Abstract
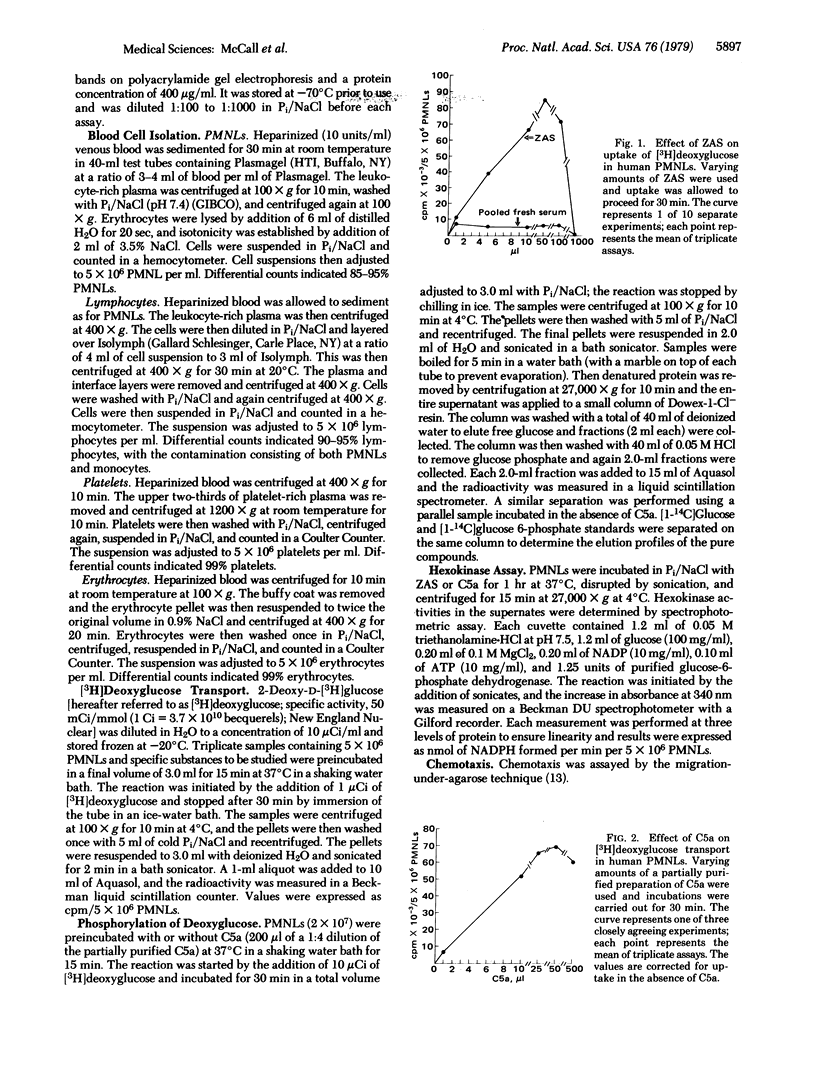
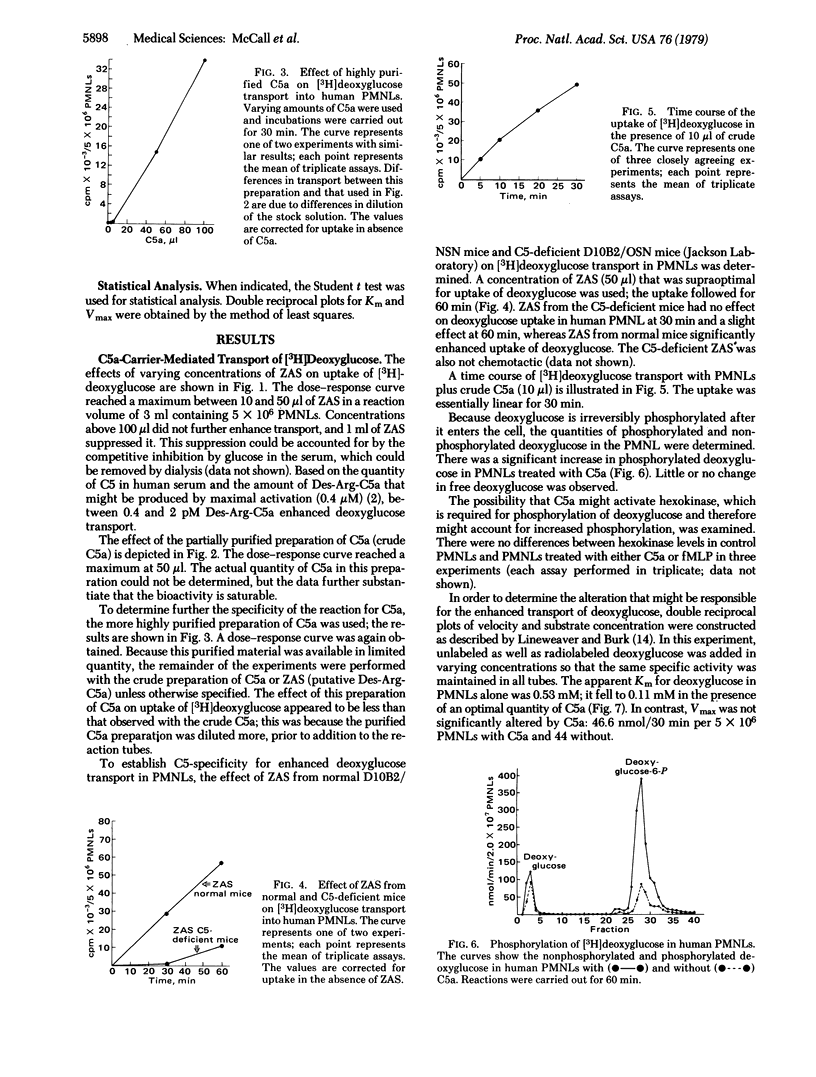
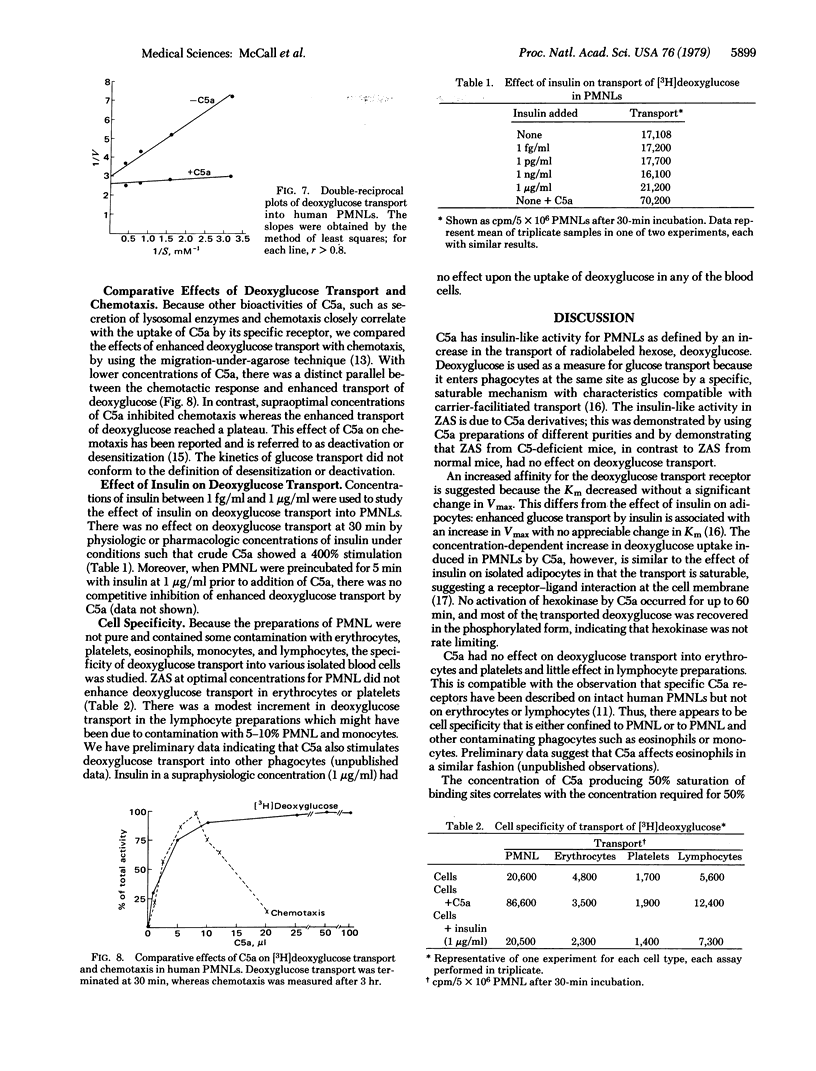
The polymorphonuclear leukocyte (PMNL) depends on glucose as a source of energy for motility, chemotaxis, phagocytosis, and bactericidal activity. Activated complement (C5a) at low concentrations stimulates carrier-mediated carbohydrate transport in PMNLs as measured by the uptake of 2-deoxy-D-[3H]glucose. Human PMNLs were preincubated at 37°C for 15 min with zymosan-activated human serum or various purified preparations of human C5a. A concentration-dependent increase in deoxyglucose transport (>700% of control) into PMNLs occurred with all test substances. Reaction was linear for 30 min, and uptake of deoxyglucose followed saturation kinetics. C5a caused a decrease in the Km for deoxyglucose, from 0.53 to 0.11 mM, without altering the Vmax (44 nmol/30 min per 5 × 106 PMNLs in control and 46.6 with C5a). The optimal concentration of C5a for enhanced carrier-mediated transport of deoxyglucose was similar to that which promoted optimal chemotaxis. Activated serum from C5-deficient mice had little or no effect on deoxyglucose transport whereas that from normal syngeneic mice enhanced deoxyglucose transport. C5a did not enhance deoxyglucose transport into isolated erythrocytes, platelets, or lymphocytes. The deoxyglucose within the cell was primarily in the phosphorylated form, and hexokinase activity was not increased in PMNLs stimulated with C5a, indicating that hexokinase was not rate limiting and that enhanced transport was the mechanism of the C5a activity. Insulin at physiologic concentration (10 ng/ml) had no effect on deoxyglucose transport in PMNL and did not act as a competitive inhibitor of C5a. This insulin-like bioactivity could be detected with the amount of C5a that would be present after activation of 0.1-0.5% of the C5 in 1 ml of serum. This suggests that uptake of [3H]deoxyglucose by PMNLs might serve as a highly sensitive test for activation of the fifth component of complement.
