Abstract
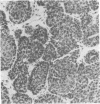
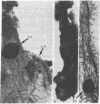
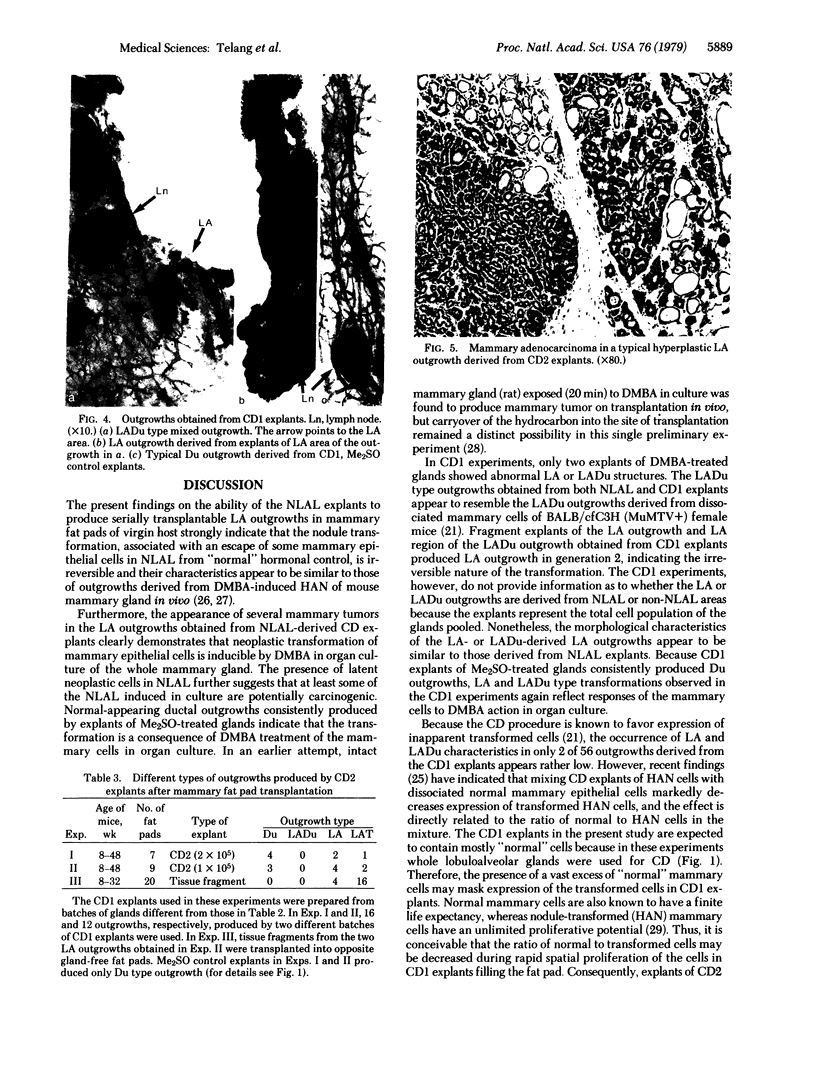
Biological characteristics of nodule-like alveolar lesions (NLAL) induced by 7,12-dimethylbenz[a]anthracene (DMBA) in organ culture of whole mammary gland (BALB/c female mice) were assessed after transplantation into gland-free mammary fat pads of syngeneic virgin mice. (i) Tissue-fragment explants from NLAL areas of the gland produced abnormal lobuloalveolar (LA) outgrowths in 3 of 10 fat pads. (ii) Transplantation of dissociated cells of NLAL-derived LA outgrowths into 36 fat pads showed 100% LA outgrowths and 3 (8%) of these 36 outgrowths produced mammary carcinomas. (iii) The explants of dissociated cells from whole mammary glands treated with DMBA in culture produced full or partial LA structures in 2 of 56 outgrowths. (iv) The explants of dissociated cells prepared from outgrowths derived from outgrowths derived from explants as in iii produced 9 LA outgrowths in 16 instances; mammary tumor incidence in these outgrowths was 3 of 16 (18%). (v) The explants of tissue fragments from LA outgrowths as in iv produced LA outgrowths in 20 of 20 fat pads; mammary carcinomas appeared in 16 of 20 (80%) of these outgrowths. No NLAL was detectable in control glands treated with dimethyl sulfoxide (solvent for DMBA); explants of the control glands consistently produced ductal outgrowths and no tumor. This accomplishment of chemical carcinogen-induced neoplastic transformation of epithelial cells in vitro provides a model for studying carcinogenesis in an entire isolated organ.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee M. R. Responses of mammary cells to hormones. Int Rev Cytol. 1976;47:1–97. doi: 10.1016/s0074-7696(08)60086-8. [DOI] [PubMed] [Google Scholar]
- Banerjee M. R., Terry P. M., Sakai S., Lin F. K. Regulation of messenger RNA and specific milk protein in mammary gland. J Toxicol Environ Health. 1977 Sep;3(1-2):281–308. doi: 10.1080/15287397709529566. [DOI] [PubMed] [Google Scholar]
- Banerjee M. R., Wood B. G., Washburn L. L. Chemical carcinogen-induced alveolar nodules in organ culture of mouse mammary gland. J Natl Cancer Inst. 1974 Nov;53(5):1387–1393. doi: 10.1093/jnci/53.5.1387. [DOI] [PubMed] [Google Scholar]
- Casto B. C., DiPaolo J. A. Virus, chemicals and cancer. Prog Med Virol. 1973;16:1–47. [PubMed] [Google Scholar]
- DEOME K. B., FAULKIN L. J., Jr, BERN H. A., BLAIR P. B. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res. 1959 Jun;19(5):515–520. [PubMed] [Google Scholar]
