Abstract
The mouse mammary gland in whole-organ culture, an in vitro system that is capable of alveolar development, differentiation, involution, and oncogenic transformation, has been used to examine the effects of the retinoid 2-retinylidene-5,5-dimethyl-1,3-cyclohexanedione (retinylidene dimedone) on epithelial transformation by a low concentration (10 nM) of the carcinogen 7,12-dimethylbenz[a]anthracene. The retinoid significantly prevented mammary gland transformation only when administered after the carcinogen. In addition, the phenotypes of the early transformed state of the mammary gland were suppressed for 20 days after removal of the retinoid. The retinoid was effective during both mammary alveolar development and regression in culture at concentrations as low as 1 nM, and itself had no significant transforming or cytotoxic activity. Mammary glands treated with both the carcinogen and the retinoid resembled, at the microscopic level, those given the solvent (dimethyl sulfoxide) only. The mouse mammary gland in whole-organ culture provides a promising model system in which to study the actions by which retinoids prevent and suppress the chemical transformation in vitro and oncogenesis in vivo of epithelial cells in general and of mammary gland in particular. The present findings support the suggestion that a search for suitable retinoids as chemopreventive agents against human breast cancer is warranted. This model system may be useful as initial indicator in that search.
Full text
PDF
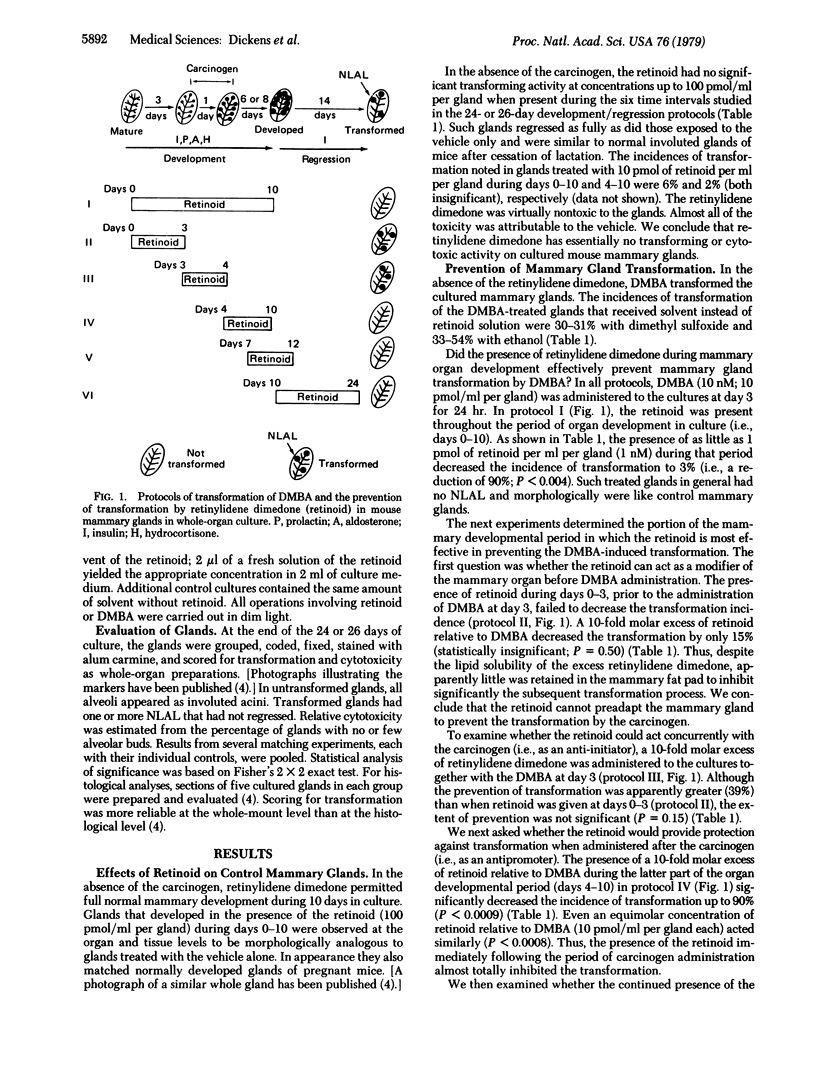
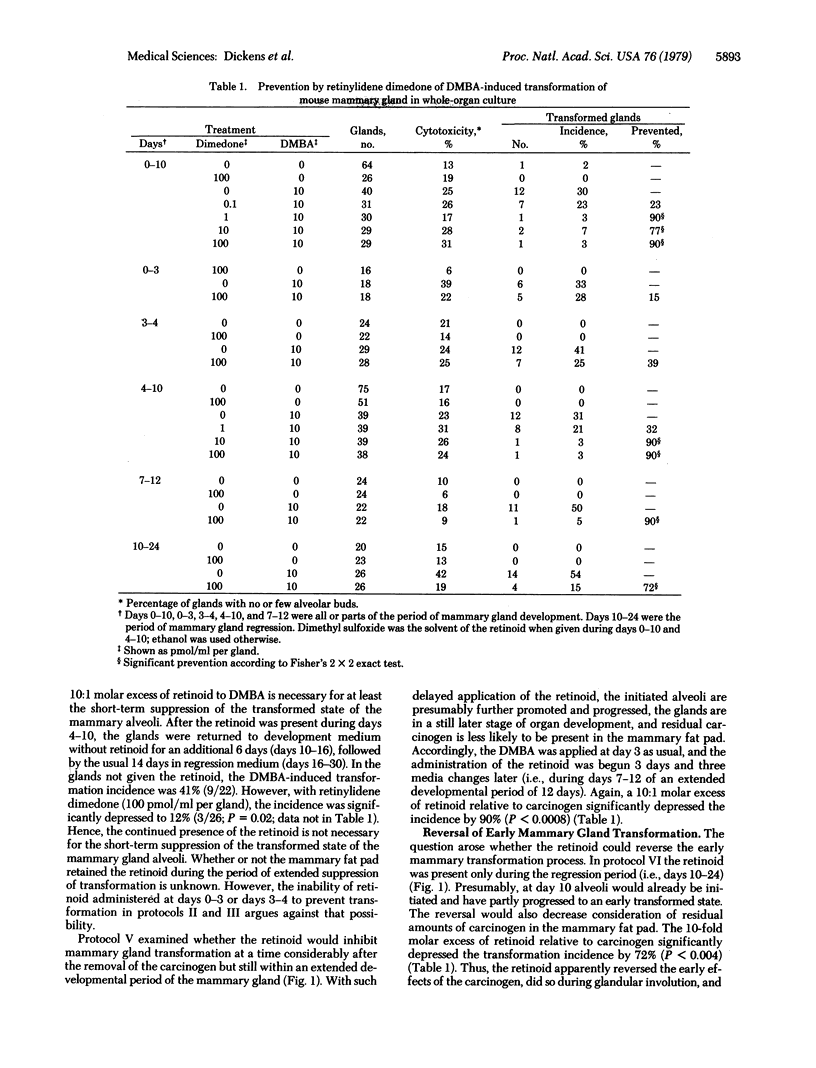


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee M. R., Wood B. G., Washburn L. L. Chemical carcinogen-induced alveolar nodules in organ culture of mouse mammary gland. J Natl Cancer Inst. 1974 Nov;53(5):1387–1393. doi: 10.1093/jnci/53.5.1387. [DOI] [PubMed] [Google Scholar]
- Bollag W. Prophylaxis of chemically induced benign and malignant epithelial tumors by vitamin A acid (retinoic acid). Eur J Cancer. 1972 Dec;8(6):689–693. doi: 10.1016/0014-2964(72)90153-3. [DOI] [PubMed] [Google Scholar]
- Chopra D. P., Wilkoff L. J. Inhibition and reversal by beta-retinoic acid of hyperplasia induced in cultured mouse prostate tissue by 3-methylcholanthrene or N-methyl-N'-nitro-N-nitrosoguanidine. J Natl Cancer Inst. 1976 Mar;56(3):583–589. doi: 10.1093/jnci/56.3.583. [DOI] [PubMed] [Google Scholar]
