Abstract
Introduction
We set out to evaluate the prognostic value of 18F-fluorodeoxyglucose positron-emission tomography (pet) in patients with advanced (non-transplant-eligible) hepatocellular carcinoma (hcc) and to evaluate the correlation between standardized uptake values (suvs) and survival outcomes.
Methods
We identified patients with hcc who, from 2005 to 2013, underwent pet imaging before any treatment. This retrospective study from our hcc database obtained complete follow-up data for the 63 identified patients.
Results
Of the 63 patients, 10 underwent surgical resection, and 59 underwent locoregional therapy. In this cohort, 28 patients were pet-positive (defined as any lesion with a suv ≥ 4.0) before any therapy was given, and 35 patients were pet negative (all lesions with a suv < 4.0). On survival analysis, median survival was greater for the pet-negative than for the pet-positive patients: 29 months (range: 16.3–41.1 months) versus 12 months (range: 4.0–22.1 months) respectively, p = 0.0241. The pet-positive patients more often had large tumours (≥5 cm), poor differentiation, and extrahepatic disease, reflecting more aggressive tumours. On multivariate analysis, only pet positivity was associated with poor survival (p = 0.049).
Conclusions
Compared with pet-positive patients, pet-negative patients with hcc experienced longer survival. Imaging by pet can be of value in early prognostication for patients with hcc, especially patients receiving locoregional therapy for whom pathologic tumour differentiation is rarely available. This potential role for pet requires further validation in a prospective study.
Keywords: Positron-emission tomography, hepatocellular carcinoma, prognosis, outcomes
1. INTRODUCTION
Hepatocellular carcinoma (hcc) is the 6th most common cause of cancer worldwide, and the 3rd most common cause of cancer-related death1,2. The combination of disease burden and hepatic functional reserve dictate therapy, which can include liver transplantation, surgical resection, locoregional therapy, or treatment with targeted agents. In selected patients, local therapies such as transarterial chemoembolization and radiofrequency or microwave ablation are associated with a 5-year survival of up to 50% in Child–Pugh class A patients3. Similarly, the 5-year overall survival can be up to 50% after liver resection and 70% after liver transplantation3.
Several prognostic factors have been associated with poor survival outcomes after hcc resection—notably, the absence of a tumour capsule, preoperative alpha-fetoprotein levels exceeding 10,000 ng/mL, microvascular invasion, and poor histologic grade4. Except for alpha-fetoprotein, which fell out of favour as a true prognostic indicator because of its low sensitivity5, most of the foregoing factors are assessable only after resection. The current limitation of anatomic imaging for prognostication is its reliance on surrogate indicators of tumour activity such as size, number of lesions, or volume; the correlation between those factors and tumour behaviour is not sufficient to direct therapy in all patients.
Positron-emission tomography with 18F-fluorodeoxyglucose (fdg-pet) is increasingly being used in the field of oncology to aid in determining disease stage and prognosis for a variety of tumours6. Lower sensitivity initially limited the use of fdg-pet in diagnosing hcc, and although the technique is not part of any treatment guideline3,7, some evidence has emerged to support a relation between fdg-pet−positive tumours and their degree of differentiation. In a series of patients who underwent surgical resection for hcc, preoperative fdg-pet imaging results were associated with tumour differentiation and appeared to predict outcomes such as tumour recurrence and patient survival8.
There might be a role for fdg-pet in patients with advanced hcc treated with local therapy. In patients receiving concurrent chemoradiotherapy, pet-positive imaging was associated with disease progression, the likelihood of the development of extrahepatic metastases, and poorer survival9. In an another study by Huang et al.10, tumour control after stereotactic ablative radiotherapy was also predicted by the combination of fdg-pet signal and tumour volume on contrast-enhanced computed tomography (ct) images. The degree of differentiation of hcc cells seems to be closely related to the enzymatic activity of glucose 6-phosphatase, which converts fluorodeoxyglucose-6-phosphate to fluorodeoxyglucose. Well-differentiated tumours therefore show glucose 6-phosphatase activity similar to that of normal liver tissue, and thus less fdg accumulation and a lower suv; poorly differentiated tumours have a higher suv11.