- Daniel C. W., De Ome K. B., Young J. T., Blair P. B., Faulkin L. J., Jr The in vivo life span of normal and preneoplastic mouse mammary glands: a serial transplantation study. Proc Natl Acad Sci U S A. 1968 Sep;61(1):53–60. doi: 10.1073/pnas.61.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao T. L., Sinha D. Mammary adenocarcinoma induced in organ culture by 7,12-dimethylbenz(a)anthracene. J Natl Cancer Inst. 1972 Aug;49(2):591–593. [PubMed] [Google Scholar]
- DeOme K. B., Miyamoto M. J., Osborn R. C., Guzman R. C., Lum K. Detection of inapparent nodule-transformed cells in the mammary gland tissues of virgin female BALB/cfC3H mice. Cancer Res. 1978 Jul;38(7):2103–2111. [PubMed] [Google Scholar]
- Dickens M. S., Custer R. P., Sorof S. Retinoid prevents mammary gland transformation by carcinogenic hydrocarbon in whole-organ culture. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5891–5895. doi: 10.1073/pnas.76.11.5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberger C. Chemical carcinogenesis. Annu Rev Biochem. 1975;44:79–121. doi: 10.1146/annurev.bi.44.070175.000455. [DOI] [PubMed] [Google Scholar]
- Heidelberger C. Chemical oncogenesis in culture. Adv Cancer Res. 1973;18:317–366. doi: 10.1016/s0065-230x(08)60756-3. [DOI] [PubMed] [Google Scholar]
- Ichinose R. R., Nandi S. Influence of hormones on lobulo-alveolar differentiation of mouse mammary glands in vitro. J Endocrinol. 1966 Aug;35(4):331–340. doi: 10.1677/joe.0.0350331. [DOI] [PubMed] [Google Scholar]
- Kundu A. B., Telang N. T., Banerjee M. R. Binding of 7,12-dimethylbenz[a]anthracene to BALB/c mouse mammary gland DNA in organ culture. J Natl Cancer Inst. 1978 Aug;61(2):465–469. [PubMed] [Google Scholar]
- LASNITZKI I. GROWTH PATTERN OF THE MOUSE PROSTATE GLAND IN ORGAN CULTURE AND ITS RESPONSE TO SEX HORMONES, VITAMIN A, AND 3-METHYLCHOLANTHRENE. Natl Cancer Inst Monogr. 1963 Oct;12:381–403. [PubMed] [Google Scholar]
- Lin F. K., Banerjee M. R., Crump L. R. Cell cycle-related hormone carcinogen interaction during chemical carcinogen induction of nodule-like mammary lesions in organ culture. Cancer Res. 1976 May;36(5):1607–1614. [PubMed] [Google Scholar]
- Medina D. Mammary tumorigenesis in chemical carcinogen-treated mice. I. Incidence in BALB-c and C57BL mice. J Natl Cancer Inst. 1974 Jul;53(1):213–221. doi: 10.1093/jnci/53.1.213. [DOI] [PubMed] [Google Scholar]
