Abstract
Background
To evaluate the predominant pattern of brain injury and the anatomic areas of injury in children with infantile spasms following neonatal hypoxic-ischemic encephalopathy (HIE).
Methods
A nested case-control study of infantile spasms in children with term neonatal HIE was performed. All patients had T1/T2-weighted magnetic resonance imaging (MRI) with diffusion-weighted imaging (DWI) performed on the third day of life. Using a validated scoring system, the MRI was classified as: normal, watershed, basal ganglia/thalamus, total, or focal-multifocal. Two study investigators scored additional anatomic areas of injury (cortical extent, levels of the brainstem, hypothalamus) on T1/T2-weighted MRI and DWI blinded to the outcome. The predominant pattern of brain injury and anatomic areas of injury were compared between patients who developed infantile spasms and randomly selected controls.
Results
Eight patients who developed infantile spasms were identified among a cohort of 176 term newborns with HIE (4.5%). There were no significant differences in the perinatal and neonatal course between newborns who developed infantile spasms and controls who did not. The development of infantile spasms after neonatal HIE was significantly associated with basal ganglia/thalamus and total brain injury (P=.001), extent of cortical injury greater than 50% (odds ratio [OR]=11.7, 95% confidence interval [CI]=1.1-158.5, P=.01), injury to the midbrain (OR=13, 95% CI=1.3 to 172, P=.007) and hypothalamic abnormalities (P=.01).
Conclusions
The development of infantile spasms after HIE is associated with injury to the basal ganglia and thalami on neonatal MRI, particularly when extensive cortical injury and/or injury to the midbrain is present.
Keywords (MeSH): Brain imaging, hypoxic-ischemic encephalopathy, magnetic resonance, neonatal, infantile spasms
Introduction
Hypoxic-ischemic injury is the most common cause of term neonatal encephalopathy, which occurs in one to six per 1000 live births.1 Hypoxic-ischemic encephalopathy (HIE) in the neonatal period results in severe motor and cognitive deficits in up to 40% of surviving newborns treated with hypothermia.2 The predominant pattern of brain injury as determined by neonatal magnetic resonance imaging (MRI) is a strong predictor of neurodevelopmental outcome.3,4 Children with more severe brain injury on neonatal MRI are at higher risk for epilepsy.5
Neonatal HIE is among the most common causes of infantile spasms, accounting for up to 10% of cases.6 Infantile spasms are characterized by clusters of spasms and hypsarrhythmia on electroencephalography (EEG) with a peak age of onset between 3 and 7 months.7,8 A unifying mechanism through which diverse etiologies culminate in infantile spasms is unknown. Lesions of the cortex, basal ganglia, hypothalamus, brainstem and spinal cord have all been implicated in the pathogenesis of infantile spasms. 8-11
Currently, little is known about the clinical characteristics and MRI findings of infants that develop infantile spasms after neonatal HIE. Identifying newborns at higher risk for infantile spasms may enable earlier treatment and improve neurodevelopmental outcomes. The objectives of this study were to (1) characterize the predominant pattern of brain injury on neonatal MRI in term newborns with HIE who developed infantile spasms, (2) evaluate the extent of injury in specific anatomic areas that are not usually scored as part of the predominant pattern of injury, and (3) explore any association between the predominant pattern of brain injury, other anatomic areas of injury and the outcome of infantile spasms in this cohort.
Methods
Study Population
The University of British Columbia Clinical Research Ethics Board approved this study. Our cohort consisted of 176 term newborns with HIE imaged with T1/T2-MRI with DWI on the third day of life (72 hours ± 24 hours) and admitted to Children's & Women's Hospital of British Columbia, a provincial tertiary level neonatal referral center. Inclusion criteria for this cohort comprised gestational age ≥36 weeks with clinically recognizable encephalopathy, and at least one of the following criteria: (1) fetal distress at delivery, (2) requirement for resuscitation at birth, (3) Apgar score ≤5 at 5 minutes, or (4) metabolic acidosis (umbilical artery pH<7.1 or base deficit >10). Newborns with confirmed congenital malformations, genetic abnormalities, antenatal infections, or inborn errors of metabolism were excluded. These criteria were meant to identify a broad cohort of neonatal encephalopathy of presumed hypoxic-ischemic origin.