Keywords: hexose transport, leukocyte metabolism
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bass D. A., Dechatelet L. R., McCall C. E. Independent stimulation of motility and the oxidative metabolic burst of human polymorphonuclear leukocytes. J Immunol. 1978 Jul;121(1):172–178. [PubMed] [Google Scholar]
- Bibi S. S., DeChatelet L. R., McCall C. E. Human toxic neutrophils. IV. Incorporation of amino acids and uptake of 2-deoxy-D-glucose. J Infect Dis. 1977 Jun;135(6):949–951. doi: 10.1093/infdis/135.6.949. [DOI] [PubMed] [Google Scholar]
- Carruthers B. M. Leukocyte motility. II. Effect of absence of glucose in medium; effect of presence of deoxyglucose, dinitrophenol, puromycin, actinomycin D, and trypsin on the response to chemotactic substance; effect of segregation of cells from chemotactic substance. Can J Physiol Pharmacol. 1967 Mar;45(2):269–280. doi: 10.1139/y67-029. [DOI] [PubMed] [Google Scholar]
- Chenoweth D. E., Hugli T. E. Demonstration of specific C5a receptor on intact human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3943–3947. doi: 10.1073/pnas.75.8.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock P. R., Fehr J., Dalmasso A. P., Brighan K. L., Jacob H. S. Hemodialysis leukopenia. Pulmonary vascular leukostasis resulting from complement activation by dialyzer cellophane membranes. J Clin Invest. 1977 May;59(5):879–888. doi: 10.1172/JCI108710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock P. R., Hammerschmidt D., White J. G., Dalmosso A. P., Jacob H. S. Complement (C5-a)-induced granulocyte aggregation in vitro. A possible mechanism of complement-mediated leukostasis and leukopenia. J Clin Invest. 