Abstract
Pseudoloma neurophilia (Microsporidia) is the most common pathogen detected in zebrafish (Danio rerio) from research facilities. The parasite infects the central nervous system and muscle and may be associated with emaciation and skeletal deformities. However, many fish exhibit sub-clinical infections. Another microsporidium, Pleistophora hyphessobryconis, has recently been detected in a few zebrafish facilities. Here, we review the methods for diagnosis and detection, modes of transmission, and approaches used to control microsporidia in zebrafish, focusing on P. neurophilia. The parasite can be readily transmitted by feeding spores or infected tissues, and we show that cohabitation with infected fish is also an effective means of transmission. Spores are released from live fish in various manners, including through the urine, feces, and sex products during spawning. Indeed, P. neurophilia infects both the eggs and ovarian tissues, where we found concentrations ranging from 12,000 to 88,000 spores per ovary. Hence, various lines of evidence support the conclusion that maternal transmission is a route of infection: spores are numerous in ovaries and developing follicles in infected females, spores are present in spawned eggs and water from spawning tanks based on polymerase chain reaction tests, and larvae are very susceptible to the infection. Furthermore, egg surface disinfectants presently used in zebrafish laboratories are ineffective against microsporidian spores. At this time, the most effective method for prevention of these parasites is avoidance.
Keywords: Danio rerio, Microsporidia, Pleistophora hyphessobryconis, Pseudoloma neurophilia, zebrafish
Introduction
The dramatic increase in the use of zebrafish (Danio rerio) in biomedical research has led to a corresponding increased interest in the diseases affecting this important biological model. Many of the laboratory animal health and pathogen control principles developed for mice and rats are applicable to aquatic laboratory animals such as the zebrafish; however, there are special considerations in working with aquatic animals. Kent and colleagues (2009) provided a general review of the control of diseases in fish research colonies. The present review focuses specifically on the transmission and control of microsporidia in zebrafish facilities. We emphasize particularly Pseudoloma neurophilia because this microsporidium is very common in zebrafish (Murray 2011), and we provide a discussion on Pleistophora hyphessobryconis, which was recently detected in a few facilities (Sanders et al. 2010).
Microsporidia
Microsporidia are obligate intracellular eukaryotic parasites with species infecting virtually all animal phyla. They have a relatively simple life cycle, consisting of two general developmental stages: merogony and sporogony. Meronts multiply inside the infected host cell, eventually forming sporonts and then spores, which are ultimately released from the host and transmit the infection. The infectious spore stage has a thick, chitinous endospore, making it extremely resistant to environmental stress and lysis, allowing the organism to maintain viability for extended periods in the aquatic environment (Shaw et al. 2000). Additionally, microsporidia are generally resistant to many standard forms of surface decontamination used for fish eggs, such as chlorine and iodophores, complicating the control of these pathogens.
Microsporidia are common pathogens of numerous aquatic organisms, including crustaceans and amphipods, and members from some 18 genera of these parasites have been described in fishes (Lom 2002; Lom and Nilsen 2003). The impacts of microsporidian infections on fish populations in the wild, in aquaculture, and in the laboratory have been documented in numerous cases (reviewed in Shaw and Kent 1999). These often focus on the more acute effects of microsporidian disease, such as mortality; however, most microsporidian species infecting aquatic animals result in chronic diseases with minimal associated host mortality (Murray et al. 2011).
Pseudoloma neurophilia
Pseudoloma neurophilia was first reported by de Kinkelin (1980) in fish purchased from a pet store for use in toxicological studies. The parasite was further described and assigned to a new genus, Pseudoloma neurophilia, by Matthews and colleagues (2001). Pseudoloma neurophilia is the most commonly observed microsporidian parasite of zebrafish; the infection was detected in >74% of the facilities examined through the Zebrafish International Resource Center diagnostic service in 2010 (Murray et al. 2011). It generally causes chronic infections in zebrafish, with clinical signs ranging from emaciation and obvious spinal deformities (lordosis, scoliosis) to subclinical infections exhibiting no outward signs of disease (Matthews et al. 2001). As with other animals used in research, experiments using zebrafish with these infections may be subject to nonexperimental variation, potentially confounding results, as has been described in laboratory colonies of rabbits and mice infected with the microsporidian parasite Encephalitozoon cuniculi (Baker 2003). Furthermore, infected fish without overt clinical disease have been shown to have reduced fecundity and size (Ramsay et al. 2009).
Pleistophora hyphessobryconis
The muscle-infecting microsporidium Pleistophora hyphessobryconis has also been observed and described in laboratory populations of zebrafish (Sanders et al. 2010). Commonly known as “neon tetra disease” for its type host, the neon tetra Paracheirodon innesi, this parasite is very common in the aquarium trade, often resulting in considerable mortality. This microsporidium has been described in a broad range of fish hosts and has been reported from many species of aquarium fishes in several families, including Danio rerio and Danio nigrofasciatus (Steffens 1962). Similar to P. neurophilia, P. hyphessobryconis can also be harbored by otherwise healthy-appearing fish, which may show clinical signs of the infection or mortality after experiencing experimental or incidental immunosuppression (Sanders et al. 2010). The presence of P. hyphessobryconis infections in laboratory zebrafish colonies highlights the importance of obtaining fish used in research from reputable sources and also illustrates the potential for introduction of otherwise novel microsporidia with a broad host range to new hosts.
