Abstract
Background
Almost 40% of people diagnosed with colorectal cancer will die from their disease, most with metastatic spread. When feasible, hepatic resection offers the greatest probability of cure for isolated liver metastases, but there are barriers to curative resection. Those barriers include the extent and distribution of lesions within the liver, extrahepatic disease, comorbidities, and age. Chemotherapy is often administered before or after resection with the intention of improving disease-free and overall survival. The timing of chemotherapy (adjuvant vs. neoadjuvant vs. perioperative) for patients undergoing potentially curative hepatic resection of metastasis of colorectal cancer origin is controversial.
Methods
Colorectal cancer patients with liver metastases resected at The Ottawa Hospital between January 1, 2003, and December 31, 2009, were identified, and their clinical records were retrospectively reviewed. Patients receiving intraoperative radiofrequency ablation (rfa) as part of their management were included. Factors associated with overall and disease-free survival were evaluated.
Results
The 168 identified patients (57% men, 43% women) had a median age of 63 years (range: 31–84 years). After hepatectomy, 10% had positive resection margins. Intraoperative rfa was used in 25 patients (15%). Chemotherapy was administered in the neoadjuvant (19%), adjuvant (31%), or “perioperative” (both neoadjuvant and adjuvant, 50%) setting. Use or omission of intraoperative rfa was not associated with a difference in overall survival (hazard ratio: 0.99; 95% confidence interval: 0.53 to 1.84; p = 0.97).
Conclusions
Compared with patients who did not receive chemotherapy, those who received chemotherapy, regardless of timing, experienced improved overall survival and disease-free survival. Use of rfa where required as an adjunct to hepatic resection appears to be effective and is not associated with worse overall survival.
Keywords: Colorectal cancer, hepatic resection, timing of chemotherapy, radiofrequency ablation
1. INTRODUCTION
Of the estimated 23,800 Canadians diagnosed with colorectal cancer (crc) annually, approximately 9200 (40%) will die from their disease, most with distant metastatic spread1. When feasible, hepatic resection offers the greatest probability of cure for patients with isolated liver metastases2. However, even for those with disease that is largely liver-limited, there are barriers to curative resection such as the extent and distribution of lesions within the liver, extrahepatic disease, comorbidities, and age3.
The maldistribution of lesions within the liver, making complete excision of all disease impossible without the risk of subsequent liver insufficiency, is one barrier that has received considerable attention. Strategies include serial resection, portal vein embolization, and the adjuvant use of radiofrequency ablation (rfa). The rfa procedure uses heat derived from radiofrequency waves at the end of a probe inserted into a metastasis to induce tumour necrosis. The use of rfa as an adjunct to hepatic resection is gaining acceptance. However, its efficacy in comparison with resection is controversial because of high rates of recurrence at the ablation site in some studies4.
Despite the increasing opportunity for potentially curative hepatic resection, recurrence in resected patients is the most frequent outcome. Chemotherapy, either before or after resection, is therefore often used with the intention of improving disease-free (dfs) and overall survival (os). Chemotherapy is also used in patients who might initially have unresectable disease, but who can be converted to resectability after response to systemic treatment5,6. The timing of chemotherapy—adjuvant or perioperative (pre- and postoperative)—for patients undergoing potentially curative hepatic resection of metastasis originating from crc is controversial.
The present retrospective review was designed to assess the roles and relationships of systemic chemotherapy and surgical resection as treatments for liver-dominant or liver-limited metastatic crc. Primary objectives of the study included examination of the impact of chemotherapy on survival in patients with resected liver metastases, evaluation of the safety and impact of rfa on survival, and comparison of the impact of preoperative, perioperative, or postoperative chemotherapy on survival. Our goal was to assess these treatments for metastatic crc with regard to improving os. A secondary endpoint of the study was to determine the utility of rfa as an adjunct to surgical resection.