A nested case-control study was performed. This is a study design in which only a subset of controls from the cohort are compared to the incident cases.12 Eight patients with neonatal HIE who developed infantile spasms were identified from 2004 to 2011. We ensured complete case-ascertainment by cross-referencing our retrospective cohort and the EEG records at our institution, which are maintained in a single database. Control patients who did not develop infantile spasms were randomly selected from the cohort at a ratio of two to one. Six patients in the current study population have previously been reported on in a study comparing computed tomography and MRI findings in term newborns with HIE (n=48).13
Medical records were reviewed systematically for details regarding the antenatal, perinatal, and neonatal course, blinded to the imaging findings. Neonatal seizures were ascertained clinically and confirmed on continuous amplitude integrated EEG or on conventional EEG. The diagnosis of infantile spasms was made by the treating Pediatric Neurologist on the basis of seizure semiology and EEG features (hypsarrhythmia or modified hypsarrhythmia). The response of infantile spasms to treatment and subsequent epilepsy evolution was documented.
Neurodevelopment and seizure control at last follow-up visit in the Neurology clinic were recorded. Development was classified as normal, mildly abnormal, or moderately to severely abnormal according to the treating Pediatric Neurologist. The median follow-up period for control patients was 20 months (range 6 – 81 months). Patients discharged from the Neurology clinic at six months of life (n=4) had normal development and a normal neurological examination. The median follow-up for patients with infantile spasms was 33.5 months (range 13 – 68 months).
Magnetic Resonance Imaging Studies
MRI studies were performed with sedation administered by an anesthesiologist. The examinations were carried out on a Siemens 1.5 Tesla Avanto using VB 13A software and included the following sequences (time of relaxation/time of echo/averages/field of view/thickness/gap): axial and coronal volumetric T1-weighted spin echo images (800/20/1/230/4 mm/0.2 mm), axial fast spin echo T2-weighted images (4000/101/2/230/4 mm/0.5 mm), and diffusion-weighted images b = 1000 and 1500 s/mm2, with apparent diffusion coefficient maps (3300/82/4/210/4 mm/0.5 mm).
An experienced pediatric neuroradiologist (K.J.P.), blinded to the newborn's medical history, prospectively coded each MRI (T1/T2-weighted MRI and DWI) according to published criteria.3,4 The extent of injury was scored 0 to 4 in the lentiform nuclei and thalami bilaterally and 0 to 5 in the watershed regions bilaterally. The subjects were then classified according to their predominant pattern of injury: normal, watershed, basal ganglia/thalamus, total, and focal-multifocal injury. Of note, focal and multifocal injuries (stroke and/or white matter injury) were distinct in location from watershedpredominant injury.13
Two study investigators (a pediatric neuroradiologist [M.S.] and a senior pediatric neurology fellow [D.G.]) assessed each MRI (T1/T2-weighted MRI and DWI) for the extent of cortical injury, as well as abnormalities of the brainstem and hypothalamus. Scans were placed in a random order prior to assessment and investigators were blinded to the newborns' medical history, as well as the classification of the predominant pattern of injury. The extent of cortical injury was scored as 0 to 4 (0 = none, 1 = 1-25%, 2 = 26-50%, 3=51-75%, 4=76-100% of cortex involved). After determining there was an adequate view of the brainstem structures in each scan, the brainstem was classified as normal or abnormal. The extent of injury in the brainstem was scored 0 to 2 (0 = none, 1 = mild, 2 = moderate or severe) at the level of the midbrain, pons and medulla. The hypothalamus was classified as normal or abnormal. Reduced diffusion along the walls of the third ventricle in the region of the hypothalamus that was not contiguous with reduced diffusion in the adjacent thalami was classified as a hypothalamic abnormality.
Data Analysis
Statistical analysis was performed with Stata software version 11.1 (Stata Corporation, College Station, Texas). Clinical characteristics of newborns who developed infantile spasms and those who did not were compared using Fisher's exact test and the Kruskal-Wallis test. Fisher's exact test was used to assess the association of the predominant pattern of injury with the occurrence of infantile spasms. The odds ratio (OR) and 95% confidence interval (CI) were calculated to explore the relationship between individual anatomic areas of injury (extent of cortical injury, brainstem and hypothalamus) and the outcome of infantile spasms.