- Chu E. W., Malmgren R. A. An inhibitory effect of vitamin A on the induction of tumors of forestomach and cervix in the Syrian hamster by carcinogenic polycyclic hydrocarbons. Cancer Res. 1965 Jul;25(6):884–895. [PubMed] [Google Scholar]
- Cone M. V., Nettesheim P. Effects of vitamin A on 3-methylcholanthrene-induced squamous metaplasias and early tumors in the respiratory tract of rats. J Natl Cancer Inst. 1973 Jun;50(6):1599–1606. doi: 10.1093/jnci/50.6.1599. [DOI] [PubMed] [Google Scholar]
- Crocker T. T., Sanders L. L. Influence of vitamin A and 3,7-dimethyl-2,6-octadienal (citral) on the effect of benzo(a)pyrene on hamster trachea in organ culture. Cancer Res. 1970 May;30(5):1312–1318. [PubMed] [Google Scholar]
- Harisiadis L., Miller R. C., Hall E. J., Borek C. A vitamin A analogue inhibits radiation-induced oncogenic transformation. Nature. 1978 Aug 3;274(5670):486–487. doi: 10.1038/274486a0. [DOI] [PubMed] [Google Scholar]
- Ichinose R. R., Nandi S. Influence of hormones on lobulo-alveolar differentiation of mouse mammary glands in vitro. J Endocrinol. 1966 Aug;35(4):331–340. doi: 10.1677/joe.0.0350331. [DOI] [PubMed] [Google Scholar]
- Kundu A. B., Telang N. T., Banerjee M. R. Binding of 7,12-dimethylbenz[a]anthracene to BALB/c mouse mammary gland DNA in organ culture. J Natl Cancer Inst. 1978 Aug;61(2):465–469. [PubMed] [Google Scholar]
- Lasnitzki I. Reversal of methylcholanthrene-induced changes in mouse prostates in vitro by retinoic acid and its analogues. Br J Cancer. 1976 Sep;34(3):239–248. doi: 10.1038/bjc.1976.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchok A. C., Cone V., Nettesheim P. Induction of squamous metaplasia (vitamin A deficiency) and hypersecretory activity in tracheal organ cultures. Lab Invest. 1975 Oct;33(4):451–460. [PubMed] [Google Scholar]
- Merriman R. L., Bertram J. S. Reversible inhibition by retinoids of 3-methylcholanthrene-induced neoplastic transformation in C3H/10T1/2 clone 8 cells. Cancer Res. 1979 May;39(5):1661–1666. [PubMed] [Google Scholar]
- Moon R. C., Grubbs C. J., Sporn M. B. Inhibition of 7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis by retinyl acetate. Cancer Res. 1976 Jul;36(7 Pt 2):2626–2630. [PubMed] [Google Scholar]
- Moon R. C., Thompson H. J., Becci P. J., Grubbs C. J., Gander R. J., Newton D. L., Smith J. M., Phillips S. L., Henderson W. R., Mullen L. T. N-(4-Hydroxyphenyl)retinamide, a new retinoid for prevention of breast cancer in the rat. Cancer Res. 1979 Apr;39(4):1339–1346. [PubMed] [Google Scholar]
