Abstract
We conducted a systematic review to determine the appropriate use of bortezomib alone or in combination with other agents in patients with multiple myeloma (mm). We searched medline, embase, the Cochrane Library, conference proceedings, and the reference lists of included studies. We analyzed randomized controlled trials and systematic reviews if they involved adult mm patients treated with bortezomib and if they reported on survival, disease control, response, quality of life, or adverse effects.
Twenty-six unique studies met the inclusion criteria. For patients with previously untreated mm and for candidates for transplantation, we found a statistically significant benefit in time to progression [hazard ratio (hr): 0.48, p < 0.001; and hr: 0.63, p = 0.006, respectively] and a better response with a bortezomib than with a non-bortezomib regimen (p < 0.001). Progression-free survival was longer with bortezomib and thalidomide than with thalidomide alone (p = 0.01). In non-candidates for transplantation, a significant benefit in overall survival was observed with a bortezomib regimen (hr compared with a non-bortezomib regimen: 0.61; p = 0.008), and in transplantation candidates receiving bortezomib, the response rate was improved after induction (p = 0.004) and after a first transplant (p = 0.016).
In relapsed or refractory mm, overall survival (p = 0.03), time to progression (hr: 1.82; p = 0.000004), and progression-free survival (hr: 1.69; p = 0.000026) were significantly improved with bortezomib and pegylated liposomal doxorubicin (compared with bortezomib alone), and bortezomib monotherapy was better than dexamethasone alone (hr: 0.77; p = 0.027). Bortezomib combined with thalidomide and dexamethasone was better than either bortezomib monotherapy or thalidomide with dexamethasone (p < 0.001).
In previously untreated or in relapsed or refractory mm patients, bortezomib-based therapy has improved disease control and, in some patients, overall survival.
Keywords: Bortezomib, multiple myeloma, proteasome inhibitors, systematic review
1. INTRODUCTION
Bortezomib (Velcade: Millennium Pharmaceuticals, Cambridge, MA, U.S.A.), a first-in-class proteasome inhibitor, has been extensively studied either alone or in combination with other agents for the treatment of multiple myeloma (mm). Bortezomib works in the ubiquitin–proteasome pathway of cellular protein homeostasis by blocking the action of the 26S proteasome, a multicatalytic enzyme that degrades abnormal or misfolded proteins targeted for destruction, particularly those involved in cell cycling and gene transcription. Because those proteins are more abundant during the processes of carcinogenesis, they are key in cancer survival; proteasome inhibition in cancer cells leads to cell apoptosis and is, therefore, a target for therapy1.
In 2008, bortezomib was approved by Health Canada for use as a first-line treatment for mm patients who are not candidates for stem-cell transplantation2. Existing consensus-based3,4 and evidence-based5,6 guidelines recommend the use of bortezomib for primary induction therapy in candidates and non-candidates for transplantation, and also for consolidation and salvage therapy after relapse.
Given that new data have recently become available, the Hematology Disease Site Group (dsg) at Cancer Care Ontario, in collaboration with the Program in Evidence-Based Care, conducted a systematic review to determine the appropriate use of bortezomib in patients with mm. This review constitutes the evidentiary basis of an updated Cancer Care Ontario guideline on bortezomib for mm and lymphoma (available at https://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=34323).
The systematic review addresses these questions:
In patients with mm, what is the efficacy of bortezomib alone or in combination, as measured by survival, quality of life (qol), disease control [for example, time to progression (ttp)], response duration, or response rate?
What is the toxicity associated with the use of bortezomib?
Which patients are more or less likely to benefit from treatment with bortezomib?
