Abstract
Purpose
Research suggests that physicians neglect preventive care for cancer survivors. A survivor’s self-motivation with respect to preventive care is unknown. Using protective skin care as a proxy, our aims were to characterize preventive care in cancer survivors and to identify factors associated with appropriate prevention.
Methods
Using data from the 2009 U.S. Health Information National Trends Survey, we compared preventive skin care patterns in cancer survivors and non-cancer patients. Primary endpoints were the use of sunscreens, long-sleeved shirts, hats, and shade.
Results
We identified 179 early cancer survivors (<5 years), 242 intermediate cancer survivors (5–10 years), 412 long-term cancer survivors (>10 years), and 5951 non-cancer patients. The use of sunscreens (60%), long-sleeved shirts (88%), hats (58%), and shade (68%) was suboptimal. Overall, cancer survivors were not more likely to adhere to preventive care (p = 0.89). A composite score showed a significant difference between the cancer survivor groups (p < 0.01) whereby intermediate survivors reported the best preventive practices.
Conclusions
A prior diagnosis of cancer does not appear to increase personal compliance with cancer prevention. Reasons for this poor engagement are not clear. Targeted strategies to increase self-motivation might improve preventive practices in cancer survivors.
Keywords: Cancer survivors, skin, sun, prevention, protection
1. INTRODUCTION
There are more than 12 million cancer survivors in the United States1. That number is expected to grow significantly in the next several decades because of an aging general population and ongoing improvements in cancer detection and treatment2. But despite the advancements, cancer survivors remain at increased risk of disease recurrence, secondary malignancies, and late effects of therapy3.
Prevention of the complications that are directly related to a primary cancer or its treatment is considered secondary prevention. As survival continues to improve, additional modifiable conditions such as cardiovascular diseases and diabetes will become competing health risks that pose a significant threat to the lives of survivors1. Thus, the notion of tertiary prevention that minimizes the development of comorbidities not related to the primary diagnosis is increasingly relevant for improving the overall long-term health of cancer survivors. Our study examines sun-protective behaviors in cancer survivors, which is an example of tertiary prevention.
Earlier research on lifestyle behaviors found similar rates of cancer-related risk factors in cancer survivors and non-cancer patients, with little variability between cancer sites1. Continued focus on the follow-up of the primary cancer can frequently shift significant attention away from other aspects of general preventive care4. To assist physicians in better managing patients who are transitioning from active cancer treatment to primary care, a number of physician-directed interventions—such as increased physician education about how best to promote smoking cessation, dissemination of evidence-based guidelines on appropriate survivor follow-up, and the introduction of survivorship care plans—have been designed1,4–6.
Although physician-based interventions are important, little is known about the effect of self-motivation on personal preventive care by cancer survivors. We used preventive skin care practices as a proxy for personal willingness to engage in prevention. To reduce the incidence of skin cancers, sun-protective behaviors have been a substantial component of patient-directed public health education campaigns. Although preventive strategies such as the use of sunscreens are easy to adopt and do not interfere with daily activities, studies indicate that sun-protective behaviors are suboptimal7. The personal values and beliefs of patients have been suggested to be barriers to such prevention (specifically the use of sunscreens)8. For example, a tendency toward tanning or misconceptions about skin cancer risk factors are correlated with lower use of sunscreens8.
We hypothesized that, compared with non-cancer patients, cancer survivors would demonstrate better sun-protective behaviors because of their enhanced exposure to cancer-related information and increased vigilance about cancer risk factors after their primary cancer diagnosis. Our study aims were to characterize and compare the use of various skin care preventive strategies in cancer survivors and non-cancer patients, and to identify the clinical factors that affect those preventive behaviors.
2. METHODS
2.1. Data Source
We analyzed data from the 2009 U.S. Health Information National Trends Survey (hints), which was developed by the National Cancer Institute in the United States. A cross-sectional survey of a representative sample of the U.S. adult population, hints is designed to gain a better understanding of how adults more than 18 years of age use various communication channels and modalities to obtain health and cancer-related information. In addition, hints aims to measure how respondents manage their own health and the degree to which people engage in healthy behaviors, with a specific focus on cancer prevention and control. The survey is distributed every 2 years so that certain information can be examined over time for temporal trends. Data for the 2009 hints survey was collected from January 2010 to May 2010. The survey sampled two populations: a group surveyed during a 30-minute computer-assisted telephone interview, and a group surveyed using a mailed questionnaire. Response rates for the telephone and mail surveys were 24% and 31% respectively.
2.2. Selection of Participants
According to the U.S. National Cancer Institute’s Office of Cancer Survivorship, an individual is considered a cancer survivor from the time of diagnosis. Our study included all hints respondents who indicated a personal history of a current or prior diagnosis of cancer. Respondents who satisfied that definition were subsequently categorized into three cancer survivor groups—early (<5 years), intermediate (5–10 years), and late (>10 years)—based on the interval between the date of their first cancer diagnosis and the date of survey completion. Respondents who denied ever having a cancer history were classified as non-cancer patients. Respondents who did not answer the question or whose cancer history was unknown were excluded from the analysis. For the purposes of a secondary analysis, we also grouped patients into survivors of melanoma, survivors of non-melanoma skin cancer, and survivors of non-skin cancers.
2.3. Main Outcomes and Covariates
Our main outcome measures were self-reported preventive skin care practices. Participants were asked to consider what they do when they go outside in the summer or on a warm sunny day. Specifically: How often do you wear sunscreen? How often do you wear a shirt with sleeves that cover your shoulders? How often do you wear a hat? How often do you stay in the shade or under an umbrella?
Each of the foregoing outcome variables was categorized into 5 possible groups based on frequency of use: Never, Rarely, Sometimes, Often, or Always. Each response category was subsequently assigned a score ranging from 0 to 4 (where 0 = Never and 4 = Always) to generate a preventive skin care score. A composite score for overall quality of preventive skin care was then derived by summing the scores for the variables (sunscreen, shirt, hat, shade), thus yielding a score ranging from a minimum of 0 (poorest quality skin care) and to a maximum of 16 (best quality skin care).