Results
Eight patients developed infantile spasms (hypsarrhythmia [n=5] or modified hypsarrhythmia [n=3]) after neonatal HIE among a cohort of 176 patients (4.5%). There were no significant differences in the antenatal, perinatal and neonatal courses between patients who developed infantile spasms and the 16 control cases (Table 1). Neonatal seizures occurred in six (75%) patients who developed infantile spasms and nine (56.3%) controls who did not.
Table 1. Clinical characteristics of infants that develop infantile spasms after neonatal HIE.
| Infantile Spasms | ||
|---|---|---|
| Present | Absent | |
| Number | 8 | 16 |
| Male sex | 3 (37.5%) | 10 (62.5%) |
| Gestational age (weeks) | 38.4 (37-39) | 39 (38.2-41) |
| Birthweight (g) | 3236 (2770-3572) | 3655 (3541-3780) |
| Head circumference (cm) | 34.5 (33.5-37) | 35.5 (33.75-36.5) |
| 5-minute Apgar score | 4 (1-6) | 6 (3-7) |
| Umbilical artery pH | 7.0 (6.8-7.4) | 7.02 (6.9-7.2) |
| Umbilical artery base excess (mmol/L) | 7 (6.9-12) | 7.6 (4.6-11.7) |
| Hypoglycemia | 1 (12.5%) | 4 (25%) |
| Therapeutic hypothermia | 2 (25%) | 2 (12.5%) |
| Neonatal seizures | 6 (75%) | 9 (56.3%) |
Data is presented as a median (interquartile range) or number (%).
There were no significant differences between the two groups.
Infantile spasms were diagnosed at a median age of 3.5 months (range 2 to 9 months). The initial therapy was vigabatrin in seven patients and nitrazepam in one patient who failed to tolerate vigabatrin. One patient responded to treatment with vigabatrin. Two patients responded to combination therapy of vigabatrin plus adrenocorticotropic hormone. In the remaining five cases, infantile spasms were refractory to multiple additional medications trialled singly or in various combinations. Adrenocorticotropic hormone was considered to be contraindicated in four patients due to frequent intercurrent illnesses.
Seven (87.5%) of eight patients with infantile spasms and three (18.8%) of the 16 control patients developed post-neonatal epilepsy consisting of partial seizures with secondary generalization (OR 30, 95% CI 2.1 – 1467, P = .001). The patient in whom spasms responded to vigabatrin monotherapy has been seizure-free for several years off of antiseizure medication. At last follow-up, seizure frequency ranged from multiple daily to weekly seizures in six patients with prior infantile spasms.
One patient with infantile spasms died secondary to respiratory complications at 13 months. All children with infantile spasms had moderate to severe developmental delay compared to 25% of controls (P < .0001). Seven patients with infantile spasms and two patients without infantile spasms were non-ambulatory (OR 49, 95% CI 2.9 – 2356, P = .0003). Six patients with infantile spasms and one patient without infantile spasms required a gastrostomy tube for feeding (OR 84, 95% CI 3.3 - 4116, P = .0002).
Pattern of Injury and Association with Infantile Spasms
Among the eight patients who developed infantile spasms after neonatal HIE, four had basal ganglia/thalamus predominant injury and four had total brain injury on MRI on the third day of life. Among newborns who did not develop infantile spasms, four had a normal MRI, three had watershed predominant injury, three had basal ganglia/thalamus predominant injury and six had F-MF injury. Basal ganglia/thalamus and total brain injury on the third day of life were significantly associated with the outcome of infantile spasms (P = .001).
Anatomic Areas of Injury and Association with Infantile Spasms
The extent of injury to the cortex, each level of the brainstem (midbrain, pons, medulla) and hypothalamus is summarized in Table 2 for the eight cases with infantile spasms. The neonatal MRI findings of a patient with infantile spasms are demonstrated in Figure 1.
Table 2. Brain injury in newborns with HIE that develop infantile spasms.
| Patient | Pattern | Cortical Extent | Midbrain | Pons | Medulla | Hypothalamus |
|---|---|---|---|---|---|---|
| 1 | BG/T | + | + | - | - | - |
| 2 | Total | ++++ | ++ | ++ | - | + |
| 3 | Total | +++ | - | - | - | - |
| 4 | BG/T | + | + | - | - | - |
| 5 | Total | ++++ | + | ++ | + | - |
| 6 | BG/T | +++ | + | - | - | + |
| 7 | Total | ++++ | ++ | - | ++ | - |
| 8 | BG/T | + | - | + | - | - |
BG/T = basal ganglia/thalamus
Extent of cortical injury:
indicates 1-25%,
26-50%,
51-75%,
76-100% of cortex involved
Levels of brainstem: - indicates absence of injury, + mild, ++ moderate to severe
Hypothalamus: - indicates absence of injury, + presence
Figure 1.