- Newberne P. M., Suphakarn V. Preventive role of vitamin A in colon carcinogenesis in rats. Cancer. 1977 Nov;40(5 Suppl):2553–2556. doi: 10.1002/1097-0142(197711)40:5+<2553::aid-cncr2820400924>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Saffiotti U., Montesano R., Sellakumar A. R., Borg S. A. Experimental cancer of the lung. Inhibition by vitamin A of the induction of tracheobronchial squamous metaplasia and squamous cell tumors. Cancer. 1967 May;20(5):857–864. doi: 10.1002/1097-0142(1967)20:5<857::aid-cncr2820200545>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Silverberg E. Cancer statistics, 1978. CA Cancer J Clin. 1978 Jan-Feb;28(1):17–32. doi: 10.3322/canjclin.28.1.17. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Dunlop N. M., Newton D. L., Smith J. M. Prevention of chemical carcinogenesis by vitamin A and its synthetic analogs (retinoids). Fed Proc. 1976 May 1;35(6):1332–1338. [PubMed] [Google Scholar]
- Sporn M. B., Newton D. L. Chemoprevention of cancer with retinoids. Fed Proc. 1979 Oct;38(11):2528–2534. [PubMed] [Google Scholar]
- Sporn M. B., Squire R. A., Brown C. C., Smith J. M., Wenk M. L., Springer S. 13-cis-retinoic acid: inhibition of bladder carcinogenesis in the rat. Science. 1977 Feb 4;195(4277):487–489. doi: 10.1126/science.835006. [DOI] [PubMed] [Google Scholar]
- Telang N. T., Banerjee M. R., Iyer A. P., Kundu A. B. Neoplastic transformation of epithelial cells in whole mammary gland in vitro. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5886–5890. doi: 10.1073/pnas.76.11.5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., De Larco J. E., Sporn M. B. Retinoids block phenotypic cell transformation produced by sarcoma growth factor. Nature. 1978 Nov 16;276(5685):272–274. doi: 10.1038/276272a0. [DOI] [PubMed] [Google Scholar]
- Tonelli Q. J., Custer R. P., Sorof S. Transformation of cultured mouse mammary glands by aromatic amines and amides and their derivatives. Cancer Res. 1979 May;39(5):1784–1792. [PubMed] [Google Scholar]
- Wood B. G., Washburn L. L., Mukherjee A. S., Banerjee M. R. Hormonal regulation of lobulo-alveolar growth, functional differentiation and regression of whole mouse mammary gland in organ culture. J Endocrinol. 1975 Apr;65(1):1–6. doi: 10.1677/joe.0.0650001. [DOI] [PubMed] [Google Scholar]
- de Larco J. E., Todaro G. J. Growth factors from murine sarcoma virus-transformed cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4001–4005. doi: 10.1073/pnas.75.8.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]