Tanning bed use was explored as an indicator for engaging in unhealthy sun-protective behavior. Respondents were asked about the number of times that they had used tanning beds in the 12 months preceding the survey: 0, 1–2, 3–10, 11–24, and more than 25 times. We controlled for a variety of sociodemographic characteristics that included age, sex, race, marital status, highest educational attainment, and annual income level. We also considered additional baseline health-related characteristics such as smoking status and self-reported general health.
2.4. Statistical Analyses
All analyses were performed using the Stata software application (version 10 SE: StataCorp LP, College Station, TX, U.S.A.). Categorical and continuous variables are summarized with frequency distributions and means or medians, as appropriate. Chi-square and Wilcoxon tests were used to detect differences between cancer survivors and non-cancer patients. Multivariate logistic regression models were subsequently constructed to examine for factors associated with preventive behavior while adjusting for confounders. Appropriate survey sampling weights were applied to generate estimates that are representative of the U.S. population. For the purposes of the multivariate analysis, each of the outcome variables was dichotomized: individuals who responded Always, Often, or Sometimes were categorized as engaging in prevention; those who responded Rarely or Never were classified as not participating in prevention. In sensitivity analyses, the way in which the outcome variables were dichotomized was varied. In those analyses, the findings were not appreciably different, and so only the main results are presented. A value of p < 0.05 was considered statistically significant.
3. RESULTS
We identified 179 early cancer survivors, 242 intermediate survivors, 412 long-term survivors, and 5951 non-cancer patients. Mean ages in those groups were 61, 63, 69, and 53 years respectively (p < 0.01). The sex distribution varied, with women constituting a higher proportion of the non-cancer patients and the long-term survivors than of the early and intermediate survivors (60% and 67% vs. 47% and 52% respectively, p < 0.01). The rate of smoking was lower in non-cancer patients (47%) than in early, intermediate, and long-term survivors (54%, 61%, 52%; p = 0.05). All other demographic characteristics—such as race, marital status, education, income, and general health—were well balanced in the patient groups (all p > 0.05). Table i summarizes these and other baseline patient characteristics.
TABLE I.
Baseline characteristics of cancer survivors and non-cancer patientsa
| Characteristic |
Participant group
|
p Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
Non-cancer patients
|
Cancer survivors
|
||||||||
|
<5 Years
|
5–10 Years
|
>10 Years
|
|||||||
| (n) | (%) | (n) | (%) | (n) | (%) | (n) | (%) | ||
| Participants (n) | 5951 | 179 | 242 | 412 | |||||
| Mean age (years) | 53 | 61 | 64 | 70 | 0.00 | ||||
| Sex | 0.01 | ||||||||
| Women | 60 | 47 | 52 | 68 | |||||
| Men | 40 | 53 | 48 | 32 | |||||
| Race | 0.56 | ||||||||
| White | 4786 | 75 | 141 | 64 | 197 | 71 | 328 | 73 | |
| Non-white | 927 | 19 | 27 | 26 | 32 | 20 | 68 | 21 | |
| Unreported | 238 | 6 | 11 | 9 | 13 | 9 | 16 | 6 | |
| Marital status | 0.65 | ||||||||
| Single | 804 | 22 | 23 | 29 | 25 | 20 | 55 | 19 | |
| Married | 3594 | 60 | 106 | 51 | 162 | 65 | 254 | 63 | |
| Divorced or separated | 1528 | 17 | 48 | 19 | 53 | 15 | 103 | 18 | |
| Unreported | 25 | 0 | 2 | 1 | 2 | 1 | 0 | 0 | |
| Education | 0.35 | ||||||||
| College or greater | 3981 | 62 | 120 | 58 | 158 | 53 | 285 | 60 | |
| High school diploma | 1431 | 25 | 49 | 35 | 59 | 33 | 96 | 27 | |
| Less than high school | 519 | 13 | 9 | 6 | 23 | 13 | 31 | 14 | |
| Unreported | 20 | 0 | 1 | 1 | 2 | 1 | 0 | 0 | |
| Income | 0.28 | ||||||||
| <$20,000 | 892 | 17 | 23 | 15 | 35 | 22 | 60 | 15 | |
| $20,000–$75,000 | 2592 | 45 | 69 | 39 | 97 | 36 | 178 | 45 | |
| >$75,000 | 1683 | 27 | 59 | 33 | 75 | 31 | 124 | 26 | |
| Unreported | 784 | 12 | 28 | 13 | 35 | 11 | 50 | 15 | |
| Smoking status | 0.02 | ||||||||
| Yes | 2799 | 47 | 93 | 54 | 129 | 61 | 195 | 52 | |
| No | 3088 | 52 | 86 | 46 | 111 | 38 | 211 | 46 | |
| Unreported | 64 | 1 | 0 | 0 | 2 | 1 | 6 | 2 | |
| General health | 0.19 | ||||||||
| Excellent | 708 | 11 | 26 | 19 | 19 | 5 | 45 | 10 | |
| Very good | 2209 | 36 | 71 | 28 | 92 | 34 | 147 | 37 | |
| Good | 2095 | 37 | 59 | 38 | 87 | 36 | 156 | 40 | |
| Fair | 749 | 14 | 17 | 13 | 31 | 19 | 57 | 11 | |
| Poor | 175 | 2 | 6 | 2 | 11 | 5 | 7 | 2 | |
| Unreported | 15 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | |
From the U.S. Health Information National Trends Survey (hints), 2009.