2. METHODS
2.1. Patients
After study approval by The Ottawa Hospital Research Ethics Board, crc patients with liver metastases resected at The Ottawa Hospital between January 1, 2003, and December 31, 2009, were identified, and their clinical records were retrospectively reviewed. Patients who underwent surgical resection of one or more liver metastases were included. Patients undergoing a noncurative-intent excision biopsy of a liver metastasis undertaken at the time of resection of the primary, but for diagnostic purposes only, were excluded. Patients with non-crc primary sites were also excluded.
Patient data were accessed using the institutional database, and patient demographics, disease characteristics, treatment descriptions, and survival outcomes were extracted. Pathology and operative reports were reviewed to determine margin status, lesion grade, and extent of resection. Margin status was reported as either positive or negative, with negative margins being defined as the absence of residual disease.
2.2. Outcomes Measures and Statistical Analysis
Primary outcome was os, defined from the date of diagnosis of liver metastasis to the date of death. Patients lost to follow-up were censored at the date they were last known to be alive. A secondary endpoint, dfs, was calculated from the date of surgical removal of liver metastases to the date of objective disease recurrence.
Survival was analyzed using the Kaplan–Meier method, with statistical significance assessed by log-rank testing. Hazard ratios (hrs) and confidence intervals were obtained at 95% significance. Cox regression analyses were used to develop univariate and multivariate models. The models were used to describe the association of independent variables with the primary outcomes. Variables that were analyzed in the models included age, sex, margin status, number of liver metastases, whether the metastases occurred synchronously or metachronously with the primary tumour, histologic grade, receipt of rfa, and receipt of any adjuvant chemotherapy, each as a binary variable. Chemotherapy was recorded as being received either before resection of the hepatic metastases, after resection, or perioperatively.
All statistical analyses were performed using the SAS software application (version 9.2. SAS Institute, Cary, NC, U.S.A.), and p < 0.05 was considered statistically significant.
3. RESULTS
Of the 225 patients who underwent liver surgery during the study period, 168 (56.6% men, 43.4% women) were eligible for the study. Table i presents the characteristics of the patients. Almost half the population (47%) had a solitary liver lesion, with the median being 2 lesions, and the maximum, 7 lesions. Timing of the development of the liver metastases was divided equally between metachronous and synchronous with the colorectal primary. Most patients had a unilobar topography of metastases. Simultaneous resection of the colorectal primary and liver in a single operation was accomplished in 40 patients (23.8%).
TABLE I.
Baseline demographic and clinical characteristics of the study patients
| Characteristic | Value |
|---|---|
| Patients (n) | 168 |
| Sex [n (%)] | |
| Men | 95 (56.6) |
| Women | 73 (43.4) |
| Age group [n (%)] | |
| <65 Years | 93 (57.1) |
| ≥65 Years | 70 (42.9) |
| Age (years) | |
| Median | 63 |
| Range | 31–84 |
| Preoperative cea group [n (%)] | |
| 0–4 ng/mL | 40 (23.8) |
| >4 ng/mL | 48 (28.6) |
| Missing | 80 (47.6) |
| Liver lesions | |
| Group [n (%)] | |
| 1 | 79 (47.0) |
| 2 | 38 (22.6) |
| 3 | 22 (13.1) |
| 4 | 10 (6.0) |
| >4 | 11 (6.5) |
| Missing | 8 (4.8) |
| Median (n) | 1 |
| Range (n) | 1–7 |
| Character [n (%)] | |
| Metachronous | 81 (48.2) |
| Synchronous | 87 (51.8) |
| Distribution [n (%)] | |
| Solitary | 79 (47.0) |
| Multiple | 81 (48.2) |
| Missing | 8 (4.8) |
| Topography [n (%)] | |
| Unilobar | 105 (62.5) |
| Bilobar | 58 (34.5) |
| Missing | 5 (3.0) |
| Survival status [n (%)] | |
| Alive | 92 (54.8) |
| Deceased | 76 (45.2) |
| Chemotherapy received [n (%)] | |
| Any adjuvant | 118 (70.2) |
| None | 47 (28.0) |
| Not reported | 3 (1.8) |
| Timing of adjuvant chemotherapy [n (%)] | |
| Preoperative | 22 (18.6) |
| Perioperative | 59 (50) |
| Postoperative | 37 (31.4) |
cea = carcinoembryonic antigen.