Current Methods of Detection
External Indicators of Infection
External indications of P. neurophilia infection in zebrafish include reduced growth, emaciation, spinal deformation (e.g., lordosis, scoliosis), or low-level mortalities with no grossly visible lesions. Typically, indicators of infection and mortality become apparent only after a stress event (Ramsay et al. 2009), such as crowding or shipping. These general clinical presentations are not pathognomonic for P. neurophilia, making external examination of fish alone of little use in the diagnosis of this infection.
The skeletal muscle–infecting microsporidium P. hyphessobryconis can also be harbored by otherwise healthy-appearing fish. Similar to fish infected with P. neurophilia, immunosuppression by various means can result in acute infection, with affected fish displaying large, depigmented regions localized around the dorsal fin. Fish presenting severe signs of P. hyphessobryconis eventually die from the infection.
Microscopy
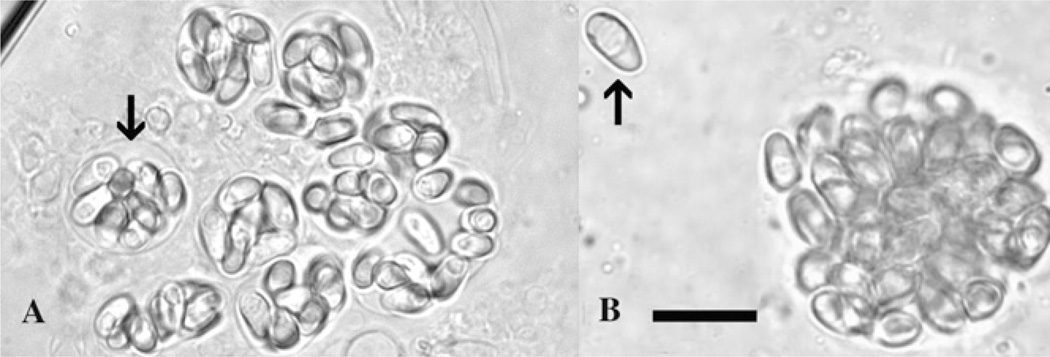
Microsporidian spores can often be seen in wet mount preparations from infected tissues. They are discernible by their generally refractile appearance and characteristic posterior vacuole. In suspected cases of infection by P. neurophilia, posterior brain and spinal cord tissue can be examined by wet mount for the presence of spores, which are approximately 3 × 5 µm in size and pyriform in shape (Figure 1A). Wet mount preparations of tissue from opaque lesions present in the skeletal muscle can be examined for the presence of P. hyphessobryconis spores, which are 4 × 6–7 µm in size, also pyriform in shape, and possess a very prominent posterior vacuole (Figure 1B).
Figure 1.
Wet mounts of microsporidian spores from zebrafish. (A) Aggregates of spores of Pseudoloma neurophilia contained within sporophorous vesicles (arrow). (B) Pleistophora hyphessobryconis from the skeletal muscle. Note prominent posterior vacuole in spores (arrow). Bar = 10 µm.
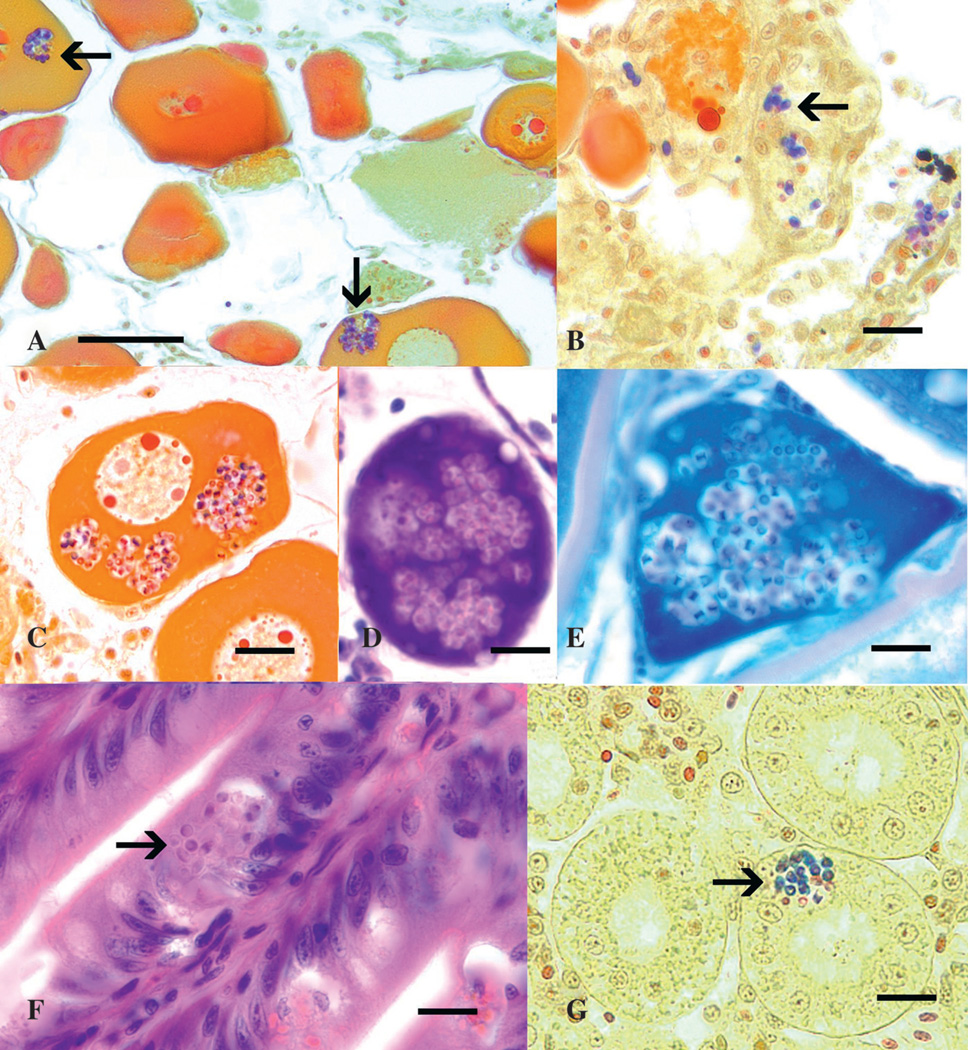
In general, microsporidian spores can be readily detected in standard hematoxylin and eosin–stained tissue sections when they occur in aggregates. However, in light infections, when only single spores are present in areas of inflammation, detection by hematoxylin and eosin is difficult. Microsporidian spores appear gram positive in Gram stains (Figure 2, A-C and G) and are generally acid fast in various acid-fast staining methods (Figure 2E). The acid-fast character of the spores can be variable depending upon the amount of decolorization. In cases where microsporidian infection is suspected, special stains, such as the Luna stain or periodic acid Schiff, can greatly increase the visibility of spores, allowing greater sensitivity of detection by histology (Peterson et al. 2011). Chitin-specific fluorescent stains such as Fungi-Fluor (Polysciences, Warrington, PA) also increase the sensitivity of spore detection by histology but require the use of a fluorescence microscope (Kent and Bishop-Stewart 2003).
Figure 2.
