) Genetic variation in Staphylococcus aureus surface and immune evasion genes is lineage associated: implications for vaccine design and host-pathogen interactions. BMC Microbiol 10: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. David MZ, Daum RS (2010) Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev 23: 616–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nimmo GR, Coombs GW (2008) Community-associated methicillin-resistant Staphylococcus aureus (MRSA) in Australia. Int J Antimicrob Agents 31: 401–410. [DOI] [PubMed] [Google Scholar]
- 4. Shorr AF (2007) Epidemiology of staphylococcal resistance. Clin Infect Dis 45: S171–176. [DOI] [PubMed] [Google Scholar]
- 5. DeLeo FR, Kennedy AD, Chen L, Wardenburg JB, Kobayashi SD, et al. (2011) Molecular differentiation of historic phage-type 80/81 and contemporary epidemic Staphylococcus aureus . Proc Natl Acad Sci USA 108: 18091–18096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brady JM, Stemper ME, Weigel A, Chyou P, Reed KD, et al. (2007) Sporadic “Transitional” community-associated methicillin-resistant Staphylococcus aureus strains from health care facilities in the United States. J Clin Microbiol 45: 2654–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Song J, Hsueh P, Chung DR, Ko KS, Kang C, et al. (2011) Spread of methicillin-resistant Staphylococcus aureus between the community and the hospitals in Asian countries: an ANSORP study. J Antimicrob Chemother 66: 1061–1069. [DOI] [PubMed] [Google Scholar]
- 8. Vandenesch F, Naimi T, Enright MC, Lina G, Nimmo Gr, et al. (2003) Community-aquierd mrthicillin-resistan Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis 9: 978–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Borg M, de Kraker M, Scicluna E, de Sande-Bruinsma N, Tiemersma E, et al. (2007) Prevalence of methicillin-resistant Staphylococcus aureus (MRSA) in invasive isolates from southern and eastern Mediterranean countries. J Antimicrob Chemo 60: 1310–1315. [DOI] [PubMed] [Google Scholar]
- 10. Tokajian ST, Abou Khalil P, Jabbour D, Rizk M, Farah M, et al. (2010) Molecular characterization of Staphylococcus aureus in Lebanon. Epidemiol Infect 138: 707–712. [DOI] [PubMed] [Google Scholar]
- 11. Harastani H, Araj G, Tokajian S (2014) Molecular characteristics of Staphylococcus aureus isolated from a major hospital in Lebanon. Int J Infect Dis 19: 33–38. [DOI] [PubMed] [Google Scholar]
- 12. Khalil W, Hashwa F, Shihabi A, Tokajian S (2012) Methicillin-resistant Staphylococcus aureus ST80-IV clone in children from Jordan. Diagn Microbiol Infect Dis 73: 228–230. [DOI] [PubMed] [Google Scholar]
- 13. Hsu LY, Tristan A, Koh TH, Bes M, Etienne J, et al. (2005) Community associated methicillin-resistant Staphylococcus aureus, Singapore. Emerg Infect Dis 11: 341–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ahmad N, Ruzan IN, Abd Ghani MK, Hussin A, Nawi S, et al. (2009) Characteristics of community- and hospital-acquired meticillin-resistant Staphylococcus aureus strains carrying SCCmec type IV isolated in Malaysia. J Med Micro 58: 1213–1218. [DOI] [PubMed] [Google Scholar]
- 15. Deresinski S (2005) Methicillin-resistant Staphylococcus aureus: an evolutionary, epidemiologic and therapeutic odyssey. Clin Infect Dis 40: 562–573. [DOI] [PubMed] [Google Scholar]
- 16. Antri K, Rouzic N, Dauwalder O, Boubekri I, Bes M, et al. (2011) High prevalence of methicillin-resistant Staphylococcus aureus clone ST80-IV in hospital and community settings in Algiers. Clin Microbiol Infect 17: 526–532. [DOI] [PubMed] [Google Scholar]
- 17. Hanssen A, Fossum A, Mikalsen J, Halvorsen DS, Bukholm G, et al. (2005) Dissemination of community-acquired methicillin-resistant Staphylococcus aureus clones in northern Norway: sequence types 8 and 80 predominate. J Clin Microbiol 43: 2118–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Larsen AR, Böcher S, Stegger M, Goering R, Pallesen LV, et al. (2008) Epidemiology of European community-associated methicillin-resistant Staphylococcus aureus clonal complex 80 type IV strains isolated in Denmark from 1993 to 2004. J Clin Microbiol 46: 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Witte W, Cuny C, Strommenger B, Braulke C, Heuck D (2004) Emergence of a new community acquired MRSA strain in Germany. Euro Surveill 9: 440. [DOI] [PubMed] [Google Scholar]