Table ii describes patterns of sun-protective behaviors in the groups. In general, preventive behaviors were similar in the cancer survivors and the non-cancer patients; an exception was the use of shade. Use of sunscreen by the early, intermediate, and long-term cancer survivors was 60%, 61%, and 64% respectively; it was 60% by the non-cancer patients (p = 0.32). Use of shoulder-sleeved shirts was 89% by both the early and intermediate cancer survivors, 91% by long-term cancer survivors, and 87% by non-cancer patients (p = 0.40). Hats were used by 63%, 64%, and 57% of early, intermediate, and long-term cancer survivors respectively and by 58% of non-cancer patients (p = 0.19). Shade was used by 62%, 72%, and 68% of early, intermediate, and long-term cancer survivors respectively and by 69% of non-cancer patients (p = 0.006).
TABLE II.
Patterns of sun-protective behavior and preventive and composite preventive scores in cancer survivors and non-cancer patients
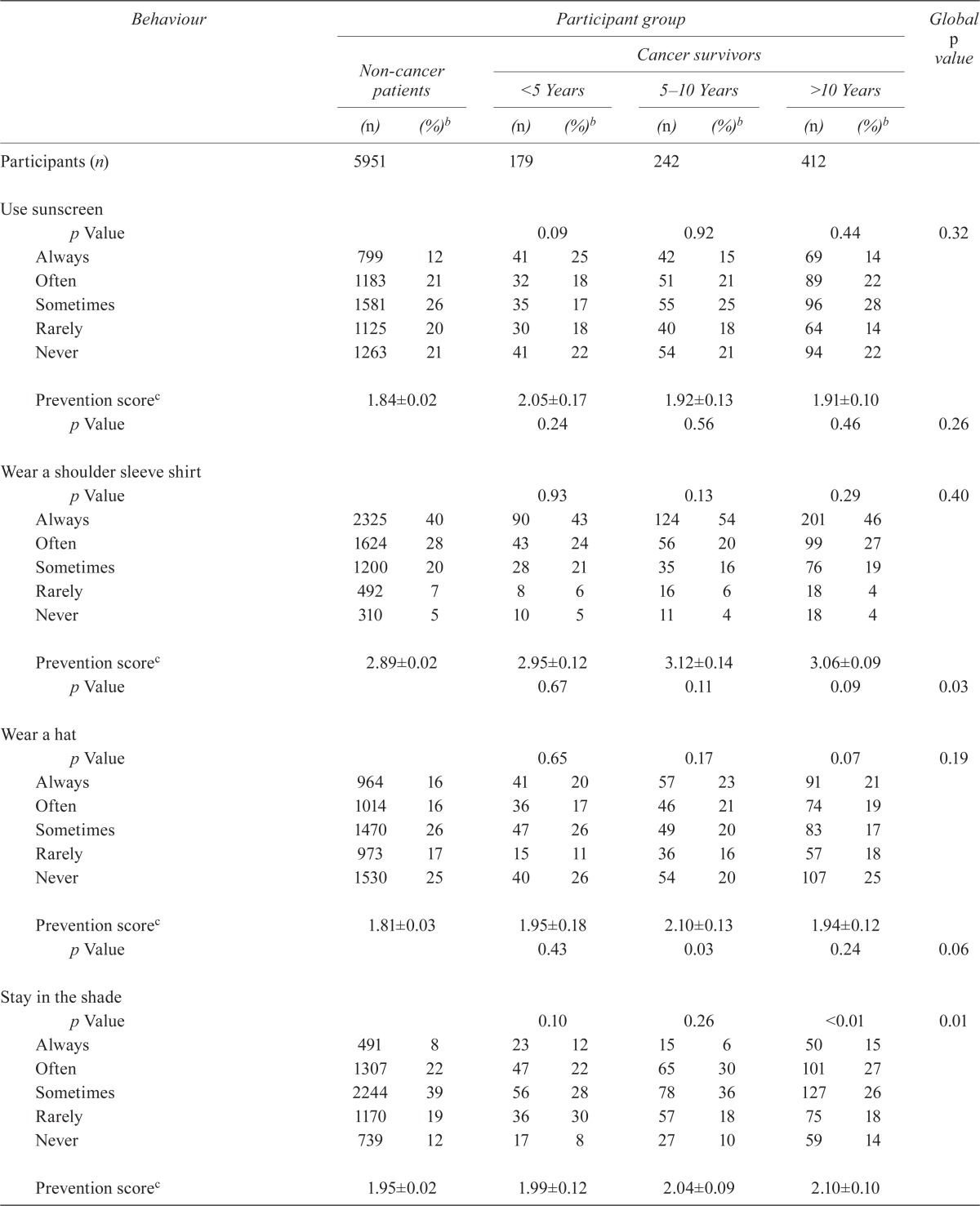
| Behaviour |
Participant group
|
Global p value | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
Non-cancer patients
|
Cancer survivors
|
||||||||
|
<5 Years
|
5–10 Years
|
>10 Years
|
|||||||
| (n) | (%)b | (n) | (%)b | (n) | (%)b | (n) | (%)b | ||
| Participants (n) | 5951 | 179 | 242 | 412 | |||||
| Use sunscreen | |||||||||
| p Value | 0.09 | 0.92 | 0.44 | 0.32 | |||||
| Always | 799 | 12 | 41 | 25 | 42 | 15 | 69 | 14 | |
| Often | 1183 | 21 | 32 | 18 | 51 | 21 | 89 | 22 | |
| Sometimes | 1581 | 26 | 35 | 17 | 55 | 25 | 96 | 28 | |
| Rarely | 1125 | 20 | 30 | 18 | 40 | 18 | 64 | 14 | |
| Never | 1263 | 21 | 41 | 22 | 54 | 21 | 94 | 22 | |
| Prevention scorec | 1.84±0.02 | 2.05±0.17 | 1.92±0.13 | 1.91±0.10 | |||||
| p Value | 0.24 | 0.56 | 0.46 | 0.26 | |||||
| Wear a shoulder sleeve shirt | |||||||||
| p Value | 0.93 | 0.13 | 0.29 | 0.40 | |||||
| Always | 2325 | 40 | 90 | 43 | 124 | 54 | 201 | 46 | |
| Often | 1624 | 28 | 43 | 24 | 56 | 20 | 99 | 27 | |
| Sometimes | 1200 | 20 | 28 | 21 | 35 | 16 | 76 | 19 | |
| Rarely | 492 | 7 | 8 | 6 | 16 | 6 | 18 | 4 | |
| Never | 310 | 5 | 10 | 5 | 11 | 4 | 18 | 4 | |
| Prevention scorec | 2.89±0.02 | 2.95±0.12 | 3.12±0.14 | 3.06±0.09 | |||||
| p Value | 0.67 | 0.11 | 0.09 | 0.03 | |||||
| Wear a hat | |||||||||
| p Value | 0.65 | 0.17 | 0.07 | 0.19 | |||||
| Always | 964 | 16 | 41 | 20 | 57 | 23 | 91 | 21 | |
| Often | 1014 | 16 | 36 | 17 | 46 | 21 | 74 | 19 | |
| Sometimes | 1470 | 26 | 47 | 26 | 49 | 20 | 83 | 17 | |
| Rarely | 973 | 17 | 15 | 11 | 36 | 16 | 57 | 18 | |
| Never | 1530 | 25 | 40 | 26 | 54 | 20 | 107 | 25 | |
| Prevention scorec | 1.81±0.03 | 1.95±0.18 | 2.10±0.13 | 1.94±0.12 | |||||
| p Value | 0.43 | 0.03 | 0.24 | 0.06 | |||||
| Stay in the shade | |||||||||
| p Value | 0.10 | 0.26 | <0.01 | 0.01 | |||||