Few data are available on the utility of fdg-pet as a prognostic factor for patients with advanced non-transplant-eligible hcc12, especially those receiving local ablative therapies, in whom pathologic tumour differentiation is rarely known. In the present study, we set out to determine the value and utility of fdg-pet as a prognostic tool for the management of patients with advanced hcc.
2. METHODS
2.1. Patients
The study included patients with hcc followed in our hcc clinic who, between 2005 and 2013, underwent fdg-pet imaging. The study excluded patients who received any treatment before their fdg-pet imaging and patients who ultimately underwent transplantation. The diagnosis of hcc was made based on criteria set out in the guideline of the American Association for the Study of Liver Diseases3. The modalities used to treat the tumour were decided by the treating physician and tumour board recommendations, and were based on current guidelines from the American Association for the Study of Liver Diseases. Demographic, clinical, laboratory, and pathology data were collected from our prospective hcc database. Survival outcomes were determined using data from the hcc clinic and from the ramq (Régie de l’assurance maladie du Québec). Approval to conduct this retrospective study was obtained from our institutional research ethics board.
2.2. FDG-PET
All patients fasted for at least 6 hours before their fdg-pet imaging. All examinations were performed using a hybrid pet-ct scanner (Discovery ST: General Electric Medical Systems, Waukesha, WI, U.S.A.). Scanning was initiated about 1 hour after administration of fluorodeoxyglucose. Noncontrast ct imaging was performed for attenuation correction and localization purposes. The ct and pet images from the neck to the proximal thighs were obtained using a spatial resolution of 5.3 mm in the centre of the field of view. Data were acquired 3-dimensionally (sagittal, transaxial, and coronal planes) after intravenous administration of the fdg. The dose of fdg ranged from 370 MBq to 740 MBq and was calculated by patient weight (7.5 MBq/kg). The resulting images were reconstructed using a ordered-subset expectation-maximization iterative algorithm.
Nuclear medicine specialists read the fdg-pet images and measured suvs. More precisely, maximal suvs were evaluated using a region-of-interest tool and a search for the most intense voxel for a given lesion on the ct images. For the present study, an imaging report with a suv of 4.0 or greater for any lesion was considered positive (pet+); the 4.0 cutoff was based on a previous report13. Conversely, a result showing suvs of less than 4.0 for all lesions was categorized as negative (pet−). When multiple tumours were present, the tumour nodule with the highest suv was recorded.
2.3. Statistical Analysis
Chi-square tests were used for categorical data, and t-tests or Mann–Whitney U-tests were used, as appropriate, for continuous data. Overall survival was calculated from the time of diagnosis until the date of last follow-up or death. Univariate and multivariate analyses were performed. Survival curves were calculated using the Kaplan–Meier method, and differences were examined using the log-rank test. A difference was considered significant at p ≤ 0.05. All statistical calculations were performed using the JMP software application (version 10: SAS Institute, Cary, NC, U.S.A.).