2. METHODS
2.1. Search Strategy
A search of the medline [Ovid (October 2004 through August 2012)], embase [Ovid (2004 week 42 through August 27, 2012], and Cochrane Library (August 2012) databases used the key words “bortezomib,” “bortezomid,” “velcade,” “ps?341,” “ldp?341,” and “mln?341” combined with key words specific to mm and with search strings identifying randomized controlled trials (rcts), systematic reviews, and practice guidelines. In addition, conference proceedings of the American Society of Clinical Oncology (2005–2012) and the American Society of Hematology (2005–2011) and reference lists from the selected sources were searched for relevant trials. The Canadian Medical Association Infobase (http://www.cma.ca/index.php/ci_id/54316/la_id/1.htm), the U.S. National Guideline Clearinghouse (http://www.guideline.gov/), and the U.K. National Institute for Health and Care Excellence (http://www.nice.org.uk/) were also searched for existing evidence-based practice guidelines.
2.2. Study Selection
Articles were selected for inclusion in this systematic review if they were published full-report articles or meeting abstracts of
randomized studies including adult patients with mm and evaluating bortezomib as a single agent or in combination with other regimens.
systematic reviews (full-report articles only), meta-analyses, or evidence-based clinical practice guidelines of bortezomib in adult patients with mm.
Trials could compare bortezomib with any agent, any combination of agents, or placebo, and could report results on any one or a combination of survival, qol, disease control (for example, ttp), response duration, response rate, and adverse effects.
Articles were excluded if they were clinical practice guidelines without a description of a systematic literature search, abstracts of noncomparative studies, abstract reports of interim analyses, or systematic reviews that were more than 2 years old. Letters, comments, books, news, editorials, or abstract publications of systematic reviews were also excluded, as were articles published in a language other than English.
The methodologist (AEH or FGB) screened the titles and abstracts of the citations identified in the electronic databases and the titles of the abstracts from conference proceedings and excluded reports of studies that did not investigate the use of bortezomib or that did not meet the inclusion criteria for design (that is, they were not randomized trials or systematic reviews for mm). The full text of each remaining article was retrieved, and two authors (AEH or FGB and DER or TCK) reviewed the articles against the selection criteria.
For the evaluation of the quality of included rcts, discrete parameters such as reporting of the sample-size calculation for the study, the randomization method, allocation concealment, blinding, intention-to-treat analysis, final analysis, early termination, losses to follow-up, and ethics approval were considered.
2.3. Data Analysis
Data appropriate for meta-analysis were not available because the heterogeneity of the studies did not allow for statistical pooling. A narrative synthesis is therefore presented, and the studies are grouped into untreated mm and into relapsed or refractory disease.