| Always | 491 | 8 | 23 | 12 | 15 | 6 | 50 | 15 | |
| Often | 1307 | 22 | 47 | 22 | 65 | 30 | 101 | 27 | |
| Sometimes | 2244 | 39 | 56 | 28 | 78 | 36 | 127 | 26 | |
| Rarely | 1170 | 19 | 36 | 30 | 57 | 18 | 75 | 18 | |
| Never | 739 | 12 | 17 | 8 | 27 | 10 | 59 | 14 | |
| Prevention scorec | 1.95±0.02 | 1.99±0.12 | 2.04±0.09 | 2.10±0.10 | |||||
| Used a tanning bed in the preceding 12 months | |||||||||
| p Value | <0.01 | 0.02 | 0.06 | <0.01 | |||||
| Yes | 399 | 6 | 3 1 | 8 2 | 13 3 | ||||
| No | 5552 | 94 | 176 | 99 | 234 | 98 | 399 | 97 | |
| Composite prevention scorec | 8.49±0.05 | 8.93±0.38 | 9.18±0.36 | 9.02±0.23 | |||||
| p Value | 0.25 | 0.06 | 0.03 | <0.01 | |||||
From the U.S. Health Information National Trends Survey (hints), 2009.
All percentages are weighted.
Mean ± standard error.
The individual preventive scores generated for each outcome measure revealed better use of shoulder-sleeved shirts by cancer survivors (p = 0.02). We also observed a nonsignificant trend toward better use of hats (p = 0.06) and of shade (p = 0.10). Use of tanning beds was significantly less frequent among cancer survivors (p < 0.01). Overall, use of prevention practices was better in early cancer survivors, but worsened over time as the length of survivorship increased.
A comparison of the composite scores indicated that the overall quality of sun-protective behavior was suboptimal for all groups: mean scores were just 8.9, 9.2, 9.0, and 8.5 (of a maximum of 16) in early, intermediate, and long-term cancer survivors and non-cancer patients respectively. Although use of individual preventive strategies did not differ greatly between cancer survivors and non-cancer patients, the mean composite scores were significantly different, with intermediate cancer survivors showing the best use of preventive skin care (p < 0.01).
In multivariate analysis (Table iii), use of preventive skin care did not correlate with cancer survivorship. Cancer survivors as a group were not more likely to engage in any of the four preventive strategies measured. The length of survivorship also did not appear to alter sun-protective behaviors. However, age and sex appear to significantly correlate with the preventive strategy used. Older respondents (more than 50 years of age) preferred using shoulder-sleeved shirts [odds ratio (or): 1.68; p < 0.01] and hats (or: 1.46; p < 0.01), but they were much less likely to use sunscreen (or: 0.77, p < 0.01). We also observed a nonsignificant trend toward using shade in the older age group. There was an association between male sex and the use of shoulder-sleeved shirts (or: 1.53; p < 0.01) and hats (or: 3.54; p < 0.01). Conversely, men were less likely than women to apply sunscreen (or: 0.47; p < 0.01) or use shade (or: 0.65; p < 0.01).
TABLE III.