- Medina D. Mammary tumorigenesis in chemical carcinogen-treated mice. II. Dependence on hormone stimulation for tumorigenesis. J Natl Cancer Inst. 1974 Jul;53(1):223–226. doi: 10.1093/jnci/53.1.223. [DOI] [PubMed] [Google Scholar]
- Medina D., Shepherd F., Gropp T. Enhancement of the tumorigenicity of preneoplastic mammary nodule lines by enzymatic dissociation. J Natl Cancer Inst. 1978 May;60(5):1121–1126. doi: 10.1093/jnci/60.5.1121. [DOI] [PubMed] [Google Scholar]
- Mehta R. G., Banerjee M. R. Action of growth-promoting hormones on macromolecular biosynthesis during lobulo-alveolar development of the entire mammary gland in organ culture. Acta Endocrinol (Copenh) 1975 Nov;80(3):501–516. doi: 10.1530/acta.0.0800501. [DOI] [PubMed] [Google Scholar]
- Richards J., Nandi S. Neoplastic transformation of rat mammary cells exposed to 7,12-dimethylbenz[alpha]anthracene or N-nitrosomethylurea in cell culture. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3836–3840. doi: 10.1073/pnas.75.8.3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röller M. R., Heidelberger C. Attempts to produce carcinogenesis in organ cultures of mouse prostate with polycyclic hydrocarbons. Int J Cancer. 1967 Sep 15;2(5):509–520. doi: 10.1002/ijc.2910020513. [DOI] [PubMed] [Google Scholar]
- Terry P. M., Banerjee M. R., Lui R. M. Hormone-inducible casein messenger RNA in a serum-free organ culture of whole mammary gland. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2441–2445. doi: 10.1073/pnas.74.6.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonelli Q. J., Custer R. P., Sorof S. Transformation of cultured mouse mammary glands by aromatic amines and amides and their derivatives. Cancer Res. 1979 May;39(5):1784–1792. [PubMed] [Google Scholar]
- Williams G. M., Weisburger E. K., Weisburger J. H. Isolation and long-term cell culture of epithelial-like cells from rat liver. Exp Cell Res. 1971 Nov;69(1):106–112. doi: 10.1016/0014-4827(71)90316-8. [DOI] [PubMed] [Google Scholar]
- Wood B. G., Washburn L. L., Mukherjee A. S., Banerjee M. R. Hormonal regulation of lobulo-alveolar growth, functional differentiation and regression of whole mouse mammary gland in organ culture. J Endocrinol. 1975 Apr;65(1):1–6. doi: 10.1677/joe.0.0650001. [DOI] [PubMed] [Google Scholar]
- Yamada T., Takoka T., Katsuta H., Namba M., Sato J. Carcinogenesis in tissue culture. 20. Electrokinetic changes in cultured rat liver cells associated with malignant transformation in vitro. Jpn J Exp Med. 1972 Aug;42(4):377–388. [PubMed] [Google Scholar]