Histological sections of ovarian, intestinal, and kidney infections of Pseudoloma neurophilia from zebrafish. Bar = 10 µm unless otherwise indicated. (A) Gram-positive (blue) staining spores in follicles (arrows). Bar = 50 µm. (B) Gram-positive spores (arrows) in stroma of ovary. (C) Numerous gram-positive spores in developing follicle. (D) Developing follicle replete with spores. Hematoxylin and eosin stain. (E) Spores within a developing follicle. Kinyoun acid-fast stain. Note the faint acid-fast appearance of spores due to over-decolorization. (F) Spores (arrow) in intestinal epithelium. Hematoxylin and eosin stain. (G) Spores in renal tubule. Gram stain.
With P. neurophilia, large aggregates of spores are primarily found in the neural tissue of the posterior brain and spinal cord. Smaller groups or individual spores can also be seen in the kidney, skeletal muscle, gut epithelium, and ovary (Kent and Bishop-Stewart 2003) or within developing follicles (Figure 2). Spores of P. neurophilia released from aggregates in myocytes or peripheral nerves in the somatic muscle typically elicit a severe inflammatory reaction (Ramsay et al. 2009).
In contrast, the muscle is the primary site of infection for P. hyphessobryconis. Massive infection by proliferative stages and spores occupy the myocyte, with inflammatory changes occurring after infections become so severe that the myocytes rupture. Spores of this parasite can also be observed in the kidney, spleen, intestine, and ovaries in heavier infections (Sanders et al. 2010).
Molecular Diagnostics
Conventional polymerase chain reaction (PCR1) (Murray et al. 2011; Whipps and Kent 2006) and real-time PCR–based (Sanders and Kent 2011) assays targeting unique portions of the small subunit ribosomal DNA gene are available for testing of zebrafish tissues for P. neurophilia. The real-time PCR assay of Sanders and Kent, in combination with sonication, has also been applied to detect P. neurophilia small subunit ribosomal DNA in water, sperm, and eggs, providing a potential nonlethal assay for screening populations of fish for this parasite. As with most PCR-based assays, these tests are very sensitive and provide a relatively fast method of screening for the presence of P. neurophilia in zebrafish. No PCR-based assays currently exist for the specific detection of P. hyphessobryconis, but this is a potential target for future studies.
Transmission
In order to control the spread of a pathogen in a population, it is important to understand its mode or modes of transmission. In general, microsporidia infecting fish are transmitted directly, presumably per os by ingestion of infected tissues or spores present in the water (Dyková and Woo 1995; Shaw and Kent 1999). The two microsporidia thus far described in zebrafish, P. neurophilia and P. hyphessobryconis, have been shown to infect fish by this method by experimental exposure (Kent and Bishop-Stewart 2003; Sanders et al. 2010). Thus removal of dead and moribund fish would be expected to limit the potential exposure of tank mates to these two parasites.