Association of clinical characteristics with odds of using various sun-protection strategiesa
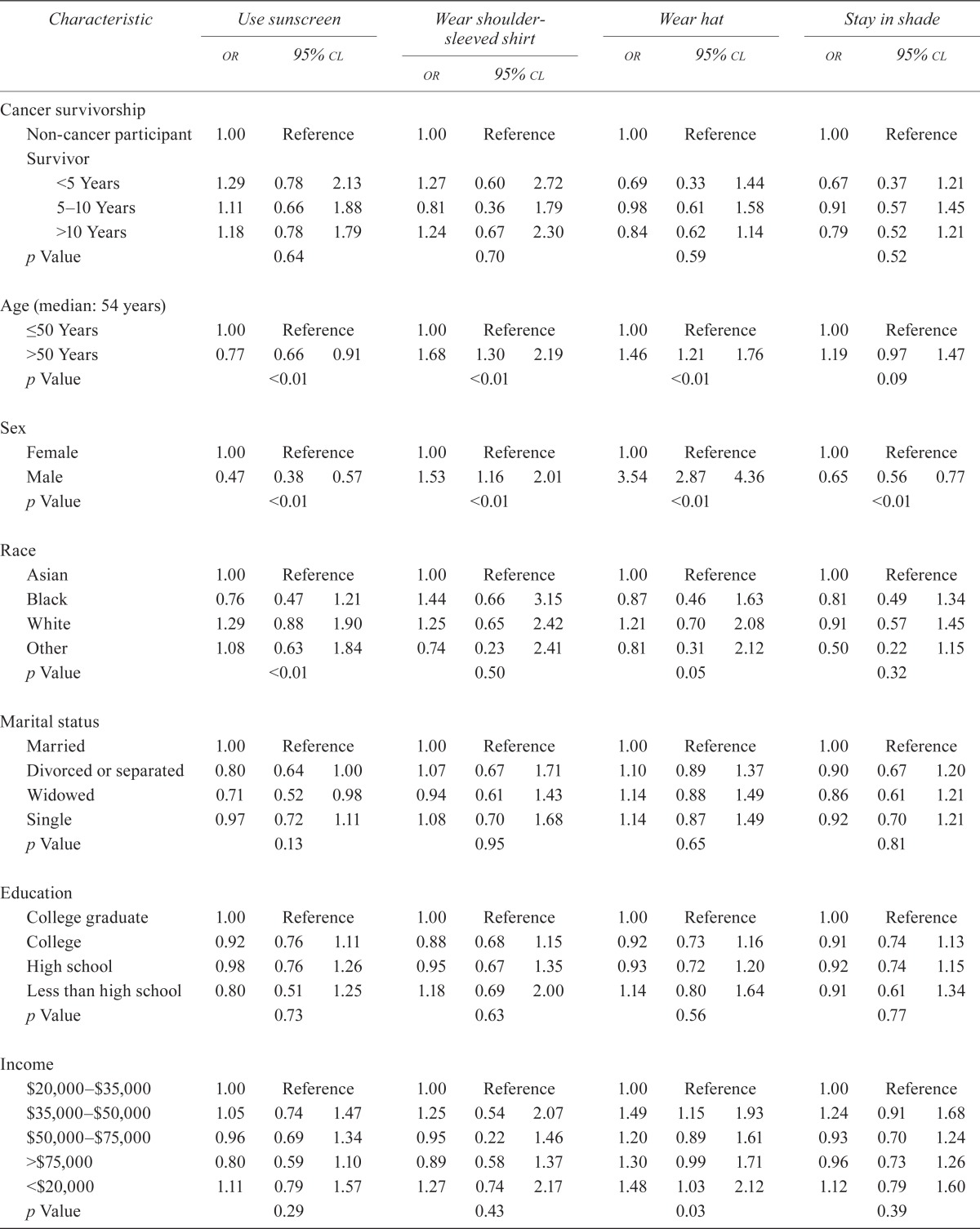
| Characteristic |
Use sunscreen
|
Wear shoulder-sleeved shirt
|
Wear hat
|
Stay in shade
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| or | 95% cl | or | 95% cl | or | 95% cl | or | 95% cl | |||||
| Cancer survivorship | ||||||||||||
| Non-cancer participant | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | ||||
| Survivor | ||||||||||||
| <5 Years | 1.29 | 0.78 | 2.13 | 1.27 | 0.60 | 2.72 | 0.69 | 0.33 | 1.44 | 0.67 | 0.37 | 1.21 |
| 5–10 Years | 1.11 | 0.66 | 1.88 | 0.81 | 0.36 | 1.79 | 0.98 | 0.61 | 1.58 | 0.91 | 0.57 | 1.45 |
| >10 Years | 1.18 | 0.78 | 1.79 | 1.24 | 0.67 | 2.30 | 0.84 | 0.62 | 1.14 | 0.79 | 0.52 | 1.21 |
| p Value | 0.64 | 0.70 | 0.59 | 0.52 | ||||||||
| Age (median: 54 years) | ||||||||||||
| ≤50 Years | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | ||||
| >50 Years | 0.77 | 0.66 | 0.91 | 1.68 | 1.30 | 2.19 | 1.46 | 1.21 | 1.76 | 1.19 | 0.97 | 1.47 |
| p Value | <0.01 | <0.01 | <0.01 | 0.09 | ||||||||
| Sex | ||||||||||||
| Female | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | ||||
| Male | 0.47 | 0.38 | 0.57 | 1.53 | 1.16 | 2.01 | 3.54 | 2.87 | 4.36 | 0.65 | 0.56 | 0.77 |
| p Value | <0.01 | <0.01 | <0.01 | <0.01 | ||||||||
| Race | ||||||||||||
| Asian | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | ||||
| Black | 0.76 | 0.47 | 1.21 | 1.44 | 0.66 | 3.15 | 0.87 | 0.46 | 1.63 | 0.81 | 0.49 | 1.34 |
| White | 1.29 | 0.88 | 1.90 | 1.25 | 0.65 | 2.42 | 1.21 | 0.70 | 2.08 | 0.91 | 0.57 | 1.45 |
| Other | 1.08 | 0.63 | 1.84 | 0.74 | 0.23 | 2.41 | 0.81 | 0.31 | 2.12 | 0.50 | 0.22 | 1.15 |
| p Value | <0.01 | 0.50 | 0.05 | 0.32 | ||||||||
| Marital status | ||||||||||||
| Married | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | ||||
| Divorced or separated | 0.80 | 0.64 | 1.00 | 1.07 | 0.67 | 1.71 | 1.10 | 0.89 | 1.37 | 0.90 | 0.67 | 1.20 |
| Widowed | 0.71 | 0.52 | 0.98 | 0.94 | 0.61 | 1.43 | 1.14 | 0.88 | 1.49 | 0.86 | 0.61 | 1.21 |
| Single | 0.97 | 0.72 | 1.11 | 1.08 | 0.70 | 1.68 | 1.14 | 0.87 | 1.49 | 0.92 | 0.70 | 1.21 |
| p Value | 0.13 | 0.95 | 0.65 | 0.81 | ||||||||
| Education | ||||||||||||
| College graduate | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | ||||
| College | 0.92 | 0.76 | 1.11 | 0.88 | 0.68 | 1.15 | 0.92 | 0.73 | 1.16 | 0.91 | 0.74 | 1.13 |
| High school | 0.98 | 0.76 | 1.26 | 0.95 | 0.67 | 1.35 | 0.93 | 0.72 | 1.20 | 0.92 | 0.74 | 1.15 |
| Less than high school | 0.80 | 0.51 | 1.25 | 1.18 | 0.69 | 2.00 | 1.14 | 0.80 | 1.64 | 0.91 | 0.61 | 1.34 |
| p Value | 0.73 | 0.63 | 0.56 | 0.77 | ||||||||
| Income | ||||||||||||
| $20,000–$35,000 | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | ||||
| $35,000–$50,000 | 1.05 | 0.74 | 1.47 | 1.25 | 0.54 | 2.07 | 1.49 | 1.15 | 1.93 | 1.24 | 0.91 | 1.68 |
| $50,000–$75,000 | 0.96 | 0.69 | 1.34 | 0.95 | 0.22 | 1.46 | 1.20 | 0.89 | 1.61 | 0.93 | 0.70 | 1.24 |
| >$75,000 | 0.80 | 0.59 | 1.10 | 0.89 | 0.58 | 1.37 | 1.30 | 0.99 | 1.71 | 0.96 | 0.73 | 1.26 |
| <$20,000 | 1.11 | 0.79 | 1.57 | 1.27 | 0.74 | 2.17 | 1.48 | 1.03 | 2.12 | 1.12 | 0.79 | 1.60 |
| p Value | 0.29 | 0.43 | 0.03 | 0.39 | ||||||||
| Smoking status | ||||||||||||
| Smoker | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | ||||
| Never smoked | 1.16 | 0.96 | 1.41 | 0.94 | 0.74 | 1.17 | 1.05 | 0.88 | 1.25 | 1.06 | 0.89 | 1.27 |
| p Value | 0.13 | 0.57 | 0.57 | 0.49 | ||||||||
| General health | ||||||||||||
| Excellent | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | ||||
| Very good | 0.98 | 0.78 | 1.23 | 0.92 | 0.69 | 1.22 | 1.04 | 0.76 | 1.42 | 0.92 | 0.69 | 1.23 |
| Fair | 1.47 | 1.08 | 2.01 | 1.10 | 0.71 | 1.69 | 1.01 | 0.69 | 1.47 | 1.07 | 0.76 | 1.51 |
| Good | 0.90 | 0.69 | 1.17 | 0.99 | 0.72 | 1.35 | 1.16 | 0.86 | 1.55 | 0.92 | 0.68 | 1.26 |
| Poor | 0.80 | 0.52 | 1.23 | 0.60 | 0.30 | 1.20 | 1.15 | 0.66 | 1.99 | 1.02 | 0.60 | 1.73 |
| p Value | <0.01 | 0.43 | 0.73 | 0.80 | ||||||||