3.1. Surgery
The extent or types of hepatic resection were as follows: bisegmentectomy (n = 22), left hepatectomy (n = 21), left lateral segmentectomy (n = 29), right hepatectomy (n = 32), wedge resection (n = 19), segmentectomy (n = 32), and right posterior sectionectomy (n = 13). There were 53 major resections (31.5%) in which more than 2 segments were removed; the remaining 115 patients underwent minor resections (2 or fewer segments removed). Margin status was available for 167 of the patients, being negative in 150 (89.3%) and positive in 17 (10.1%). In addition to liver resection, 25 of the 168 patients (15%) also underwent intraoperative rfa.
Table ii sets out the surgical and pathology findings for the study group. Length of stay was a mean of 9 days and a median of 7 days (range: 1–49 days), with 29.2% of patients having a stay of 10 days or more. Two postoperative deaths (1.2%) occurred within 60 days of surgical resection. One patient experienced a tear of the inferior vena cava leading to coagulopathy and death within 24 hours. The second patient developed a subphrenic abscess complicated by sepsis and an upper gastrointestinal bleed, with death at 38 days. Neither of the patients had undergone rfa of their metastatic sites. Compared with a length of stay less than 10 days, a stay greater than 10 days was associated with a hr of 1.51 (95% confidence interval: 0.92 to 2.49, p = 0.09).
TABLE II.
Surgical and pathology outcomes
| Variable | Value |
|---|---|
| Combined resection (primary and liver) [n (%)] | |
| Yes | 35 (20.8) |
| No | 133 (79.2) |
| Histologic grade (primary) [n (%)] | |
| 1 | 46 (27.4) |
| 2 | 100 (59.5) |
| 3 | 12 (7.1) |
| X | 10 (6.0) |
| Nodal status (primary) [n (%)] | |
| 0 | 61 (36.3) |
| 1 | 96 (57.1) |
| X | 11 (6.5) |
| Positive nodes (n) | |
| Median | 1 |
| Liver metastases removed (n) | |
| Range | 1–7 |
| Intraoperative rfa [n (%)] | |
| Yes | 25 (14.9) |
| No | 142 (84.5) |
| Unknown | 1 (0.6) |
| Length of stay (days) | |
| Median | 7 |
| Range | 1–49 |
| Length-of-stay group [n (%)] | |
| <10 Days | 119 (70.8) |
| ≥10 Days | 49 (29.2) |
| Resection margin status (liver) | |
| Negative | 150 (89.3) |
| Positive | 17 (10.1) |
| Unknown | 1 (0.6) |
rfa = radiofrequency ablation.
3.2. Chemotherapy
Chemotherapy was administered to 118 patients (70.2%) as an adjunct to hepatic resection. Chemotherapy was administered preoperatively in 22 of those patients (18.6%), perioperatively (pre- and postoperatively) in 59 (50%), and postoperatively in 37 (31.4%). Preoperative chemotherapy regimens included folfox (leucovorin, 5-fluorouracil, oxaliplatin) or xelox (capecitabine, oxaliplatin) in 45.3%, folfiri (leucovorin, 5-fluorouracil, irinotecan) in 27.4%, and others in 15.4%. Postoperative regimens included folfox or xelox (57.2%), folfiri (32.7%), and others (23.2%). Bevacizumab was a component of the preoperative regimen in 29 patients (35.8%) and of the postoperative regimen in 22 patients (22.9%). The median number of preoperative cycles was 8 for folfox, 6 for folfiri, and 6 for capecitabine-based therapy. The median number of postoperative cycles was 6 for folfox, 8 for folfiri, and 4 for capecitabine. The lack of chemotherapy in the period before or after surgery for the other 50 patients (29.8%) was observed to be related to either a patient or a physician decision (or both) not to pursue that option.