Murray and colleagues (2011) reported spread of the parasite within a tank from <6% to 77% prevalence over 1 year. They also showed that detritus from positive tanks placed in tanks containing parasite-free fish could spread the infection. We have found that live, infected fish transmit P. neurophilia by shedding it in the water, infecting recipient fish held in the same water but separated from each other by a screen cage. Five flow-through cohabitation tanks were set up using infected “donor” fish, segregated within a suspended breeding cage with a screen bottom, and uninfected recipient zebrafish obtained from the P. neurophilia–specific pathogen-free colony housed at the Sinnhuber Aquatic Research Laboratory at Oregon State University (Kent et al. 2011). One control tank, which consisted of both recipient and donor fish from the negative fish stock, was set up. After 2 months of cohabitation, donor fish were removed, and posterior brains and spinal cords were examined by wet mount for the presence of P. neurophilia. The overall prevalence of P. neurophilia in the donor fish was 81%, with no spores detected in the 10 negative control donor fish. Histological examination of the donor fish revealed that three experimental tanks and the negative control tank contained both male and female donor fish, whereas a fourth experimental tank contained all males except for one immature female. After an additional 2 months, the recipient fish were killed and examined by histology to determine infection status. Recipient fish from all positive tanks were infected, with an overall incidence of 66%. No infection was detected in the negative controls. Tank 4, which contained no sexually mature female donor fish, showed a 57% incidence of infection.
These results provide evidence that P. neurophilia is shed by live, infected fish and illustrate the route by which the parasite can spread throughout a population of fish in a single tank. This finding is consistent with other reports that Loma salmonae, a microsporidian parasite of salmonids, is similarly transmitted to tank mates by cohabitation (Ramsey et al. 2003; Shaw et al. 1998). The potential routes by which P. neurophilia may be transmitted by live, infected fish become apparent by observing the tissue distribution of the parasite. Although P. neurophilia primarily targets neural and muscle tissue, we occasionally observe spores in the gut epithelium (Figure 2F) and the kidney tubules (Figure 2G), each of these tissues providing a portal through which infectious spores can be shed into the water through feces or urine. Additionally, Kent and Bishop-Stewart (2003) reported the frequent occurrence of spores in the ovarian stroma (Figure 2B), and since that report, we have also detected spores within developing follicles (Figure 2, A and C-E), supporting maternal transmission during spawning as another likely route of infection.
It is difficult to quantify microsporidian spores in histological sections, and thus entire ovaries from females from nine separate infected populations were surveyed to more precisely determine the concentration of P. neurophilia (unpublished observations). Ovaries of 10 fish from each population were pooled and homogenized, and a sample of spores was counted by hemocytometer. The average number of P. neurophilia spores seen was 44,000 per fish (range, 12,000–88,000). Zebrafish frequently spawn spontaneously in aquaria, and hence release of eggs, ovarian fluids, and tissues at spawning provides an important potential route of horizontal transmission. However, the fact that recipient fish were positive from the tank in which donor fish had no sexually mature females suggests that spores are also released from infected fish by routes other than spawning. Observation of spores in the renal tubules and the intestinal epithelium (Figure 2, F and G) supports this hypothesis.
Sex products not only provide an important source of infection to tank mates of the same age cohort but also a source of infection to progeny by maternal transmission. Indeed, this route of infection has been reported for other microsporidia of fishes. The potential for maternal transmission, either transovum or transovarial, has been reported for L. salmonae (Docker et al. 1997) and Ovipleistophora ovariae (Phelps and Goodwin 2008). Phelps and Goodwin (2008) provided the most conclusive evidence for vertical transmission of fish microsporidia, showing the presence of the DNA from O. ovariae within spawned eggs of the golden shiner Notropis chrysoleucas by real-time PCR. Further evidence for the maternal transmission of P. neurophilia was observed in the experiment described by Sanders and Kent (2011), where parasite DNA was detected in the eggs and water from a group spawn of infected zebrafish. We have tested the spawn water and eggs of several other groups of fish and consistently found PCR-positive water and eggs (unpublished observations).
There are other experimental and observational lines of evidence that suggest maternal transmission of P. neurophilia, either transovarial (pseudovertical, outside of the egg or sperm) or transova (true vertical, within the egg or sperm). Evidence of true vertical transmission of P. neurophilia was observed in a follow-up experiment performed from a laboratory study described by Ramsey and colleagues (2009). Six-week-old zebrafish (AB strain) obtained from the Zebrafish International Resource Center were experimentally exposed to P. neurophilia spores at 10,000 spores per fish. At 8 weeks postexposure, six pairs of fish were separately spawned, and the embryos were reared in individual covered beakers in sterile water. Three pairs of unexposed fish were spawned separately as a negative control, with the progeny reared under identical conditions. After spawning, all adult fish were processed for histology, and slides were stained using the Kinyoun acid-fast method to determine infection status and tissue distribution of the parasite (Ramsay et al. 2009). At 8 weeks posthatch, juvenile fish were euthanized and the viscera removed; the remaining tissues (spinal cord, somatic muscle, head) were placed in pools of five fish, and DNA was extracted for PCR analysis using the method of Whipps and Kent (2006). Pseudoloma neurophilia was detected in two of three pools of fry from one spawning pair. Histological analysis of the adult pairs showed the presence of microsporidian spores in the spinal cord, ovary, and, most important, in developing follicles of the spawning female (Figure 2E). Because these fry were raised in isolation from the original spawning pair and the parasite was seen developing in eggs from the female, there is evidence that the infection was transmitted vertically, either by infection of the eggs prior to fertilization or by the exposure of the larval fish to spores present in high numbers in eggs that did not develop further. However, because P. neurophilia spores were also observed in the ovarian stroma, transovarial transmission (i.e., by spores outside of eggs) cannot be excluded.