3. RESULTS
3.1. Literature Search
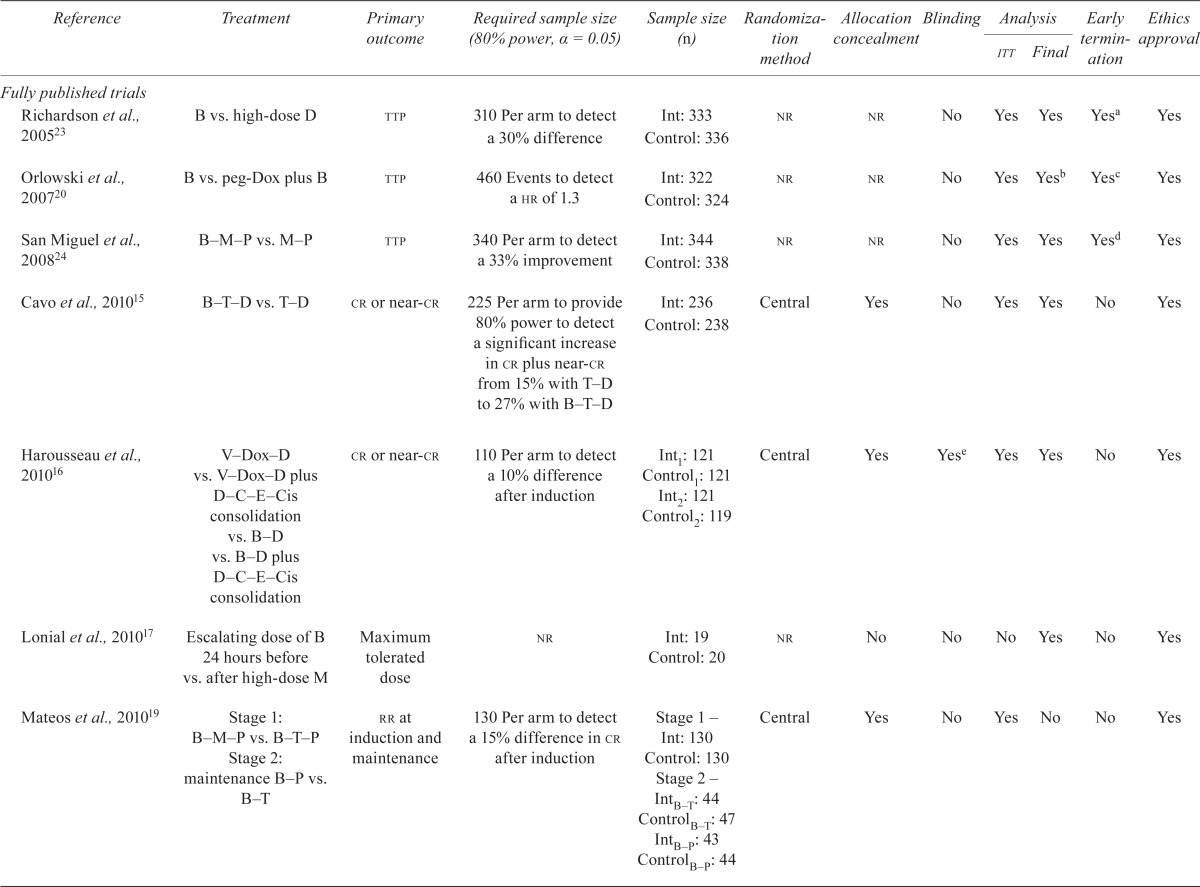
The literature search identified twenty-six unique studies: three guidelines based on systematic reviews7–9, six systematic reviews5,10–14, seventeen rcts15–31 and forty-seven related publications32–78. Figure 1 shows the study flow chart, and Table i presents the studies with their related publications and objectives.
Figure 1.
Systematic review on bortezomib for multiple myeloma (mm): study flow chart. ASH = American Society of Hematology; ASCO = American Society of Clinical Oncology; RCT = randomized controlled trial; abs = abstract.
TABLE I.
Primary and additional publications of identified randomized trials of bortezomib in multiple myeloma




| Study | Primarypublication and objective | Additional publications and objectives (when available) |
|---|---|---|
| Fully published trials | ||
| apex | Richardson et al., 200523 | |
| Efficacy: bortezomib compared with high-dose dexamethasone in relapsed disease | ||
| Richardson et al., 200732 | Update of apex results | |
| Richardson et al., 200733 | Extended follow-up | |
| San-Miguel et al., 200834 | Subgroup analysis of patients with renal impairment | |
| Chanan–Khan et al., 200835 | Herpes zoster events in bortezomib-treated patients | |
| Lee et al., 200836 | Health-related qol analysis | |
| Niesvizky et al., 200837 | Response and clinical benefit in bortezomib-treated patients | |
| Richardson et al., 200938 | Reversibility of peripheral neuropathy in bortezomib-treated patients | |
| Vogl et al., 200939 | Prior exposure to specific therapies | |
| San Miguel et al., 201140 (abs.) | Analysis of four phase iii studies: apex, mmy300, vista, and hovon65 to assess the risk of second primary malignancies after bortezomib treatment | |
| Kaura and Dranitsaris, 201278 (abs.) | Compare the number needed to treat as a measure of drug benefit from data in the apex study and a study of lenalidomide50 | |
| mmy-3001 | Orlowski et al., 200720 | |
| Efficacy and safety: pegylated liposomal doxorubicin plus bortezomib compared with bortezomib monotherapy in relapsed or refractory | ||
| disease | Sonneveld et al., 200841 | Subgroup analysis of patients who received prior thalidomide or lenalidomide |
| Shah et al., 200842 (abs.) | Subgroup analysis of patients with paraprotein heavy- and light-chain types | |
| Shah et al., 200843 (abs.) | Subgroup analysis of patients with bone marrow involvement | |
| Buda et al., 200944 (abs.) | Investigate association between gene polymorphisms and response | |
| vista | San Miguel et al., 200824 | |
| Efficacy: melphalan prednisone compared with melphalan prednisone bortezomib in previously untreated disease | ||
| San Miguel et al., 200845 (abs.) | Updated follow-up and results of subsequent therapy | |
| Harousseau et al., 200846 | Assess the prognostic impact of response on time-to-event parameters | |
| Dhawan et al., 200947 (abs.) | Health-related qol | |
| Dimopoulos et al., 200948 | Subgroup of patients with renal impairment | |