1977 Jul;60(1):260–264. doi: 10.1172/JCI108763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChatelet L. R. Initiation of the respiratory burst in human polymorphonuclear neutrophils: a critical review. J Reticuloendothel Soc. 1978 Jul;24(1):73–91. [PubMed] [Google Scholar]
- Fernandez H. N., Henson P. M., Otani A., Hugli T. E. Chemotactic response to human C3a and C5a anaphylatoxins. I. Evaluation of C3a and C5a leukotaxis in vitro and under stimulated in vivo conditions. J Immunol. 1978 Jan;120(1):109–115. [PubMed] [Google Scholar]
- Fussganger R. D., Kahn C. R., Roth J., De Meyts P. Binding and degradation of insulin by human peripheral granulocytes. Demonstration of specific receptors with high affinity. J Biol Chem. 1976 May 10;251(9):2761–2769. [PubMed] [Google Scholar]
- Gambhir K. K., Archer J. A., Bradley C. J. Characteristics of human erythrocyte insulin receptors. Diabetes. 1978 Jul;27(7):701–708. doi: 10.2337/diab.27.7.701. [DOI] [PubMed] [Google Scholar]
- Goetzl E. J., Austen K. F. Stimulation of human neutrophil leukocyte aerobic glucose metabolism by purified chemotactic factors. J Clin Invest. 1974 Feb;53(2):591–599. doi: 10.1172/JCI107594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Brai M., Osler A. G., Weissmann G. Lysosomal enzyme release from human leukocytes: mediation by the alternate pathway of complement activation. J Immunol. 1973 Jul;111(1):33–37. [PubMed] [Google Scholar]
- Henson P. M. The immunologic release of constituents from neutrophil leukocytes. II. Mechanisms of release during phagocytosis, and adherence to nonphagocytosable surfaces. J Immunol. 1971 Dec;107(6):1547–1557. [PubMed] [Google Scholar]