There is limited evidence of the potential for maternal transmission of P. hyphessobryconis. Schäperclaus (1941) found infections in 8-day-old neon tetras, which had been derived from infected parents, suggesting the possibility of maternal transmission. We observed spores of this microsporidium in the ovarian tissue of infected females (Sanders et al. 2010), but no spores were seen in developing follicles in this study. The low prevalence of this parasite in laboratory zebrafish colonies would seem to minimize the importance of this mode of transmission for P. hyphessobryconis.
Parasite Surveillance
Routine Monitoring
Routine disease and pathogen monitoring is important not only for the control of microsporidian parasites but also for the detection of other pathogens and the monitoring of the overall health of the colony (Kent et al. 2009). It is only through routine monitoring of healthy and moribund fish that colony managers can detect potential health problems in fish. No serological tests are presently available for zebrafish. Histological analysis is the best overall method for routine health monitoring of zebrafish because of the ability to assess all tissues and to detect novel pathogens, which would not be detected by specific PCR-based assays. Screening of fish in specific tanks by PCR to determine the presence or prevalence of P. neurophilia is also recommended; however, careful consideration of sample size is required to ensure the statistical relevance of these data (Kent et al. 2009, 2011).
Sentinel Program
The use of a sentinel program is a very effective means to monitor microsporidian infections in laboratory colonies. Exposing a population of known uninfected fish to the untreated effluent from other tanks on the system allows facility managers to assess the infection status of fish in the system on a large scale. For the monitoring of chronic microsporidian infections such as P. neurophilia, it is recommended that sentinel fish be held at least 3 months prior to sampling (Kent et al. 2009). The presence of P. neurophilia or other microsporidian parasites in the sentinel fish is an indication that infected fish are present somewhere in the facility. Ultraviolet (UV1) sterilization is a common feature in recirculating water systems. It is useful to hold a sentinel population exposed to effluent after UV treatment in order to assess the efficacy of the filtration and disinfection of effluent water.
Facility Design Considerations
Receiving Fish into the Facility and/or Quarantine
The practice of “eggs only” movement of fish between facilities has been successfully used for years in salmonid aquaculture to exclude pathogens from salmon facilities (Kent and Kieser 2003). It is recommended that fish received in a facility as embryos be held in quarantine and a subset examined before introduction into the main facility. Also, if possible, the parents of these fish should be examined for pathogens that may be maternally transmitted (e.g., P. neurophilia). It is recommended that the quarantine area be physically separated from the main housing area, with restrictions on staff entering the main facility from the quarantine area. After determining that the broodstock is not infected with a microsporidian parasite, the progeny may be moved into the main facility. The short generation time of zebrafish facilitates this process greatly, allowing managers to bring adults into quarantine, spawn them, and then move only the progeny of those adults that are screened and determined to be microsporidian free into the main facility. This approach was used to establish a specific pathogen–free (SPF1) laboratory for P. neurophilia zebrafish at Oregon State University (Kent et al. 2011). Now two wild-type lines of these fish are available to the research community through the Sinnhuber Aquatic Research Laboratory at Oregon State University.2
Separation of Tanks in the Main Facility
The separation of tanks in the main facility is very important in the control of microsporidia. Because microsporidian spores are transmitted by water and horizontally by infected fish, splashes and mixing of fish in tanks may result in the spread of these parasites throughout the facility. In fact, we have observed the spread of P. neurophilia from a single tank of infected fish to other fish in separate tanks housed in the same unit in which the effluent water was discharged into an open tray and frequently splashed (unpublished observations). We have also seen P. hyphessobryconis transmitted in a similar way to fish housed on the same rack as infected fish (Sanders et al. 2010). The transmission of another aquatic parasite, Ichthyophthirius multifiliis, between tanks via aerosolization of water in a laboratory has also been demonstrated (Wooster et al. 2001). Thus, covering tanks and minimizing splashing of effluent are key to controlling the spread of microsporidiosis, as is the isolation of tanks with known infected fish from those that are microsporidian free or of unknown infection status.
UV Sterilization of Water in Recirculating Systems
UV sterilization of municipal drinking water has been used for several years to inactivate protozoan pathogens such as Cryptosporidium and Giardia. UV sterilization at a dose of 6 mJ/cm2 has also been shown to be effective in the inactivation of microsporidian parasites of human health concern, such as Encephalitozoon intestinalis (Huffman et al. 2002). The effectiveness of UV sterilization is highly dependent on the proper prefiltration of incoming water to remove particulates, the cleaning of the quartz sheath that the UV bulb is inserted into, and the replacement of UV bulbs at regular intervals. As stated previously, it is important to maintain a group of sentinel fish downstream of the UV treatment in order to assess its efficacy.