3. RESULTS
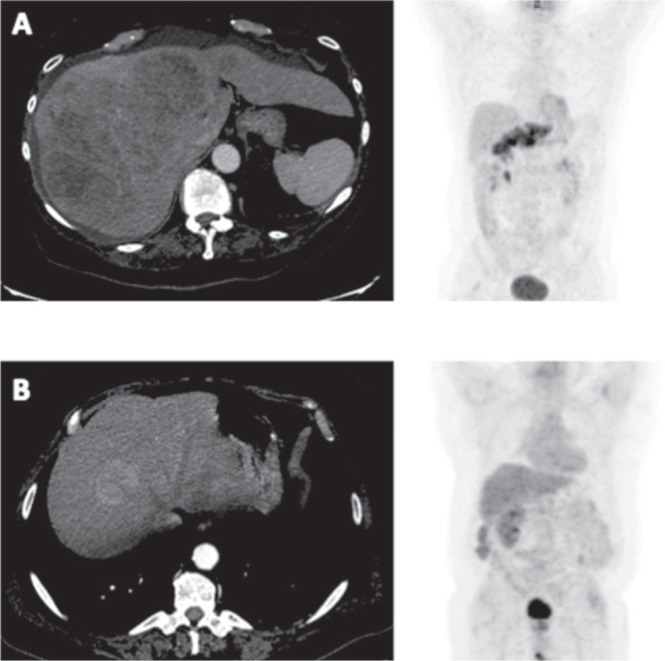
Of 234 hcc patients who did not undergo transplantation between 2005 and 2013, 63 underwent fdg-pet imaging before receiving any therapy and so were included in the present study. Of those 63 patients, 53 underwent one or more types of locoregional treatment, and 10 received surgical resection in addition to local therapy. Baseline clinical characteristics are described for the overall patient population and compared by suv group (Table i). The main cause of liver disease overall was hepatitis C virus (27%, n = 17). More than half the patients (55.5%) were pet−. The suv subgroups were similar in terms of the causes of liver disease (hepatitis C virus being the most common). Moreover, the distribution of local therapies was similar in the two groups, except that a larger proportion of patients in the pet+ group received sorafenib. In terms of tumour characteristics, the distribution of the number of lesions was similar in the two groups; however, in the pet+ group, more patients had lesions 5 cm or larger in size, poorly differentiated tumours, and extrahepatic disease (Table ii). Figure 1 shows examples of computed tomography and corresponding fdg-pet images for pet+ and pet− patients.
TABLE I.
Demographics and clinical characteristics of the patients, overall and by standardized uptake value (suv) subgroup
| Variable |
Patient group
|
p Valuea | ||
|---|---|---|---|---|
| Overall | suv≥4.0 | suv<4.0 | ||
| Patients (n) | 63 | 28 | 35 | |
| Mean age (years) | 66±11.6 | 64.2±2.1 | 66.8±2.1 | 0.3838 |
| Sex [n (%) men] | 48 (76.2) | 22 (78.6) | 26 (82.3) | 0.7718 |
| Child–Pugh class [n (%)] | ||||
| A | 48 (76.2) | 24 (85.7) | 24 (68.6) | 1.0 |
| B | 13 (20.6) | 4 (14.3) | 9 (25.7) | 0.3534 |
| C | 2 (3.2) | 0 | 2 (5.7) | 0.1223 |
| Cause of liver disease [n (%)] | ||||
| Hepatitis C virus | 17 (27.0) | 11 (39.3) | 6 (17.1) | 0.0850 |
| Hepatitis B virus | 13 (20.6) | 9 (32.1) | 4 (11.4) | 0.0615 |
| nash | 6 (9.5) | 1 (3.6) | 5 (14.3) | 0.2138 |
| Alcohol | 4 (6.3) | 1 (3.6) | 3 (8.6) | 0.6224 |
| Other | 23 (36.5) | 3 (10.7) | 10 (28.6) | 0.1190 |
| Unknown | 10 (15.9) | 3 (10.7) | 7 (20.0) | 0.4903 |
| History of therapyb [n (%)] | ||||
| tace or tae | 20 (31.7) | 8 (22.9) | 12 (34.3) | 0.4004 |
| rfa or mwa | 12 (19.0) | 3 (10.7) | 9 (25.7) | 0.1985 |
| 90Y Glass microspheres | 13 (20.6) | 8 (22.9) | 5 (14.3) | 0.2153 |
| External-beam rt | 2 (3.2) | 0 | 2 (5.7) | 1.0000 |
| Sorafenib | 19 (30.2) | 13 (46.4) | 6 (17.1) | 0.0149 |
| Resected patients [n (%)] | 10 (15.9) | 5 (17.9) | 5 (14.3) | 0.7400 |
| Extrahepatic disease [n (%)] | 9 (14.3) | 8 (28.6) | 1 (2.9) | 0.0079 |
Boldface type indicates significance.
Includes overlapping treatments.
nash = non-alcoholic steatohepatitis; tace = transarterial chemoembolization; tae = transarterial embolization (bland); rfa = radiofrequency ablation; mwa = microwave ablation; rt = radiation therapy.
TABLE II.