3.3. OS
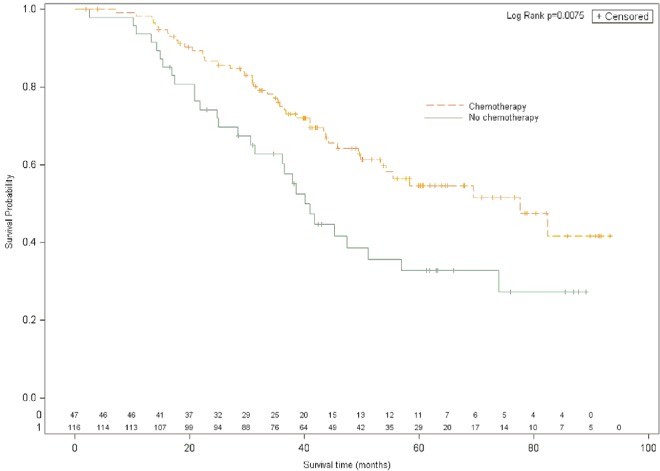
Compared with no use of chemotherapy, administration of any type of chemotherapy as an adjunct to surgery was associated with a statistically significant improvement in os. Median os duration was 77.6 months with chemotherapy and 40.9 months without chemotherapy (hr: 0.53; 95% confidence interval: 0.33 to 0.85; p = 0.0075; Figure 1). That finding was confirmed in multivariate analysis, in which receipt of any type of chemotherapy was associated with a hr of 0.61 (95% confidence interval: 0.37 to 0.99; p = 0.049) after adjustment for age, sex, baseline number of metastases, metachronous or synchronous status, resection margin status, and length of stay after resection (Table iii). Compared with the 50 patients who did not receive any chemotherapy in the period before or after surgery (os duration of 40.9 months), the patients treated with preoperative chemotherapy only (excluding patients receiving perioperative treatment) had a median os duration of 53.3 months and a hr for os of 0.72 (95% confidence interval: 0.33 to 1.58; p = 0.42). Compared with no chemotherapy, receipt of perioperative chemotherapy was also associated with improved os duration (median: 77.6 months; hr: 0.48; 95% confidence interval: 0.28 to 0.84; p = 0.0098), as was receipt of postoperative chemotherapy (median os: not reached; hr: 0.51; 95% confidence interval: 0.27 to 0.96; p < 0.05; Table iv). Overall, preoperative use of the various adjuvant and neoadjuvant chemotherapy approaches did not significantly differ from their perioperative (p = 0.67) or postoperative use (p = 0.70).
Figure 1.
Overall survival for patients with resected liver metastases treated with (any) chemotherapy or without chemotherapy.
TABLE III.
Multivariate analysis for overall survival
| Variable | Comparator | Hazard ratio |
95% Confidence limits
|
p Value | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Adjuvant chemotherapy received (any) | No chemotherapy | 0.61 | 0.37 | 0.99 | 0.049 |
| Age < 65 years | ≥65 years | 0.67 | 0.40 | 1.11 | 0.12 |
| Male sex | Female sex | 0.82 | 0.51 | 1.33 | 0.43 |
| Intraoperative rfa | No rfa | 0.86 | 0.44 | 1.67 | 0.65 |
| Negative margin status (R0) | R1 status | 0.45 | 0.10 | 0.98 | 0.046 |
| Metachronous | Synchronous | 0.57 | 0.31 | 1.05 | 0.071 |
| Baseline number of metastases | — | 1.41 | 0.88 | 2.25 | 0.19 |
| Length of stay (continuous) | — | 1.04 | 0.99 | 1.11 | 0.13 |
rfa = radiofrequency ablation.