From the U.S. Health Information National Trends Survey (hints), 2009.
or = odds ratio; cl = confidence limits.
We also conducted two additional exploratory analyses:
A comparison of controls versus survivors of melanoma versus survivors of non-melanoma skin cancer versus survivors of non-skin cancers
A comparison of controls versus all skin cancer survivors versus all other cancer survivors
The results of the foregoing analyses were essentially consistent with our main analysis in that they did not demonstrate any major significant differences between the various groups.
4. DISCUSSION
Survivorship care is increasingly being recognized as an important aspect of comprehensive, long-term cancer management, so that improvements gained from recent cancer advances are not lost because of poor follow-up. An essential component of cancer survivorship is the promotion of lifelong preventive health care. However, many patient-, physician-, and health system–related barriers pose challenges to adopting a healthy lifestyle.
To our knowledge, investigations examining sun protection as a measure of tertiary prevention are very limited to date, although some studies have shown that sunscreen use is associated with a reduction in the appearance of new skin lesions9. In addition, data in the setting of primary prevention demonstrate that sun protection is closely correlated with a patient’s level of self-motivation to engage in their own care10–12. In the present study, we used sun-protective behaviors as a proxy to evaluate personal willingness to participate in tertiary prevention8. We examined four different preventive strategies and observed that each was suboptimally used by cancer survivors. Importantly, sun-protective behaviors were not different between cancer survivors and non-cancer patients.
Our findings that use of preventive skin care practices by cancer survivors is similar to that by non-cancer patients are consistent with those in previous studies. Coups and Ostroff13 reported that overall engagement in sun protection was identical between cancer survivors and non-cancer patients. However, the purpose of their study was to establish the baseline prevalence of a number of healthy lifestyle behaviors among cancer survivors. Although skin care was explored, the emphasis of the study was on other high-risk behaviors, such as smoking and physical inactivity13.
Overall, data on skin care behaviors in adult cancer survivors as a whole are limited. A few studies have looked at sun protection in melanoma survivors. Earlier survey studies showed that the use of sun protection and skin self-examination were both suboptimal in melanoma survivors14,15. One population study examined the use of sun protection in early survivors of melanoma and found no difference in their behaviors compared with behaviors in the general population16. That result is consistent with our findings and with other behavioral studies comparing all cancer survivors to non-cancer controls. By contrast, Mayer et al. found that melanoma survivors, although still engaging poorly in prevention, performed better than did non-cancer patients17. However, interpretation of the numbers in their study might be limited by the large difference in the number of patients in the melanoma survivor group and in the non-cancer control group17. Additional population-based studies have also observed that rates of poor dietary habits, obesity, and physical inactivity were the same in cancer survivors as in the general population1–3. Those observations are contrary to our initial hypothesis that cancer survivors might be more cognizant about their health given their increased exposure to information about cancer risk factors and their apprehension about the need for further treatments. More recently, some researchers have actually shown higher rates of high-risk behaviors (for example, smoking) in specific survivor groups (uterine cancer survivors, among others)3.