- 20. Ben Nejma M, Mastouri M, Jrad B, Nour M (2013) Characterization of ST80 Panton-Valentine Leukocidin-positive community-acquired methicillin-resistant Staphylococcus aureus clone in Tunisia. Diagn Microbiol Infect Dis 77: 20–24. [DOI] [PubMed] [Google Scholar]
- 21. Larsen AR, Stegger M, Böcher S, Sørum M, Monnet DL, et al. (2009) Emergence and characterization of community-associated methicillin-resistant Staphylococcus aureus infections in Denmark, 1999 to 2006. J Clin Microbiol 47: 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Denis O, Malaviolle X, Titeca G, Struelens MJ, Garrino MG, et al. (2004) Emergence of Panton-Valentine Leukocidin positive community-acquired MRSA infections in Belgium. Euro Surveill 8: 2483. [Google Scholar]
- 23. Harbarth S, Francois P, Schrenzel J, Fankhauser-Rodriguez C, Hugonnet S, et al. (2005) Community-associated methicillin-resistant Staphylococcus aureus, Switzerland. Emerg Intfet Dis 11: 962–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maier J, Melzl H, Reischl U, Drubel I, Witte W, et al. (2005) Panton-Valentine Leukocidin methicillin-resistant Staphylococcus aureus in Germany associated with travel of foreign family origin. Eur J Clin Microbiol Infect Dis 24: 637–639. [DOI] [PubMed] [Google Scholar]
- 25. Urth T, Juul G, Skov R, Schønheyder HC (2005) Spread of methicillin-resistant Staphylococcus aureus ST80-IV clone in a Danish community. Infect Control Hosp Epidemiol 26: 144–149. [DOI] [PubMed] [Google Scholar]
- 26. Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, et al. (1995) Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: Criteria for bacterial strain typing. J Clin Microbiol 33: 2233–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Struelens MJ, Deplano A, Godard C, Maes N, Serruys E (1992) Epidemiological typing and delineation of genetic relatedness of methicillin resistant Staphylococcus aureus by macro-restriction analysis of genomic DNA by using pulsed-field gel electrophoresis. J Clin Microbiol 30: 2599–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twentieth Informational Supplement. CLSI document M100-S20, Wayne, Pa, USA.
- 29. McClure J, Conly J, Lau V, Elsayed S, Louie T, et al. (2006) Novel multiplex PCR assay of the staphylococcal virulence marker Panton–Valentine Leukocidin genes and simultaneous discrimination of methicillin-susceptible from methicillin-resistant staphylococci. J Clin Microbiol 44: 1141–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Johnson WM, Tyler SD, Ewan EP, Ashton FE, Pollard DR, et al. (1991) Detection of genes for enterotoxins, exofoliative toxins, and toxic shock syndrome toxin 1 in Staphylococcus aureus by the polymerase chain reaction. J Clin Microbiol 29: 426–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Monday SR, Bohach GA (1999) Use of multiplex PCR to detect classical and newly described pyrogenic toxin genes in Staphylococcal isolates. J Clin Microbiol 37: 3411–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang K, McClure J, Elsayed S, Louie T, Conly JM (2005) Novel multiplex PCR assay for characterization and concomitant subtyping of staphylococcal cassette chromosome mec types I to V in methicillin-resistant Staphylococcus aureus. . J Clin Microbiol 43: 5026–5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ito T, Katayama Y, Asada K, Mori N, Tsutsumimoto K, et al. (2001) Structural comparison of three types of staphylococcal cassette chromosome mec integrated in the chromosome in methicillin-resistant Staphylococcus aureus . Antimicrob Agents Chemother 45: 1323–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Harmsen M, Claus H, Witte W, Rothganger J, Turnwald D, et al. (2003) Typing of methicillin resistant Staphylococcus aureus in a university hospital setting by novel software for spa repeat determination and database management. J Clin Microbiol 41: 5442–5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Strommenger B, Braulke C, Pasemann B, Schmidt C, Witte W (2008) Multiplex PCR for rapid detection of Staphylococcus