TABLE IV.
Hazard ratios and 95% confidence intervals for overall and disease-free survival
| Chemotherapy status | Overall survival | Disease-free survival | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Months | hr | 95% ci | p Value | Months | hr | 95% ci | p Value | |
| None | 40.9 | Reference | 0.005 | 8.2 | Reference | 0.04 | ||
| Preoperative | 53.3 | 0.81 | 0.4 to 1.7 | 6.5 | 1.23 | 0.69 to 2.19 | ||
| Perioperative | 77.6 | 0.49 | 0.29 to 0.85 | 12.7 | 0.99 | 0.65 to 1.53 | ||
| Postoperative | Not reached | 0.5 | 0.27 to 0.95 | 27.7 | 0.45 | 0.26 to 0.78 | ||
hr = hazard ratio; ci = confidence interval.
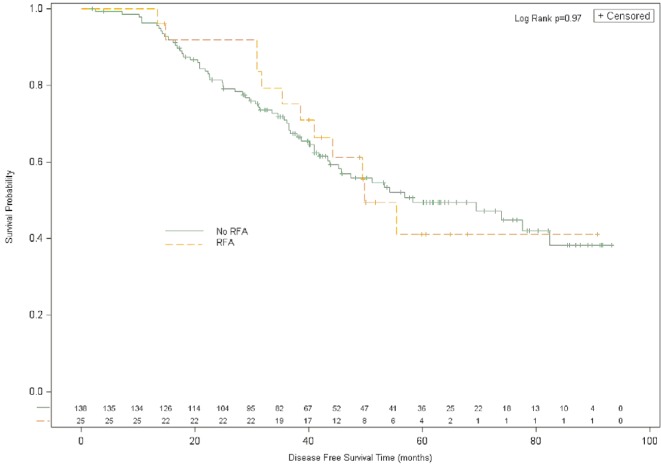
Overall survival was not significantly different in patients treated with or without rfa. Median os duration was 49.8 months for rfa and 58.4 months for no rfa (hr: 0.99; 95% confidence interval: 0.53 to 1.84; p = 0.97; Figure 2). After adjustment for important baseline factors, rfa was not found to be independently associated with improved os (Table iii).
Figure 2.
Overall survival for patients with resected liver metastases treated with or without intraoperative radiofrequency ablation (rfa).
3.4. DFS
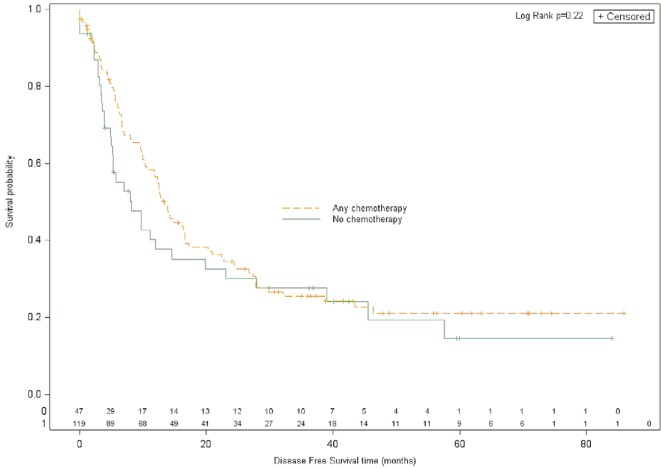
Median dfs was 13.3 months for patients who received any chemotherapy (n = 118) and 8.2 months for those who received no chemotherapy in the perioperative period (n = 50), for a hr of 0.81 (95% confidence interval: 0.54 to 1.20; p = 0.22; Figure 3). When analyzed specifically with respect to the timing of chemotherapy delivery, and compared with the patients who received no chemotherapy in the period around the time of surgery, patients who received chemotherapy in the preoperative setting had the shortest median dfs duration (6.5 months; hr: 1.23; 95% confidence interval: 0.69 to 2.19; p = 0.44). Patients treated perioperatively had a median dfs duration of 12.7 months (hr: 0.99; 95% confidence interval: 0.65 to 1.53), and patients treated postoperatively had a median dfs duration of 27.7 months (hr: 0.45; 95% confidence interval: 0.26 to 0.78; Table iii).