Tumour and pathology characteristics by standardized uptake value (suv) subgroup
| Variable |
Patient group
|
p Valuea | |
|---|---|---|---|
| suv≥4.0 (n=28) | suv<4.0 (n=35) | ||
| Lesion [n (%)] | |||
| Size | |||
| <5 cm | 13 (46.4) | 28 (80.0) | 0.0079 |
| ≥5 cm | 15 (53.6) | 7 (20.0) | |
| Number | |||
| 1 | 18 (64.3) | 17 (48.6) | 0.3078 |
| 2 | 4 (14.2) | 7 (20.0) | 0.7409 |
| 3 | 4 (14.2) | 6 (17.1) | 1.0 |
| ≥4 | 2 (7.1) | 5 (14.3) | 0.4478 |
| Tumour differentiation b | |||
| Good | 4 (25.0) | 9 (69.2) | 0.0130 |
| Moderate | 5 (31.2) | 3 (23.1) | 0.6968 |
| Poor | 7 (43.8) | 1 (7.7) | 0.0443 |
| Alpha-fetoprotein (ng/mL) at time of imaging | |||
| Median | 84 | 8 | 0.1587 |
| Interquartile range | 24–1416 | 4–190 | |
Boldface type indicates significance.
Based on available samples (16 for suv≥4.0, 13 for suv<4.0).
Figure 1.
Examples of computed tomography (ct) images (left panels) and their corresponding 18F–fluorodeoxyglucose (fdg) positron-emission tomography (pet) images (right panels) for studies with a standardized uptake value of (A) 4.0 or greater and (B) less than 4.0.
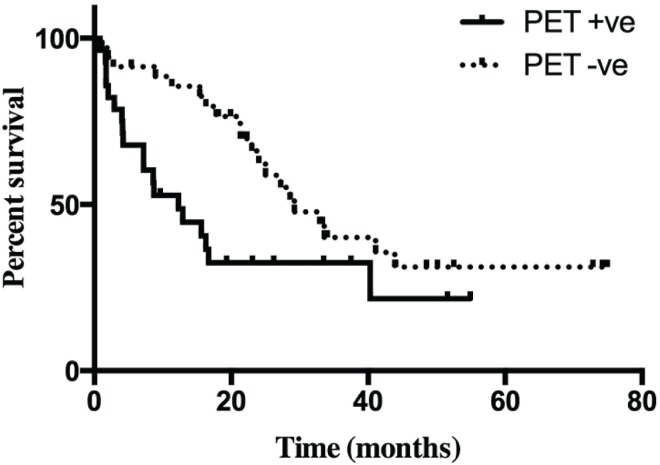
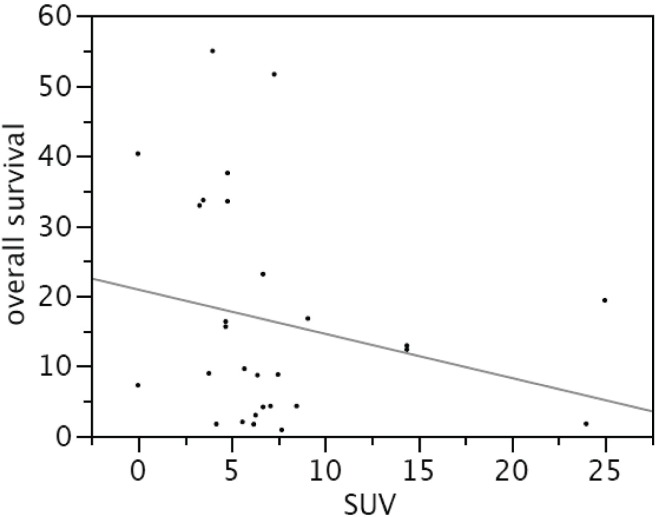
The Kaplan–Meier survival curve (Figure 2) demonstrates better median survival in the pet− than in the pet+ patients: 29 months (range: 16.3–41.1 months) versus 12 months (range: 4.0–22.1 months) respectively, p = 0.0215. Interestingly, we observed a significant inverse correlation between overall survival and suv (p = 0.0002, Figure 3). Although many variables—such as pet+, poor tumour differentiation, tumour size, major vascular invasion, presence of extrahepatic disease, and Child–Pugh score—were associated with poorer survival on univariate analysis, only positivity on fdg-pet imaging (suv ≥ 4.0) was shown to be significantly associated with worse survival on multivariate analysis (p = 0.049, Table iii).