Although information on high-risk sun behaviors in adult survivors is limited, some studies have examined this topic in childhood and young adult survivors. Although childhood survivors might, compared with their sibling controls, demonstrate less participation in high-risk activities, they continue to have significant ultraviolet exposure18. The same situation holds for young adult survivors19. Notably, one study found that young survivors continued to use tanning beds after a diagnosis of basal cell carcinoma, with 8% being considered frequent users20. In our univariate analysis on tanning bed use, we found that cancer survivors were less likely than controls to use tanning beds. That observation became nonsignificant in multivariate analysis, suggesting that, compared with the general population, cancer survivors were engaged in high-risk behaviors to the same degree. However, our analysis is limited by the small number of cases in some of the groups.
A noteworthy feature of our analysis is the observation that differences in preventive behavior were dependent on time since the cancer diagnosis. Specifically, preventive skin care was best in intermediate cancer survivors (5–10 years from their initial cancer diagnosis). Several factors could explain this observation. First, poor engagement in tertiary prevention among early cancer survivors can be attributed to continued attention on the primary cancer by health care providers and patients alike, given that the risks of recurrence and of side effects are highest during the first 5 years. Earlier studies examining the transition to survivorship have shown that, during the initial phase of follow-up, survivors are more likely to be followed by oncologists than by primary care physicians; the latter group of providers focuses more on general preventive care21 and their involvement at or after 5 years is associated with increased uptake of preventive measures such as influenza vaccines and screening for cholesterol, bone density, and other cancers22. Conversely, long-term survivors (10 years or more beyond their original cancer diagnosis) might no longer be as vigilant about their continued risk of cancer, especially given that, at this point, almost all cancer patients are discharged from the care of their cancer specialists and are fully returned to their general practitioners, whom they may not be regularly visiting. It is therefore possible that their adherence to preventive care is closer to that observed for the general population.
Although earlier studies and the present analysis reveal no major differences in self-reported preventive behaviors between cancer survivors and non-cancer patients, a mailed survey of 1667 cancer survivors conducted by Denmark–Wahnefried et al.23 found that survivors expressed a clear interest in prevention and that they were also very receptive to health promotion programs. Another study in melanoma survivors also found that patients expressed anxiety and became more conscious of sun exposure24. Those findings indicate that an interest in prevention does not always translate into actual engagement in prevention, but the precise reasons for this apparent disconnect are unclear. One possible reason is that survivors might not always feel confident in their ability to participate in prevention. A small cross-sectional survey study of breast cancer survivors noted that, although 70% and 80% of survivors respectively expressed an interest in improving their dietary habits and activity levels, fewer than 50% of survivors proceeded to make actual modifications to their behaviors25. Importantly, the authors demonstrated that respondents with low self-efficacy and low motivational readiness experienced the most barriers to change25. Thus, cancer survivors might benefit from active interventions that empower them with skills that improve self-efficacy and motivational readiness. For example, a small clinical trial showed that, compared with a control group that received standard follow-up care, colorectal cancer survivors randomized to a 12-week supervised exercise and dietary program experienced significant improvements in long-term adherence to healthy lifestyles26. The trial confirmed that a brief intervention to improve lifestyle behaviors is feasible in modifying longer-term preventive patterns26.
One of the main strengths of the present study is its analysis of a nationally representative sample. By applying survey weights to the analyses, our findings are meant to reflect the general U.S. population, which has not previously been examined to this extent. However, there are several limitations. First, we decided to use sun protection as a proxy to assess a survivor’s self-motivation to engage in cancer prevention. Although there is evidence to indicate that this correlate is reasonable, that assumption was not incorporated into the general design of the questions in hints. Sun-protective behaviors might therefore not be a reliable representation of personal willingness to participate in cancer prevention. Second, data from hints are self-reported, and responses might not correspond with actual practice patterns. However, patient-reported outcomes, including those for sun protection and ultraviolet light exposure, have previously been documented to be reasonably accurate and precise27. Third, we generated a composite score to obtain an overall impression of the quality of preventive skin practices, thus allowing us to compare groups of cancer survivors and non-cancer patients. Nevertheless, that score is not a validated scale and might be applicable only to our study cohort. Finally, hints is a cross-sectional study; we are therefore unable to make causal inferences from our analyses. Furthermore, we could not perform a temporal trend analysis to explore for changes in preventive behaviors over time.
5. CONCLUSIONS
Using sun protection as a proxy for personal motivation to participate in cancer prevention, our study revealed that, compared with their non-cancer counterparts, cancer survivors are not more engaged in preventive behaviors. Many prior interventions for health promotion and cancer screening have focused on physicians and how health care providers can better facilitate and streamline processes for cancer prevention. Our findings suggest that, despite a prior cancer diagnosis and access to cancer-related information from their past experiences with the cancer care team, cancer survivors might lack the willingness to participate in tertiary prevention. Future studies should identify strategies that might increase self-efficacy and motivational readiness in survivors so that they feel more equipped to participate in their own preventive care.
6. CONFLICT OF INTEREST DISCLOSURES
The authors have no financial relationships to disclose. The authors declare that they have full control of the data and would allow the journal to review the data upon request.