Figure 3.
Disease-free survival for patients with resected liver metastases treated with (any) chemotherapy or without chemotherapy.
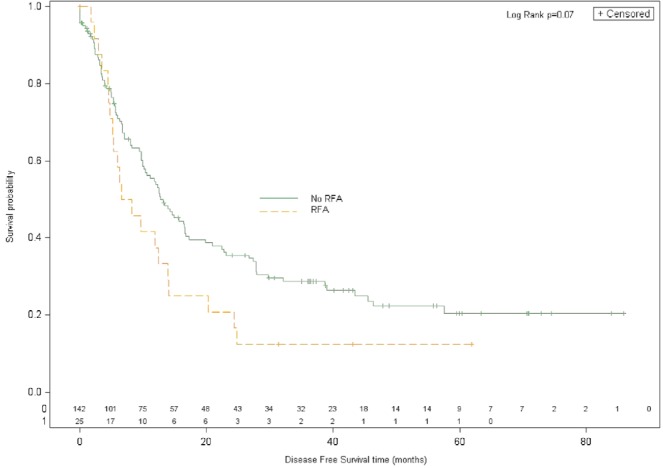
Compared with no use of rfa (n = 142), use of rfa (n = 25) was associated with an insignificant trend toward inferior dfs (rfa status was unknown in 1 patient). Median dfs duration was 7.4 months with rfa and 12.7 months without rfa (hr: 1.54; 95% confidence interval: 0.98 to 2.44; p = 0.076; Figure 4).
Figure 4.
Disease-free survival for patients treated with or without intraoperative radiofrequency ablation (rfa).
First recurrences were experienced by 71 patients (42.3%) in the liver and by 97 patients (57.7%) extra-hepatically. Of the extrahepatic recurrences, 7 (7.2%) occurred in bone, 17 (17.5%) in lymph nodes, 6 (6.2%) in other gastrointestinal sites, 3 (3.1%) in the brain, and 10 (10.3%) in other sites. There were 26 patients who experienced first recurrences to multiple sites (15.5%). No patients who underwent rfa experienced recurrence at the rfa site.
4. DISCUSSION
Hepatectomy is performed to remove metastases isolated to the liver. In some cases, patients deemed initially to be unresectable are converted to resectability after receiving systemic treatment.
We found that, compared with patients who did not receive chemotherapy around the time of surgery, those who received chemotherapy, regardless of timing, experienced improved os and dfs. In addition, our results show that the adjunct use of rfa was effective, with no significant reduction in os and no local recurrence at the ablation site. In addition, rfa was not found to increase length of stay or influence perioperative mortality. It was also observed to be an acceptable procedure—meaning that there were no apparent deleterious effects of substituting rfa of a limited number of metastases for excision of all lesions.
In addition to analyzing the impact of chemotherapy on survival, we evaluated the effect of chemotherapy timing (preoperative, perioperative, or postoperative).
Preoperative chemotherapy is used to convert previously unresectable patients to resectability. Studies have shown that converted patients experience os similar to that in patients found initially to be resectable. That finding also held true in a study by Adam et al.7, in which patients with initially unresectable crc metastases received neoadjuvant treatment and then underwent hepatic resection. Long-term survival in those patients was found to be similar to that reported for a priori surgical candidates.