Husbandry Considerations
Egg Disinfection
The purpose of egg disinfection is to kill pathogens that are present on the surface of the eggs, preventing their spread to progeny and potentially other fish in the facility. This method has been successful in the control of many pathogens in salmon aquaculture (Kent and Kieser 2003). For zebrafish eggs, bath treatment with 25 to 50 ppm sodium hyphochlorite for 10 minutes is generally the method recommended for disinfection (Harper and Lawrence 2010). Unfortunately, this level of bleach is ineffective at killing P. neurophilia (Ferguson et al. 2007). A similar situation can be seen with the disinfection procedures for salmonid eggs in which the iodine treatments used were shown to be ineffective at eliminating 100% of spores of L. salmonae, even at very high levels of iodine (Shaw et al. 1999). Microsporidian spores are highly resistant to current methods of surface sterilization of eggs, and these methods cannot be relied upon to eliminate P. neurophilia or other microsporidia from a population, nor can current methods of surface sterilization be relied upon to effectively prevent the spread of microsporidian parasites between fish colonies. Further compounding this problem is the potential for transmission of the parasite within eggs. Transovum (true vertical transmission) of this parasite would prevent the efficacy of any surface decontamination of eggs for P. neurophilia, thus requiring careful screening of fish and the use of SPF fish stocks to prevent the spread or introduction of the parasite.
Screening of Sperm, Eggs, and Larval Fish
Current molecular diagnostic methods can easily be applied to the testing of eggs, sperm, and larval fish. In fact, the method of Whipps and Kent (2008) was used to screen eggs and larval fish in the development of a P. neurophilia SPF zebrafish colony at Oregon State University (Kent et al. 2011). The real-time PCR method of Sanders and Kent (2011) was shown to be effective in testing sperm and eggs with a sensitivity of 10 spores per microliter and 2 spores per egg, respectively. The cryopreservation of zebrafish sperm presents a special problem for preventing the spread of microsporidians. Although P. neurophilia has not been seen in the testes of fish (Murray et al. 2011), there is the potential for contamination of sperm from the kidneys or gut of the fish during manual stripping. Further compounding this problem is the potential for survival of the parasite during cryopreservation. Although the ability of P. neurophilia to survive during cryopreservation is unknown, Nucleospora salmonis, a microsporidian parasite infecting salmonids, is maintained for long periods by cryopreservation in tissue culture (Wongtavatchai et al. 1994). Also, cryopreserved spores of mammalian microsporidia, which are viable, are readily available from the American Type Culture Collection (Manassas, VA).
Disinfection of Equipment
The resistance of infectious microsporidian spores to environmental conditions requires the use of appropriate disinfection procedures to control the spread of these pathogens. Chlorine is commonly used to disinfect tanks and other equipment in zebrafish facilities. Ferguson and colleagues (2007) found that 100 ppm chlorine (pH 7) effectively kills >95% of P. neurophilia spores. Unfortunately, this is lethal for embryos, and this is not suitable for egg disinfection. We are not aware of any studies that specifically test the efficacy of chlorine on P. hyphessobryconis, but it is likely that it would be killed at similar concentrations.
Other Considerations
Several zebrafish lines that are SPF for Pseudoloma neurophilia have been developed at the colony housed at the Sinnhuber Aquatic Research Laboratory (Kent et al. 2011). The development of these SPF lines was facilitated by the construction of a new fish facility, which enabled the introduction of fish only after they were determined to be free of P. neurophilia. These fish are rigorously screened in order to maintain their SPF status. Obviously, the control of this parasite in existing facilities is much more complex and requires systematic screening and isolation of zebrafish with known infections in order to eliminate or reduce the presence of P. neurophilia infections in the colony (Murray et al. 2011).
There are currently no known treatments for microsporidiosis in zebrafish. However, Fumigillin DCH, an agent used to treat the microsporidium Nosema apis in honey bees, has been shown to be effective for several microsporidia infecting fishes (Shaw and Kent 1999). Albendazole and monensin also have some efficacy in the treatment of salmonids for infections by L. salmonae (Speare et al. 1999, 2000). The use of these drugs on experimental fish, although potentially eliminating the pathogen, could also introduce other changes in the host, confounding research (Baker 2003). Toxic effects of Fumigillin DCH have been observed in salmonids (Laurén et al. 1989); thus its utility would be limited to the treatment of fish not used as experimental animals (e.g., broodstock). Ultimately, the elimination of P. neurophilia from existing lines of zebrafish may require rederivation of those lines using the methods described by Kent and colleagues (2011).