aureus isolates suspected to represent community acquired strains. J Clin Microbiol 46: 582–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Enright C, Robinson A, Randle G, Feil J, Grundmann H, et al. (2002) The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA). Proc Natl Acad Sci U.S.A 99: 7687–7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goering R, Winters A (1992) Rapid method for epidemiological evaluation of Gram-positive cocci by field inversion gel electrophoresis. J Clin Microbiol 30: 577–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aires de Soussa M, de Lencastre H (2003) Evolution of sporadic isolates of methicilin-resistant Staphylococcus aureus (MRSA) in hospitals and their similarities to isolates of community-acquired MRSA. J Clin Microbiol 41: 3806–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Goering RV (2009) Comparative genomic analysis of European and Middle Eastern community-associated methicillin-resistant Staphylococcus aureus (CC80: ST80-IV) isolates by high-density microarray. Clin Microbiol Infect 15: 748–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Adler A, Givon-Lavi N, Moses AE, Block C, Dagan R (2010) Carriage of community-associated methicillin-resistant Staphylococcus aureus in a cohort of infants in southern Israel: risk factors and molecular features. J Clin Microbiol 48: 531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Al-Bakri AG, Al-Hadithi H, Kasabri V, Othman G, Kriegeskorte A, Becker K (2013) The epidemiology and molecular characterization of methicillin-resistant staphylococci sampled from a healthy Jordanian population. Epidemiol Infect 141: 2384–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. El-Mahdy TS, El-Ahmady M, Goering RV (2013) Molecular characterization of methicillin-resistant Staphylococcus aureus isolated over a 2-year period in a Qatari hospital from multinational patients. Clin Microbiol Infect doi: 10.1111/1469-0691.12240 [DOI] [PubMed] [Google Scholar]
- 43. Enany S, Yaoita E, Yoshida Y, Enany M, Yamamoto T (2010) Molecular characterization of Panton-Valentine Leukocidin-positive community-acquired methicillin-resistant Staphylococcus aureus isolates in Egypt. Microbiol Res 165: 152–162. [DOI] [PubMed] [Google Scholar]
- 44. Sonnevend Á, Blair I, Alkaabi M, Jumaa P, al Haj M, et al. (2012) Change in meticillin-resistant Staphylococcus aureus clones at a tertiary care hospital in the United Arab Emirates over a 5-year period. J Clin Pathol 65: 178–182. [DOI] [PubMed] [Google Scholar]
- 45. Udo EE, Sarkhoo E (2010) The dissemination of ST80-SCCmec-IV community-associated methicillin resistant Staphylococcus aureus clone in Kuwait hospitals. Ann Clin Microbiol Antimicrob 9: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Larsen AR, Skov RL, Jarlier V, Henriksen AS (2008) Epidemiological differences between the UK and Ireland versus France in Staphylococcus aureus isolates resistant to fusidic acid from community-acquired skin and soft tissue infections. J Antimicrob Chemother 61: 589–594. [DOI] [PubMed] [Google Scholar]
- 47. Stam-Bolink EM, Mithoe S, Baas WH, Arends JP, Möller AVM (2007) Spread of methicillin-resistant Staphylococcus aureus ST80 strain in the community of northern Netherlands. Eur J Clin Microbiol Infect Dis 26: 723–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Boakes E, Kearns AM, Ganner M, Perry C, Hill RL, et al. (2011) Distinct bacteriophages encoding Panton-Valentine Leukocidin (PVL) among international methicillin-resistant Staphylococcus aureus clones harboring PVL. J Clin Microbiol 49: 684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chambers HF, DeLeo FR (2009) Waves of resistance: Staphylococcus aureus in the antibiotic area. Nat Rev Microbiol 7: 629–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dufuor P, Gillet Y, Bes M, Lina G, Vandenesch F, et al. (2002) Community-acquired methicillin-resistant Staphylococcus aureus infections in France: Emergence of a single clone that produces Panton-Valentine Leukocidin. Clin Infect Dis 35: 819–824. [DOI] [PubMed] [Google Scholar]