- McCall C. E., De Chatelet L. R., Brown D., Lachmann P. New biological activity following intravascular activation of the complement cascade. Nature. 1974 Jun 28;249(460):841–843. doi: 10.1038/249841a0. [DOI] [PubMed] [Google Scholar]
- Müller-Eberhard H. J. Complement abnormalities in human disease. Hosp Pract. 1978 Dec;13(12):65–76. doi: 10.1080/21548331.1978.11707450. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Silverstein S. C., Brukner L. H., Cohn Z. A. Extracellular cytolysis by activated macrophages and granulocytes. II. Hydrogen peroxide as a mediator of cytotoxicity. J Exp Med. 1979 Jan 1;149(1):100–113. doi: 10.1084/jem.149.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Flaherty J. T., Craddock P. R., Jacob H. S. Effect of intravascular complement activation on granulocyte adhesiveness and distribution. Blood. 1978 Apr;51(4):731–739. [PubMed] [Google Scholar]
- O'Flaherty J. T., Kreutzer D. L., Ward P. A. Chemotactic factor influences on the aggregation, swelling, and foreign surface adhesiveness of human leukocytes. Am J Pathol. 1978 Mar;90(3):537–550. [PMC free article] [PubMed] [Google Scholar]
- Olefsky J. M. Mechanisms of the ability of insulin to activate the glucose-transport system in rat adipocytes. Biochem J. 1978 Apr 15;172(1):137–145. doi: 10.1042/bj1720137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P., Mason R. J., Hartwig J., Vaughan M. Quantitative studies of phagocytosis by polymorphonuclear leukocytes: use of emulsions to measure the initial rate of phagocytosis. J Clin Invest. 1972 Mar;51(3):615–624. doi: 10.1172/JCI106851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P., Murad F., Mason R. J., Vaughan M. Regulation of glycogen metabolism in polymorphonuclear leukocytes. J Biol Chem. 1970 Nov 25;245(22):6228–6234. [PubMed] [Google Scholar]