The benefit of perioperative chemotherapy has been controversial. Results by Nordlinger et al.8 comparing perioperative chemotherapy with surgery alone demonstrated that perioperative chemotherapy with folfox4 is compatible with major liver surgery and reduces the risk of disease progression in eligible and resected patients. The 5-year os was not significantly better in the chemotherapy group, but the study was not powered for os as an endpoint. Results from our review in a large Canadian academic centre support their finding that chemotherapy is compatible with major liver surgery. In our study, patients who received chemotherapy had better outcomes, but no significant differences in os were observed between preoperative and perioperative chemotherapy, indicating that both approaches are effective. In other studies, postoperative chemotherapy provided a significant survival benefit for patients with resected liver metastases9–11, which is also supported by our results.
In addition to evaluating the effect of chemotherapy, our study evaluated the efficacy of rfa when used as part of a curative hepatectomy. Intraoperatively, rfa is used after incomplete resection of metastases in patients with metastases from crc. Although this procedure is routine at our institution as an adjunct to surgery, other institutions question the efficacy of the approach. Our results show that rfa is an acceptable procedure and that no statistically significant difference in os is observed for patients receiving and not receiving rfa. An association of rfa with a shorter dfs time was observed, but the difference was not found to be statistically significant. None of the patients who received rfa treatment had hepatic recurrence at the rfa site. Our results are supported by Gillams and Lees, who demonstrated that 5-year survival is comparable in patients who receive rfa and in patients who undergo surgical resection of their hepatic disease12. Comparability is further supported by Solbiati et al.13, who demonstrated that adding rfa to systemic chemotherapy achieved local control in patients who did not undergo surgical resection, with survival rates comparable to those for most surgical patients.
In contrast to the foregoing studies and our findings, other investigators have suggested that rfa alone or in combination with hepatic resection is inferior to complete resection. Other factors, such as size of the tumour treatment margins and operator-dependent variables, can contribute to the efficacy of the technique14,15. One study compared 190 patients resected without rfa and 101 receiving rfa plus resection14. That study found a true local recurrence rate of 5% when rfa was used compared with 2% when resection alone was used. Overall recurrence was 64% after rfa plus resection compared with 52% after resection only. Survival at 4 years was 65% for hepatectomy that also included rfa compared with 36% for hepatectomy alone. Another study evaluated 87 patients managed with rfa only15. Local (hepatic) failure occurred in 47.2% of patients. Lesions more likely to recur were metachronous, central, and larger in size.
Results from our study must be interpreted considering some of its limitations. First, the retrospective design could be associated with selection bias and heterogeneity within the patient population. The small sample size is the result of data being available from only a single institution. In addition, there is some bias in studying surgical patients having a single lesion within the liver compared with patients having several lesions, making it more complex to compare our results with the published literature. Furthermore, in most of the results, absence of statistical significance is more likely to be attributable to type ii error than to clinical error (specifically, noninferiority of dfs in the group that received rfa). To allow for the assessment of the role of rfa in this patient population, further study in terms of a randomized controlled clinical trial with a larger sample size would be required.
Metastasectomy is the treatment most likely to improve dfs and os in patients with crc, but most patients are not eligible for surgery. As demonstrated by the results of our review, a multidisciplinary approach combining surgery and chemotherapy with selective use of rfa allows for the best survival outcomes in patients with metastatic crc. Such an approach is the current standard of care16.
5. CONCLUSIONS
We found that administration of chemotherapy in patients with liver-limited crc metastases is associated with improved survival outcomes. When required, the use of rfa as an adjunct to hepatic resection appears to be effective and is not associated with worse os. Given that metastatic crc remains an incurable diagnosis for most patients, further study is warranted. New strategies will likely require a personalized approach that will be elucidated only through molecular study of both the primary tumour and the liver metastases.
6. ACKNOWLEDGMENTS
We thank everyone at our institution who helped with this study.
7. CONFLICT OF INTEREST DISCLOSURES
The authors have no financial conflicts of interest to declare.