- 51. Labandeira-Rey M, Couzon F, Boisset S, Brown EL, Bes M, et al. (2007) Staphylococcus aureus Panton-Valentine Leukocidin causes necrotizing pneumonia. Science 315: 1130–1133. [DOI] [PubMed] [Google Scholar]
- 52. Djoudi F, Bonura C, Benallaoua S, Touati A, Touati D, et al. (2013) Panton-Valentine Leukocidin positive sequence type 80 methicillin-resistant Staphylococcus aureus carrying a staphylococcal cassette chromosome mec type IVc is dominant in neonates and children in an Algiers hospital. New Microbiol 36: 49–56. [PubMed] [Google Scholar]
- 53. Tristan A, Bes M, Meugnier H, Lina G, Bozdogan B, et al. (2007) Global distribution of Panton-Valentine Leukocidin-positive methicillin-resistant Staphylococcus aureus, 2006. Emerg Infect Dis 13: 594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ramdani-Bouguessa N, Bes M, Meugnier H, Forey F, Reverdy M, et al. (2006) Detection of methicillin-resistant Staphylococcus aureus strains resistant to multiple antibiotics and carrying the Panton-Valentine Leukocidin genes in an Algiers hospital. Antimicrob Agents Chemother 50: 1083–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shands KN, Schmid GP, Dan BB, Blum D, Guidotti RJ, et al. (1980) Toxic-shock syndrome in menstruating women: association with tampon use and Staphylococcus aureus and clinical features in 52 cases. N Engl J Med 303: 1436–1442. [DOI] [PubMed] [Google Scholar]
- 56. Jekle A, Yoon J, Zuck M, Najafi R, Wang L, et al. (2013) NVC-422 inactivates Staphylococcus aureus toxins. Antimicrob Agents Chemother 57: 924–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lindsay JA, Ruzin, Ross HF, Kurepina N, Novick RP (1998) The gene for toxic shock toxin is carried by a family of mobile pathogenicity islands in Staphylococcus aureus . Mol Microbiol 29: 527–543. [DOI] [PubMed] [Google Scholar]
- 58. Zhi L, Stevens DL, Hamilton SM, Parimon T, Ma Y, et al. (2010) Fatal S. aureus hemorrhagic pneumonia: Genetic analysis of a unique clinical isolate producing both PVL and TSST-1. PLoS One 6: e27246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Holmes A, Ganner M, McGuane S, Pitt TL, Cookson BD, et al. (2005) Staphylococcus aureus isolates carrying Panton-Valentine leucocidin genes in England and Wales: frequency, characterization, and association with clinical disease. J Clin Microbiol 43: 2384–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Scicluna EA, Shore AC, Thürmer A, Ehricht R, Slickers P, et al. (2010) Characterisation of MRSA from Malta and the description of a Maltese epidemic MRSA strain. Eur J Clin Microbiol Infect Dis 29: 163–170. [DOI] [PubMed] [Google Scholar]
- 61. Yamaguchi T, Nishifuji K, Sasaki M, Fudaba Y, Aepfelbacher M, et al. (2002) Identification of the Staphylococcus aureus etd pathogenicity island which encodes a novel exfoliative toxin, ETD, and EDIN-B. Infect Immun 70: 5835–5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cucarella C, Tormo MA, Úbeda C, Trotonda MP, Monzón M, et al. (2004) Role of biofilm-associated protein Bap in the pathogenesis of bovine Staphylococcus aureus . Infect Immun 72: 2177–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Budimir A, Deurenberg RH, Bošnjak Z, Stobberingh EE, Cetkovic H, et al. (2009) A variant of the Southern German clone of methicillin-resistant Staphylococcus aureus is predominant in Croatia. Clin Microbiol Infect 16: 1077–1083. [DOI] [PubMed] [Google Scholar]
- 64. Denis O, Deplano A, De Beenhouwer H, Hallin M, Huysmans G, et al. (2005) Polyclonal emergence and importation of community-acquired methicillin-resistant Staphylococcus aureus strains harbouring Panton-Valentine leucocidin genes in Belgium. J Antimicrob Chem 56: 1103–1106. [DOI] [PubMed] [Google Scholar]
- 65. Hadjihannas L, Psichogiou M, Empel J, Kosmidis C, Goukos D, et al. (2012) Molecular characteristics of community-associated methicillin-resistant Staphylococcus aureus colonizing surgical patients in Greece. Diagn Microbiol Infect Dis 74: 420–422. [DOI] [PubMed] [Google Scholar]
- 66. Bekkhoucha SN, Cady A, Gautier P, Itim F, Donnio PY (2009) A portrait of Staphylococcus aureus from the other side of the Mediterranean Sea: molecular characteristics of isolates from Western Algeria. Eur J Clin Microbiol Infect Dis 28: 553–555. [DOI] [PubMed] [Google Scholar]