7. REFERENCES
- 1.Hewitt M, Greenfield S, Stovali E. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: The National Academies Press; 2005. [Google Scholar]
- 2.Fairley TL, Hawk H, Pierre S. Health behaviors and quality of life of cancer survivors in Massachusetts, 2006: data use for comprehensive cancer control. Prev Chronic Dis. 2010;7:A09. [PMC free article] [PubMed] [Google Scholar]
- 3.Bellizzi KM, Rowland JH, Jefferey DD, McNeel T. Health behaviors of cancer survivors: examining opportunities for cancer control intervention. J Clin Oncol. 2005;23:8884–93. doi: 10.1200/JCO.2005.02.2343. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert SM, Miller DC, Hollenbeck BK, Montie JE, Wei JT. Cancer survivorship: challenges and changing paradigms. J Urol. 2008;179:431–8. doi: 10.1016/j.juro.2007.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Earle CC. Failing to plan is planning to fail: improving the quality of care with survivorship care plans. J Clin Oncol. 2006;24:5112–16. doi: 10.1200/JCO.2006.06.5284. [DOI] [PubMed] [Google Scholar]
- 6.Ganz PA. Survivorship: adult cancer survivors. Prim Care. 2009;36:721–41. doi: 10.1016/j.pop.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Eide MJ, Weinstock MA. Public health challenges in sun protection. Dermatol Clin. 2006;24:119–24. doi: 10.1016/j.det.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Craciun C, Schuz N, Lippke S, Schwarzer R. Facilitating sunscreen use in women by a theory-based online intervention: a randomized controlled trial. J Health Psychol. 2012;17:207–16. doi: 10.1177/1359105311414955. [DOI] [PubMed] [Google Scholar]
- 9.Kuhn KG, Bœsen E, Ross L, Johansen C. Evaluation and outcome of behavioural changes in the rehabilitation of cancer patients: a review. Eur J Cancer. 2005;41:216–24. doi: 10.1016/j.ejca.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 10.Branstrom R, Ullen H, Brandberg Y. Attitudes, subjective norms and perception of behavioural control as predictors of sun-related behaviors in Swedish adults. Prev Med. 2004;39:992–9. doi: 10.1016/j.ypmed.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 11.De Vries H, Lezwijn J, Hol M, Honing C. Skin cancer prevention: behavior and motives in Dutch adolescents. Eur J Cancer Prev. 2005;14:39–50. doi: 10.1097/00008469-200502000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Martin SC, Jacobsen PB, Lucas DJ, Branch KA, Ferron JM. Predicting children’s sunscreen use: application of the theories of reasoned action and planned behavior. Prev Med. 1999;29:37–44. doi: 10.1006/pmed.1999.0500. [DOI] [PubMed] [Google Scholar]
- 13.Coups EJ, Ostroff JS. A population-based estimate of the prevalence of behavioral risk factors among adult cancer survivors and noncancer controls. Prev Med. 2005;40:702–11. doi: 10.1016/j.ypmed.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Bowen D, Jabson J, Haddock N, Hay J, Edwards K. Skin care behaviors among melanoma survivors. Psychooncology. 2012;21:1285–91. doi: 10.1002/pon.2017. [DOI] [PubMed] [Google Scholar]
- 15.Manne S, Lessin S. Prevalence and correlates of sun protection and skin self-examination practices among cutaneous malignant melanoma survivors. J Behav Med. 2006;29:419–34. doi: 10.1007/s10865-006-9064-5. [DOI] [PubMed] [Google Scholar]
- 16.Mujumdar UJ, Hay JL, Monroe–Hinds YC, et al. Sun protection and skin self-examination in melanoma survivors. Psychooncology. 2009;18:1106–15. doi: 10.1002/pon.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayer D, Layman A, Carlson J. Sun-protection behaviors of melanoma survivors. J Am Acad Dermatol. 2012;66:e9–10. doi: 10.1016/j.jaad.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buchanan N, Leisenring W, Mitby PA, et al. Behaviors associated with ultraviolet radiation exposure in a cohort of adult survivors of childhood and adolescent cancer: a report from the Childhood Cancer Survivor Study. Cancer. 2009;155(suppl):4374–84. doi: 10.1002/cncr.24581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zwener EK, Mahler HI, Werchniak AE, Recklitis CJ. Sun exposure in young adult cancer survivors on and off the beach: results from Project reach. J Cancer Surviv. 2012;6:63–71. doi: 10.1007/s11764-011-0201-y. [DOI] [PubMed] [Google Scholar]
- 20.Cartmel B, Ferrucci LM, Spain P, et al. Indoor tanning and tanning dependence in young people after a diagnosis of basal cell carcinoma. JAMA Dermatol. 2013;149:1110–11. doi: 10.1001/jamadermatol.2013.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snyder CF, Earle CC, Herbert RJ, Neville BA, Blackford AL, Frick KD. Trends in follow up and preventive care for colorectal cancer survivors. J Gen Intern Med. 2008;23:254–9. doi: 10.1007/s11606-007-0497-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snyder CF, Frick KD, Peairs KS, et al. Comparing care for breast cancer survivors to non-cancer controls: a five year longitudinal study. J Gen Intern Med. 2009;24:469–74. doi: 10.1007/s11606-009-0903-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denmark–Wahnefried W, Peterson B, McBride C, Lipkus I, Clipp E. Current health behaviors and readiness to pursue life-style changes among men and women diagnosed with early stage prostate and breast carcinomas. Cancer. 2000;88:674–84. doi: 10.1002/(SICI)1097-0142(20000201)88:3<674::AID-CNCR26>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 24.Oliveria SA, Shuk E, Hay JL, et al. Melanoma survivors: health behaviors, surveillance, psychosocial factors, and family concerns. Psychooncology. 2013;22:106–16. doi: 10.1002/pon.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinto BM, Maruyama NC, Clark MM, Cruess DG, Park E, Roberts M. Motivation to modify lifestyle risk behaviors in women treated for breast cancer. Mayo Clin Proc. 2002;77:122–9. doi: 10.1016/S0025-6196(11)62326-4. [DOI] [PubMed] [Google Scholar]
- 26.Bourke L, Thompson G, Gibson DJ, et al. Pragmatic lifestyle intervention in patients recovering from colon cancer: a randomized controlled pilot study. Arch Phys Med Rehabil. 2011;92:749–55. doi: 10.1016/j.apmr.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 27.O’Riordan DL, Lunde KB, Steffen AD, Maddock JE. Validity of beachgoers’ self-report of their sun habits. Arch Dermatol. 2006;142:1304–11. doi: 10.1001/archderm.142.10.1304. [DOI] [PubMed] [Google Scholar]