Figure 2.
Kaplan–Meier survival curves by use of positron-emission tomography (pet). pet +ve = patients whose pet imaging showed at least one lesion with a standardized uptake value (suv) of 4.0 or greater; pet −ve = patients whose pet imaging showed no lesion with a suv of 4.0 or greater.
Figure 3.
Correlation between overall survival and standardized uptake value (suv).
TABLE III.
Univariate and multivariate analyses of variables significantly associated with poor survival in 63 patients
| Variable | p Value
|
|
|---|---|---|
| Univariate | Multivariate | |
| suv≥4.0 | 0.003 | 0.049 |
| Poorly differentiated tumour | 0.001 | |
| Age | <0.001 | |
| Extrahepatic disease | 0.001 | |
| Major vascular invasion | 0.016 | |
| Lesion size ≥5 cm | 0.024 | |
| Child-Pugh class A | 0.036 | |
| Child-Pugh class B | 0.048 | |
4. DISCUSSION
The spectrum of hcc treatment includes resection, liver transplantation, local ablation, and systemic cytotoxic or targeted therapy. Each modality has indications and limitations determined by the location, number, and size of the lesions and the cause of the cancer and the underlying liver function.
Survival varies with the treatment group, which itself is dictated by the nature of the disease. For example, in advanced-stage disease, the 5-year survival rate falls into the 5%–8% range without treatment. Vascular invasion and extrahepatic involvement are defining features of advanced disease and poor outcome14–16. On the other hand, in early hcc, transplantation can result in a 4-year survival rate of 75%17. However, in addition to size and multicentricity, microvascular invasion and poor differentiation are associated with treatment failure in early-stage disease18,19. Staging systems for hcc are numerous, and none is clearly superior, reflecting incomplete understanding of the tumour’s biology. Moreover, some of the prognostic factors can be identified only after a complete pathology examination of the liver and so have not been used to screen patients preoperatively18–20. Better initial prognostic information could help to determine the most favourable treatment course.
Preliminary evidence has shown that traditional pet imaging can distinguish the grade of the tumour21, predict microvascular invasion22, diagnose recurrence after radiofrequency ablation23, and diagnose extrahepatic metastases24–26. It might also help in assessing systemic treatment response27. Although a limitation of the technique is its low sensitivity (50%–55%), that limitation seems to apply to well-differentiated tumours21. The use of fdg-pet has been found to be highly sensitive in diagnosing extrahepatic hcc metastasis, likely because the metastatic lesions are often poorly differentiated23,24.
We hypothesized that the signal intensity (suv) of hepatomas on fdg-pet might potentially correlate with more aggressive behaviour and, subsequently, with poor outcome. Our results revealed a significant survival difference according to pet positivity in patients undergoing locoregional therapy. Our comparisons of the two suv groups (pet+ vs. pet−) showed that more patients with positive fdg-pet scans had lesions 5 cm or larger in size, poorly differentiated tumours, and extrahepatic disease. All of the foregoing characteristics reflect more aggressive tumours. The larger proportion of pet+ patients receiving sorafenib might be attributable to the greater presence of extrahepatic disease in that group, which would be expected, given that a proportion of sorafenib-treated patients have extra-hepatic disease. However, after controlling for the associated clinical factors, only positive fdg-pet imaging seemed to be predictive of poor outcome.
Not all patients undergo liver biopsy, and for patients not eligible for surgical resection, adjunct noninvasive methods are needed for prognostication. Although fdg-pet would not be a good tool for staging hcc patients (because up to 60% will not show fdg uptake), our data suggest that fdg-pet positivity might predict outcome in patients with hcc regardless of other clinical factors such as number of lesions or treatment modality. That is to say, patients with a similar tumour burden, for whom the same treatment is planned, might be able to be prognosticated with the addition of fdg-pet. However, given the study’s main limitations, which are related to the small sample size and the retrospective nature of the analysis, our findings cannot yet be generalized. Larger-scale prospective studies are needed to confirm the validity of our findings and to potentially bring this imaging modality into clinical practice.