| Mateos et al., 201049 | Confirm os for melphalan–prednisone–bortezomib compared with melphalan–prednisone. Explore whether melphalan–prednisone–bortezomib induces relapses that are more resistant, and whether melphalan–prednisone–bortezomib used upfront was more effective than melphalan–prednisone | |
| Dimopoulos et al., 201150 | Frequency, characteristics, reversibility, and potential risk factors of peripheral neuropathy | |
| Favis et al., 201151 | Genetic variation associated with bortezomib-induced peripheral neuropathy | |
| Delforge et al., 201152 | Effects of bortezomib on bone events, remodelling, and healing | |
| San Miguel et al., 201153 (abs.) | 5-Year follow-up data | |
| Ludwig | Ludwig et al., 200918 (abs.) | |
| Efficacy and toxicity: bortezomib thalidomide dexamethasone compared with bortezomib thalidomide dexamethasone plus cyclophosphamide in previously untreated patients | ||
| gimema | Cavo et al., 201015 | |
| Effectiveness: bortezomib–thalidomide–dexamethasone compared with thalidomide–dexamethasone as front-line therapy | ||
| Cavo et al., 200958 (abs.) | Preliminary publication | |
| Brioli et al., 201159 (abs.) | Impact of novel agents on peripheral stem-cell collection | |
| Cavo et al., 201160 (abs.) | Per-protocol analysis of 321 patients who received the entire treatment program | |
| Cavo et al., 201261 | Efficacy and safety: bortezomib thalidomide dexamethasone compared with thalidomide dexamethasone as consolidation therapy after asct in newly diagnosed disease | |
| Tacchetti et al., 201162 (abs.) | Analysis of bortezomib- and thalidomide-induced peripheral neuropathy | |
| ifm2005/01 | Harousseau et al., 201016 | |
| Induction: bortezomib–dexamethasone compared with vincristine–doxorubicin–dexamethasone before asct | ||
| Avet–l’Oiseau et al., 201055 | Effectiveness in overcoming the poor prognosis linked to translocation t(4;14)(p16;q32) in elderly patients | |
| Moreau et al., 201056 | Evaluate stem-cell collection in the dexamethasone arm | |
| Moreau et al., 201157 | Achievement of very good partial response at induction as a prognostic factor for longer pfs | |
| Lonial | Lonial et al., 201017 | |
| Evaluate the safety and efficacy of combining bortezomib with high dose melphalan and the conditioning for high-dose therapy and asct | ||
| Mateos, Spanish Myeloma Group, gem2005mas65 | Mateos et al., 201019 | |
| Compare bortezomib–melphalan–prednisone plus maintenance with bortezomib–thalidomide–prednisone plus maintenance to investigate a bortezomib-based regimen that is less intensive than the regimen used in vista to maintain efficacy and to reduce toxic effects | ||
| Mateos et al., 201164 (abs.) | Phase ii of the 2010 study; all arms were randomly assigned to maintenance with bortezomib–prednisone or bortezomib–thalidomide | |
| Palumbo | Palumbo et al., 201021 | |
| Compare bortezomib–melphalan–prednisone–thalidomide plus maintenance with bortezomib–thalidomide with bortezomib–melphalan–prednisone and no maintenance in newly diagnosed patients | ||
| Bringhen et al., 201054 | Assess the impact of schedule change on clinical outcomes and safety | |
| ifm-2007–02 | Moreau et al., 201128 | |
| Bortezomib–dexamethasone compared with reduced-dose bortezomib–thalidomide–dexamethasone before asct in newly diagnosed disease | ||