Going Forward
The chronic and often subclinical nature of P. neurophilia infections in zebrafish requires the use of rigorous screening methodologies in order to ascertain the true prevalence of this parasite in laboratory zebrafish colonies. Its continued presence in laboratory zebrafish facilities highlights the need for increased surveillance, implementation of biosecurity protocols, and further research into the transmission and control of these pathogens. Future studies to determine the efficacy of decontamination protocols, such as the dosage of UV required to inactivate spores of P. neurophilia in water and the survivability of the parasite during cryopreservation, are needed. Additionally, the potential for introduction of novel microsporidia to zebrafish facilities underscores the need to obtain fish from reputable suppliers who are able to provide a health history of the fish. We also strongly recommend that zebrafish be obtained from suppliers who do not maintain zebrafish with other aquarium fish species. Because the treatment of zebrafish with antimicrosporidial drugs may exacerbate impacts on research outcomes, the only effective method of controlling P. neurophilia infections in zebrafish is identification and removal of infected fish and avoidance of introduction of the parasite by proper quarantine and screening of incoming fish.
Whereas both methods to avoid the infection and SPF zebrafish are now available, we have seen little enthusiasm for using parasite-free zebrafish by some researchers. This is often due to the perception that subclinical infections have little or no impact on research endpoints (see Kent et al. 2011). Therefore, another research need is the demonstration of the specific physiologic, immunologic, molecular, and behavior changes associated with subclinical infections by this extremely common parasite of zebrafish.
Acknowledgments
This study was supported by grants from the National Institutes of Health (NIH NCRR 5R24RR017386-02 and NIH NCRR P40 RR12546-03S1). We would like to thank C. Kent for assistance reviewing this manuscript.
Footnotes
Abbreviations that appear ≥3x throughout this article: PCR, polymerase chain reaction; SPF, specific pathogen free; UV, ultraviolet
Fish may be ordered through the website: http://ehsc.science.oregonstate.edu/orderzebrafish.
Contributor Information
Justin L. Sanders, Department of Microbiology, all at Oregon State University, Corvallis..
Virginia Watral, Kent Laboratory, all at Oregon State University, Corvallis..
Michael L. Kent, Departments of Microbiology and Biomedical Sciences, all at Oregon State University, Corvallis..
References
- Baker DG. Natural Pathogens of Laboratory Animals: Their Effects on Research. Washington: ASM Press; 2003. [Google Scholar]
- de Kinkelin P. Occurrence of a microsporidian infection in zebra Danio, Brachydanio rerio (Hamilton-Buchanan) J Fish Dis. 1980;3:71–73. [Google Scholar]
- Docker MF, Devlin RH, Richard J, Khattra J, Kent ML. Sensitive and specific polymerase chain reaction assay for detection of Loma salmonae (Microsporea) Dis Aquat Org. 1997;29:41–48. [Google Scholar]
- Dyková I. Phylum Microspora. In: Woo PTK, editor. Fish Diseases and Disorders. Volume 1: Protozoan and Metazoan Infections. Cambridge MA: CABI; 1995. pp. 149–179. [Google Scholar]
- Ferguson JA, Watral V, Schwindt AR, Kent ML. Spores of two fish microsporidia (Pseudoloma neurophilia and Glugea anomala) are highly resistant to chlorine. Dis Aquat Org. 2007;76:205. doi: 10.3354/dao076205. [DOI] [PubMed] [Google Scholar]
- Harper C, Lawrence C. The Laboratory Zebrafish. Boca Raton FL: CRC Press; 2010. [Google Scholar]
- Huffman DE, Gennaccaro A, Rose JB, Dussert BW. Low- and medium-pressure UV inactivation of microsporidia Encephalitozoon intestinalis. Water Res. 2002;36:3161–3164. doi: 10.1016/s0043-1354(01)00528-0. [DOI] [PubMed] [Google Scholar]
- Kent ML, Bishop-Stewart JK. Transmission and tissue distribution of Pseudoloma neurophilia (Microsporidia) of zebrafish, Danio rerio (Hamilton) J Fish Dis. 2003;26:423–426. doi: 10.1046/j.1365-2761.2003.00467.x. [DOI] [PubMed] [Google Scholar]
- Kent ML, Buchner C, Watral VG, Sanders JL, LaDu J, Peterson TS, Tanguay RL. Development and maintenance of a specific pathogen free (SPF) zebrafish research facility for Pseudoloma neurophilia. Dis Aquat Org. 2011;95:73–79. doi: 10.3354/dao02333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent ML, Feist SW, Harper C, Hoogstraten-Miller S, Law JM, Sánchez-Morgado JM, Tanguay RL, Sanders GE, Spitsbergen JM, Whipps CM. Recommendations for control of pathogens and infectious diseases in fish research facilities. Comp Biochem Physiol C Toxicol Pharmacol. 2009;149:240–248. doi: 10.1016/j.cbpc.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent ML, Kieser D. Avoiding the introduction of exotic pathogens with Atlantic salmon, Salmo salar, reared in British Columbia. In: Cheng-Sheng L, O’Bryen PJ, editors. Biosecurity in Aquaculture Production Systems: Exclusion of Pathogens and Other Undesirables. Baton Rouge LA: World Aquaculture Society; 2003. pp. 43–50. [Google Scholar]