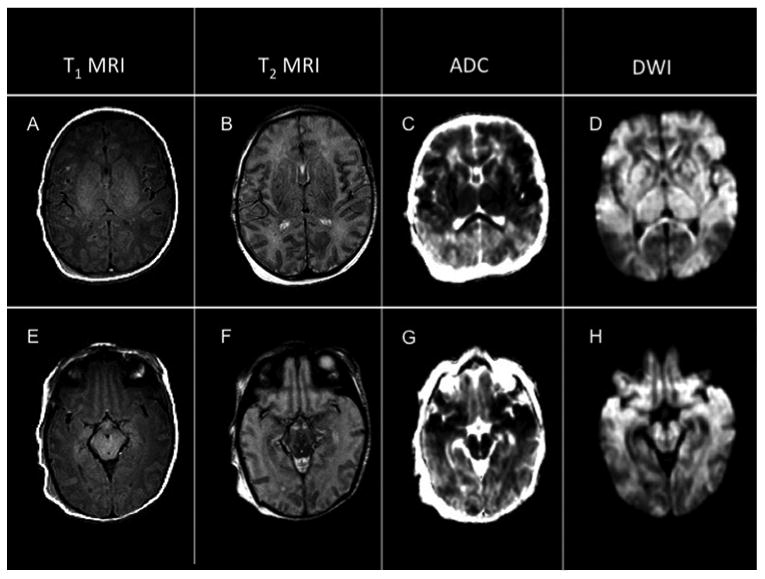
Neonatal MRI of a patient with infantile spasms
ADC=apparent diffusion coefficient map, DWI=diffusion-weighted imaging.
Neonatal MRI findings in a patient with total brain injury, including extensive injury to the cortex, subcortical white matter, basal ganglia and thalami (A-D) and injury to the midbrain (E-H).
Extent of cortical injury
Cortical injury was present in all newborns who developed infantile spasms and half of the control newborns. The extent of cortical injury was greater than 50% in five (62.5%) cases who developed infantile spasms and two (12.5%) controls (OR 11.7, 95% CI 1.1 -158.5, P = .01). Injury to the Rolandic cortex was present in all newborns who developed infantile spasms compared to five newborns (31.3%) who did not develop infantile spasms (P = .002).
Brainstem Injury
Injury to the brainstem at any level (midbrain, pons, medulla) was present in seven (87.5%) newborns with infantile spasms and four (25%) newborns without infantile spasms (OR 21, 95% CI 1.6 – 1022, P = .0004). Injury to the midbrain was present in six (75%) cases with infantile spasms and three (18.8%) controls (OR 13, 95% CI 1.3 to 172, P = .007). There was no significant association between injury to the pons or medulla and the development of infantile spasms.
Hypothalamic Injury
Hypothalamic injury was identified in two newborns who developed infantile spasms and none of the controls (P = .01).
Discussion
In this study, we describe the clinical and imaging features of term newborns with hypoxic-ischemic encephalopathy who developed infantile spasms. Among eight term newborns with HIE who developed infantile spasms, we found that four had the basal ganglia/thalamus pattern and four had total brain injury on MRI on the third day of life. The predominant pattern of injury was significantly associated with the development of infantile spasms. We also assessed brain regions that are not usually included in the standard scoring system for the predominant pattern of injury. More extensive cortical injury, injury to the midbrain and hypothalamic abnormalities were strongly associated with the development of infantile spasms. Additionally, infantile spasms were significantly associated with medically refractory epilepsy and moderate to severe developmental delay.
Neonatal HIE is one of the most common etiologies of infantile spasms.6 A shared mechanism of dysfunctional cortical-brainstem circuitry has been hypothesized to give rise to the characteristic seizure semiology and EEG pattern of this disorder.8 Subcortical disinhibition of the basal ganglia has been proposed to contribute to aberrant interactions between the cortex and brainstem in infantile spasms.9 In children with infantile spasms, hypermetabolism of the lenticular nuclei is the most consistent finding on positron emission tomography, regardless of the etiology.10 Among children in our cohort, bilateral basal ganglia injury was seen on neonatal MRI in all cases of infantile spasms. Our findings suggest that more extensive cortical injury and/or injury to the midbrain, in conjunction with basal ganglia injury, may contribute to the development of infantile spasms following neonatal HIE.