Because fdg-pet is noninvasive and, in patients with multiple lesions, can describe all the tumour nodules (unlike a biopsy, which usually samples just one lesion and is invasive), it could potentially provide additional information to help in patient management. If confirmed in larger cohorts of patients, fdg-pet could, to that end, be inserted into treatment algorithms.
5. ACKNOWLEDGMENTS
A Pfizer grant (WS1234469) supported the present work, which was also registered and approved by our institutional research ethics board.
This manuscript is based on an abstract titled “The utility of position emission tomography in hepatocellular carcinoma,” presented at the Americas Hepato-Pancreato-Biliary Association annual meeting in Miami, Florida, U.S.A., March 2012, and at the International Hepato-Pancreato-Biliary Association meeting in Paris, France, July 2012.
6. CONFLICT OF INTEREST DISCLOSURES
The authors have no financial conflicts of interest to declare.
7. REFERENCES
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [Erratum in: CA Cancer J Clin 2011;61:134] [DOI] [PubMed] [Google Scholar]
- 2.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–91. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruix J, Sherman M, on behalf of the Practice Guidelines Committee, American Association for the Study of Liver Diseases Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–36. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 4.Poon RT, Ng IO, Fan ST, et al. Clinicopathologic features of long-term survivors and disease-free survivors after resection of hepatocellular carcinoma: a study of a prospective cohort. J Clin Oncol. 2001;19:3037–44. doi: 10.1200/JCO.2001.19.12.3037. [DOI] [PubMed] [Google Scholar]
- 5.Paul SB, Gulati MS, Sreenivas V, et al. Evaluating patients with cirrhosis for hepatocellular carcinoma: value of clinical symptomatology, imaging and alpha-fetoprotein. Oncology. 2007;72(suppl 1):117–23. doi: 10.1159/000111717. [DOI] [PubMed] [Google Scholar]
- 6.Bomanji JB, Costa DC, Ell PJ. Clinical role of positron emission tomography in oncology. Lancet Oncol. 2001;2:157–64. doi: 10.1016/S1470-2045(00)00257-6. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network (nccn) NCCN Clinical Practice Guidelines in Oncology: Hepatobiliary Cancers. Fort Washington, PA: NCCN; 2012. Ver. 2.2012. [Current version available online at: http://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf (free registration required); cited January 13, 2014] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seo S, Hatano E, Higashi T, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography predicts tumor differentiation, P-glycoprotein expression, and outcome after resection in hepatocellular carcinoma. Clin Cancer Res. 2007;13:427–33. doi: 10.1158/1078-0432.CCR-06-1357. [DOI] [PubMed] [Google Scholar]
- 9.Kim BK, Kang WJ, Kim JK, et al. 18F-fluorodeoxyglucose uptake on positron emission tomography as a prognostic predictor in locally advanced hepatocellular carcinoma. Cancer. 2011;117:4779–87. doi: 10.1002/cncr.26099. [DOI] [PubMed] [Google Scholar]
- 10.Huang WY, Kao CH, Huang WS, et al. 18F-fdg pet and combined 18F-fdg-contrast ct parameters as predictors of tumor control for hepatocellular carcinoma after stereotactic ablative radiotherapy. J Nucl Med. 2013;54:1710–16. doi: 10.2967/jnumed.112.119370. [DOI] [PubMed] [Google Scholar]
- 11.Torizuka T, Tamaki N, Inokuma T, et al. In vivo assessment of glucose metabolism in hepatocellular carcinoma with fdg-pet. J Nucl Med. 1995;36:1811–17. [PubMed] [Google Scholar]