- Laurén DJ, Wishkovsky A, Groff JM, Hedrick RP, Hinton DE. Toxicity and pharmacokinetics of the antibiotic fumagillin in yearling rainbow trout (Salmo gairdneri) Toxicol Appl Pharmacol. 1989;98:444–453. doi: 10.1016/0041-008x(89)90173-7. [DOI] [PubMed] [Google Scholar]
- Lom J. A catalogue of described genera and species of microsporidians parasitic in fish. Syst Parasitol. 2002;53:81–99. doi: 10.1023/a:1020422209539. [DOI] [PubMed] [Google Scholar]
- Lom J, Nilsen F. Fish microsporidia: Fine structural diversity and phylogeny. Internat J Parasitol. 2003;33:107–127. doi: 10.1016/s0020-7519(02)00252-7. [DOI] [PubMed] [Google Scholar]
- Matthews JL, Brown AMV, Larison K, Bishop-Stewart JK, Rogers P, Kent ML. Pseudoloma neurophilia ng, n. sp., a new microsporidium from the central nervous system of the zebrafish (Danio rerio) J Eukaryot Microbiol. 2001;48:227–233. doi: 10.1111/j.1550-7408.2001.tb00307.x. [DOI] [PubMed] [Google Scholar]
- Murray KN, Dreska M, Nasiadka A, Rinne M, Matthews JL, Carmichael C, Bauer J, Varga ZM, Westerfield M. Transmission, diagnosis, and recommendations for control of Pseudoloma neurophilia infections in laboratory zebrafish (Danio rerio) facilities. Comp Med. 2011;61:1–8. [PMC free article] [PubMed] [Google Scholar]
- Peterson TS, Spitsbergen JM, Feist SW, Kent ML. Luna stain, an improved selective stain for detection of microsporidian spores in histologic sections. Dis Aquat Org. 2011;95:175–180. doi: 10.3354/dao02346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay JM, Watral V, Schreck CB, Kent ML. Pseudoloma neurophilia infections in zebrafish Danio rerio: Effects of stress on survival, growth, and reproduction. Dis Aquat Org. 2009;88:69–84. doi: 10.3354/dao02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders JL, Kent ML. Development of a sensitive assay for the detection and quantification of Pseudoloma neurophilia in laboratory populations of the zebrafish Danio rerio. Dis Aquat Org. 2011 doi: 10.3354/dao02375. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders JL, Lawrence C, Nichols DK, Brubaker JF, Peterson TS, Murray KN, Kent ML. Pleistophora hyphessobryconis (Microsporidia) infecting zebrafish Danio rerio in research facilities. Dis Aquat Org. 2010;91:47–56. doi: 10.3354/dao02245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäperclaus W. Eine neue Mikrosporidien-Krankheit beim Neonfisch und seinen Verwandten. Wochenschrift für Aquarienund Terrarienkunde. 1941;39/40:381–384. [Google Scholar]
- Shaw RW, Kent ML. Fish Microsporidia. In: Wittner M, Weiss L, editors. The Microsporidia and Microsporidiosis. Washington: ASM Press; 1999. pp. 418–444. [Google Scholar]
- Shaw RW, Kent ML, Adamson ML. Iodophor treatment is not completely efficacious in preventing Loma salmonae (Microsporidia) transmission in experimentally challenged chinook salmon, Oncorhynchus tshawytscha (Walbaum) J Fish Dis. 1999;22:311–312. [Google Scholar]
- Shaw RW, Kent ML, Adamson ML. Viability of Loma salmonae (Microsporidia) under laboratory conditions. Parasitol Res. 2000;86:978–981. doi: 10.1007/pl00008529. [DOI] [PubMed] [Google Scholar]
- Speare DJ, Athanassopoulou F, Daley J, Sanchez JG. A preliminary investigation of alternatives to fumagillin for the treatment of Loma salmonae infection in rainbow trout. J Comp Pathol. 1999;121:241–248. doi: 10.1053/jcpa.1999.0325. [DOI] [PubMed] [Google Scholar]
- Speare DJ, Daley J, Dick P, Novilla M, Poe S. Ionophore mediated inhibition of xenoma expression in trout challenged with Loma salmonae (Microspora) J Fish Dis. 2000;23:231–233. [Google Scholar]
- Steffens W. Der heutige Stand der Verbreitung von Plistophora hyphessobryconis Schäperclaus 1941 (Sporozoa, Microsporidia) Parasitol Res. 1962;21:535–541. doi: 10.1007/BF00260258. [DOI] [PubMed] [Google Scholar]
- Whipps CM, Kent ML. Polymerase chain reaction detection of Pseudoloma neurophilia, a common microsporidian of zebrafish (Danio rerio) reared in research laboratories. JAALAS. 2006;45:36–39. [PMC free article] [PubMed] [Google Scholar]
- Wongtavatchai J, Conrad PA, Hedrick RP. In vitro cultivation of the microsporidian: Enterocytozoon salmonis using a newly developed medium for salmonid lymphocytes. J Tissue Cult Methods. 1994;16:125–131. [Google Scholar]
- Wooster GA, Bishop TM, Bowser PR. Proceedings from the 26th Eastern Fish Health Workshop. Leetown: WV; 2001. The aerobiological dissemination of the fish parasite Ichthyophthirius multifiliis. [Google Scholar]