A distinct finding in two of our cases was the presence of reduced diffusion along the walls of the third ventricle in the region of the hypothalamus. This observation helps support a role for hypothalamic involvement in the pathogenesis of infantile spasms in some cases, which has previously been suggested by imaging studies of rodents with infantile spasms induced by N-methyl-D-aspartic acid,11 the corticotropin releasing hormone model,14 as well as the association of hypothalamic hamartoma with infantile spasms.15
The recognition of imaging features associated with infantile spasms after neonatal HIE may help identify patients at higher risk for the development of this severe epileptic encephalopathy. The United Kingdom Infantile Spasms Study has shown that increased lead time to treatment for infantile spasms was associated with decreased developmental scores at four years, independent of treatment allocation and etiology.16 Earlier diagnosis of infantile spasms after neonatal HIE and prompt initiation of therapy may improve developmental outcome. For the earlier diagnosis of infantile spasms in infants with a history of neonatal HIE, we suggest that future research should focus on the utility of serial EEGs, and more refined analysis of MRI findings that may indicate a higher risk for infantile spasms.
This study was limited to a small number of cases because infantile spasms are uncommon after neonatal HIE (4.5% in our cohort). In half of the infantile spasms cases in our cohort, spasms started at three months of age or younger. Our data suggest an earlier onset of infantile spasms among children with neonatal HIE compared to other etiologies. In addition, children in our cohort were more likely to have medically-refractory infantile spasms compared to other studies, 17,18 which is consistent with recent evidence that has shown that infantile spasms due to a bilateral or diffuse pathology have a worse treatment response rate.19
Control newborns with HIE who did not develop infantile spasms were randomly selected from a cohort that reflected the variability of term neonatal encephalopathy, but were not matched by severity of injury on MRI in order to evaluate this as a risk factor. Last, although dedicated imaging of the hypothalamus was not done on our patients, reduced diffusion in the region of the hypothalamus was identified and classified as a hypothalamic abnormality by two blinded study investigators.
In conclusion, we found that the development of infantile spasms after neonatal hypoxic-ischemic encephalopathy was strongly associated with injury to the basal ganglia and thalami on neonatal MRI, particularly when extensive cortical injury and/or injury to the midbrain was present. These data support the hypothesis of aberrant interactivity between the cortex and brainstem in the genesis of infantile spasms, potentially modulated by basal ganglia disinhibition.
Acknowledgments
This work was supported by the SickKids Foundation and Institute of Human Development, Child and Youth Health-Canadian Institutes of Health Research National Grants Program. SPM is supported by the Bloorview Children's Hospital Chair in Paediatric Neuroscience, with previous support from a Canada Research Chair (Tier 2) and Michael Smith Foundation for Health Research Scholar award. DG is supported by the University of British Columbia Clinician-Investigator Program. NIH/NINDS K23NS066137 and the Neonatal Brain Research Institute at UCSF support HCG.