8. REFERENCES
- 1.Canadian Cancer Society’s Advisory Committee on Cancer Statistics . Canadian Cancer Statistics 2013. Toronto, ON: Canadian Cancer Society; 2013. [Google Scholar]
- 2.Vigano L, Ferrero A, Lo Tesoriere R, Capussotti L. Liver surgery for colorectal metastases: results after 10 years of follow-up. Long-term survivors, late recurrences, and prognostic role of morbidity. Ann Surg Oncol. 2008;15:2458–64. doi: 10.1245/s10434-008-9935-9. [DOI] [PubMed] [Google Scholar]
- 3.Ksienski D, Woods R, Speers C, Kennecke H. Patterns of referral and resection among patients with liver-only metastatic colorectal cancer (mcrc) Ann Surg Oncol. 2010;17:3085–93. doi: 10.1245/s10434-010-1304-9. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen K, van Tilborg AA, Meijerink MR, et al. Incidence and treatment of local site recurrences following rfa of colorectal liver metastases. World J Surg. 2013;37:1340–7. doi: 10.1007/s00268-013-1997-6. [DOI] [PubMed] [Google Scholar]
- 5.Adam R, Wicherts DA, de Haas RJ, et al. Patients with initially unresectable colorectal liver metastases: is there a possibility of cure? J Clin Oncol. 2009;27:1829–35. doi: 10.1200/JCO.2008.19.9273. [DOI] [PubMed] [Google Scholar]
- 6.Gallinger S, Biagi JJ, Fletcher GG, Nhan C, Ruo L, McLeod RS. Liver resection for colorectal cancer metastases. Curr Oncol. 2013;20:e255–65. doi: 10.3747/co.20.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adam R, Avisar E, Ariche A, et al. Five-year survival following hepatic resection after neoadjuvant therapy for nonresectable colorectal. Ann Surg Oncol. 2001;8:347–53. doi: 10.1007/s10434-001-0347-3. [DOI] [PubMed] [Google Scholar]
- 8.Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with folfox4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (eortc Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007–16. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallagher DJ, Kemeny N. Metastatic colorectal cancer: from improved survival to potential cure. Oncology. 2010;78:237–48. doi: 10.1159/000315730. [DOI] [PubMed] [Google Scholar]
- 10.Parks R, Gonen M, Kemeny N, et al. Adjuvant chemotherapy improves survival after resection of hepatic colorectal metastases: analysis of data from two continents. J Am Coll Surg. 2007;204:753–61. doi: 10.1016/j.jamcollsurg.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 11.Portier G, Elias D, Bouche O, et al. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: ffcd achbth aurc 9002 trial. J Clin Oncol. 2006;24:4976–82. doi: 10.1200/JCO.2006.06.8353. [DOI] [PubMed] [Google Scholar]
- 12.Gillams AR, Lees WR. Five-year survival in 309 patients with colorectal liver metastases treated with radiofrequency ablation. Eur Radiol. 2009;19:1206–13. doi: 10.1007/s00330-008-1258-5. [DOI] [PubMed] [Google Scholar]
- 13.Solbiati L, Ahmed M, Cova L, Ierace T, Brioschi M, Goldberg SN. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology. 2012;265:958–68. doi: 10.1148/radiol.12111851. [DOI] [PubMed] [Google Scholar]
- 14.Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–25. doi: 10.1097/01.sla.0000128305.90650.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Duijnhoven FH, Jansen MC, Junggeburt JM, et al. Factors influencing the local failure rate of radiofrequency ablation of colorectal liver metastases. Ann Surg Oncol. 2006;13:651–8. doi: 10.1245/ASO.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 16.Nikfarjam M, Shereef S, Kimchi ET, et al. Survival outcomes of patients with colorectal liver metastases following hepatic resection or ablation in the era of effective chemotherapy. Ann Surg Oncol. 2009;16:1860–7. doi: 10.1245/s10434-008-0225-3. [DOI] [PubMed] [Google Scholar]