- 12.Pant V, Sen IB, Soin AS. Role of 18F-fdg pet ct as an independent prognostic indicator in patients with hepatocellular carcinoma. Nucl Med Commun. 2013;34:749–57. doi: 10.1097/MNM.0b013e3283622eef. [DOI] [PubMed] [Google Scholar]
- 13.Ahn SG, Kim SH, Jeon TJ, et al. The role of preoperative [18F] fluorodeoxyglucose positron emission tomography in predicting early recurrence after curative resection of hepatocellular carcinomas. J Gastrointest Surg. 2011;15:2044–52. doi: 10.1007/s11605-011-1660-1. [DOI] [PubMed] [Google Scholar]
- 14.Llovet JM, Bustamante J, Castells A, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62–7. doi: 10.1002/hep.510290145. [DOI] [PubMed] [Google Scholar]
- 15.Imamura H, Matsuyama Y, Tanaka E, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200–7. doi: 10.1016/S0168-8278(02)00360-4. [DOI] [PubMed] [Google Scholar]
- 16.Wayne JD, Lauwers GY, Ikai I, et al. Preoperative predictors of survival after resection of small hepatocellular carcinomas. Ann Surg. 2002;235:722–30. doi: 10.1097/00000658-200205000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–9. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 18.Zavaglia C, De Carlis L, Alberti AB, et al. Predictors of long-term survival after liver transplantation for hepatocellular carcinoma. Am J Gastroenterol. 2005;100:2708–16. doi: 10.1111/j.1572-0241.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- 19.Poon RT, Ng IO, Fan ST, et al. Clinicopathologic features of long-term survivors and disease-free survivors after resection of hepatocellular carcinoma: a study of a prospective cohort. J Clin Oncol. 2001;19:3037–44. doi: 10.1200/JCO.2001.19.12.3037. [DOI] [PubMed] [Google Scholar]
- 20.Mazzaferro V, Llovet JM, Miceli R, et al. on behalf of the Metroticket Investigator Study Group Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35–43. doi: 10.1016/S1470-2045(08)70284-5. [DOI] [PubMed] [Google Scholar]
- 21.Khan MA, Combs CS, Brunt EM, et al. Positron emission tomography scanning in the evaluation of hepatocellular carcinoma. J Hepatol. 2000;32:792–7. doi: 10.1016/S0168-8278(00)80248-2. [DOI] [PubMed] [Google Scholar]
- 22.Kornberg A, Freesmeyer M, Bärthel E, et al. 18F-fdg-uptake of hepatocellular carcinoma on pet predicts microvascular tumor invasion in liver transplant patients. Am J Transplant. 2009;9:592–600. doi: 10.1111/j.1600-6143.2008.02516.x. [Erratum in: Am J Transplant 2009;9:1255] [DOI] [PubMed] [Google Scholar]
- 23.Paudyal B, Oriuchi N, Paudyal P, et al. Early diagnosis of recurrent hepatocellular carcinoma with 18F-fdg pet after radiofrequency ablation therapy. Oncol Rep. 2007;18:1469–73. [PubMed] [Google Scholar]
- 24.Sugiyama M, Sakahara H, Torizuka T, et al. 18F-fdg pet in the detection of extrahepatic metastases from hepatocellular carcinoma. J Gastroenterol. 2004;39:961–8. doi: 10.1007/s00535-004-1427-5. [DOI] [PubMed] [Google Scholar]
- 25.Nagaoka S, Itano S, Ishibashi M, et al. Value of fusing pet plus ct images in hepatocellular carcinoma and combined hepatocellular and cholangiocarcinoma patients with extrahepatic metastases: preliminary findings. Liver Int. 2006;26:781–8. doi: 10.1111/j.1478-3231.2006.01296.x. [DOI] [PubMed] [Google Scholar]
- 26.Hain SF, Fogelman I. Recent advances in imaging hepatocellular carcinoma: diagnosis, staging and response assessment: functional imaging. Cancer J. 2004;10:121–7. doi: 10.1097/00130404-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Siemerink EJ, Mulder NH, Brouwers AH, Hospers GA. 18F-Fluorodeoxyglucose positron emission tomography for monitoring response to sorafenib treatment in patients with hepatocellular carcinoma. Oncologist. 2008;13:734–5. doi: 10.1634/theoncologist.2008-0063. [DOI] [PubMed] [Google Scholar]