Abbreviations
- ADC
Apparent diffusion coefficient
- CI
Confidence interval
- DWI
Diffusion-weighted imaging
- EEG
Electroencephalography
- HIE
Hypoxic-ischemic encephalopathy
- MRI
Magnetic resonance imaging
- OR
Odds ratio
Footnotes
Disclosure of Conflicts of Interest: The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Volpe JJ. Neurology of the newborn. 5th. Philadelphia: W.B. Saunders Company; 2008. [Google Scholar]
- 2.Shankaran S, Pappas A, McDonald SA, et al. Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med. 2012;366(22):2085–2092. doi: 10.1056/NEJMoa1112066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barkovich AJ, Hajnal BL, Vigneron D, et al. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR Am J Neuroradiol. 1998;19(1):161–166. [PMC free article] [PubMed] [Google Scholar]
- 4.Miller SP, Ramaswamy V, Michelson D, et al. Patterns of brain injury in term neonatal encephalopathy. J Pediatr. 2005;146:453–460. doi: 10.1016/j.jpeds.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 5.Glass HC, Hong KJ, Rogers EE, et al. Risk factors for epilepsy in children with neonatal encephalopathy. Pediatr Res. 2011;70(5):535–540. doi: 10.1203/PDR.0b013e31822f24c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osborne JP, Lux AL, Edwards SW, et al. The underlying etiology of infantile spasms (West syndrome): Information from the United Kingdom Infantile Spasms Study (UKISS) on contemporary causes and their classification. Epilepsia. 2010;51(10):2168–2174. doi: 10.1111/j.1528-1167.2010.02695.x. [DOI] [PubMed] [Google Scholar]
- 7.Gastaut H, Roger J, Soulayrol R, Pisnard N. L'encephalopathie myoclonique infantile avec hypsarythmie (syndrome de West) Paris: Masson; 1964. [DOI] [PubMed] [Google Scholar]
- 8.Pellock JM, Hrachovy R, Shinnar S, et al. Infantile spasms: a U.S. consensus report. Epilepsia. 2011;51(10):2175–2189. doi: 10.1111/j.1528-1167.2010.02657.x. [DOI] [PubMed] [Google Scholar]
- 9.Lado FA, Moshe SL. Role of subcortical structures in the pathogenesis of infantile spasms: what are possible subcortical mediators? Int Rev Neurobiol. 2002;49:115–140. doi: 10.1016/s0074-7742(02)49010-1. [DOI] [PubMed] [Google Scholar]
- 10.Chugani HT, Shewmon DA, Shankar R, Chen BC, Phelps ME. Infantile spasms: II. Lenticular nuclei and brainstem activation on positron emission tomography. Ann Neurol. 1992;31(2):212–219. doi: 10.1002/ana.410310212. [DOI] [PubMed] [Google Scholar]
- 11.Velisek L, Jehle K, Asche S, Velísková J. Model of infantile spasms induced by N-methyl-D-aspartic acid in prenatally impaired brain. Ann Neurol. 2007;61(2):109–119. doi: 10.1002/ana.21082. [DOI] [PubMed] [Google Scholar]
- 12.Cummings SR, Browner WS, Grady DG, Newman TB. Designing Clinical Research. 4th. Philadelphia: Lippincott Williams & Wilkins; 2013. [Google Scholar]
- 13.Chau V, Poskitt KJ, Sargent MA, et al. Comparison of computer tomography and magnetic resonance imaging scans on the third day of life in term newborns with neonatal encephalopathy. Pediatrics. 2009;123:319–326. doi: 10.1542/peds.2008-0283. [DOI] [PubMed] [Google Scholar]
- 14.Baram TZ, Schultz L. Corticotropin-releasing hormone is a rapid potent convulsant in the infant rat. Brain Res Dev Brain Res. 1991;61(1):97–101. doi: 10.1016/0165-3806(91)90118-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerrigan JF, Ng YT, Prenger E, Krishnamoorthy KS, Wang NC, Rekate HL. Hypothalamic harmartoma and infantile spasms. Epilepsia. 2007;48(1):89–95. doi: 10.1111/j.1528-1167.2006.00835.x. [DOI] [PubMed] [Google Scholar]
- 16.O'Callaghan FJK, Lux AL, Darke K, et al. The effect of lead time to treatment and of age of onset on developmental outcome at 4 years in infantile spasms: evidence from the United Kingdom Infantile Spasms Study. Epilepsia. 2011;52(7):1359–1364. doi: 10.1111/j.1528-1167.2011.03127.x. [DOI] [PubMed] [Google Scholar]
- 17.Lux AL, Edwards SW, Hancock E, et al. The United Kingdom Infantile Spasms Study (UKISS) comparing hormone treatment with vigabatrin on developmental and epilepsy outcomes to age 14 months: a multicentre randomized trial. Lancet Neurol. 2005;4(11):712–717. doi: 10.1016/S1474-4422(05)70199-X. [DOI] [PubMed] [Google Scholar]
- 18.Greiner HM, Lynch ER, Fordyce S, et al. Vigabatrin for childhood partial-onset epilepsies. Pediatr Neurol. 2012;46(2):83–88. doi: 10.1016/j.pediatrneurol.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 19.Vendrame M, Guilhoto LM, Loddenkemper T, Gregas M, Bourgeois BF, Kothare SV. Outcomes of epilepstic spasms in patients aged less than 3 years: singlecenter United States experience. Pediatr Neurol. 2012;46(5):276–280. doi: 10.1016/j.pediatrneurol.2012.02.022. [DOI] [PubMed] [Google Scholar]