| Moreau et al., 201069 (abs.) | Prior abstract publication of main study | |
| Moreau, mmy-3021 | Moreau et al., 201129 | |
| E efficacy and safety: subcutaneous compared with intravenous administration of bortezomib | ||
| Moreau et al., 201168 (abs.) | Pharmacokinetics and pharmacodynamics of subcutaneous compared with intravenous administration of bortezomib | |
| Reece | Reece et al., 201122 | |
| Pharmacokinetics and pharmacodynamics of bortezomib | ||
| mmvar/ifm 2005–04 | Garderet et al., 201230 | |
| Efficacy and safety: triple combination (bortezomib thalidomide dexamethasone) compared with dual combination (thalidomide dexamethasone) in disease progressing or relapsing after asct | ||
| Hjorth | Hjorth et al., 201225 | |
| Low-dose thalidomide–dexamethasone compared with bortezomib–dexamethasone in melphalan-refractory disease | ||
| evolution | Kumar et al., 201226 | |
| Evaluate safety and efficacy of bortezomib dexamethasone lenalidomide, dexamethasone–cyclophosphamide–lenalidomide in newly diagnosed patients | ||
| Kumar et al., 200965 | Abstract report of main study | |
| Kumar et al., 201166 | Abstract report of main study | |
| Kumar et al., 201167 | Minimal residual disease assessment with multiparameter flow cytometry | |
| Sharma | Sharma et al., 201227 | |
| Determine the efficacy and safety of adding bortezomib to a preparative regimen of arsenic trioxide, ascorbic acid, and melphalan in newly diagnosed patients | ||
| Sharma et al., 200963 (abs.) | Previous publication | |
| hovon-65/gmmg-hd4 | Sonneveld et al., 201231 | |
| Induction: vincristine–doxorubicin–dexamethasone compared with bortezomib–doxorubicin–dexamethasone plus high dose melphalan and asct; maintenance: thalidomide compared with bortezomib in newly diagnosed disease | ||
| Sonneveld et al., 200870 (abs.) | Abstract of interim analysis | |
| Neben et al., 201271 | Prognostic value of 12 chromosomal abnormalities | |
| Abstracts of interim analyses | ||
| Mellqvist | Mellqvist et al., 200979 (abs.) | |
| Explore the effect of a 21-week consolidation period of single-agent bortezomib given during months 3–8 after asct | ||
| Mellqvist et al., 201172 (abs.) | Updated results | |
| Vantage088 | Dimopoulos et al., 201180 (abs.). | |
| Efficacy and safety: bortezomib plus vorinostat compared with bortezomib plus placebo in relapsed or refractory disease | ||
| Dimopoulos | Dimopoulos et al., 201181 (abs.) | |
| Efficacy: bortezomib dexamethasone compared with bortezomib dexamethasone cyclophosphamide as second-line treatment | ||
| upfront | Niesvizky et al., 201182 (abs.) | |
| Safety and efficacy: bortezomib thalidomide dexamethasone compared with bortezomib dexamethasone and with bortezomib melphalan prednisone in newly diagnosed elderly patients | ||
| Niesvizky et al., 200975 (abs.) | Interim analysis | |
| Niesvizky et al., 201176 (abs.) | Patient-reported qol | |
| Orlowski | Orlowski et al., 201283 (abs.) | |
| Efficacy and safety: siltuximab plus bortezomib compared with bortezomib plus placebo in relapsed or refractory disease | ||
| pethema/gem05 | Rosinol et al., 201284 (abs.) | |
| Effectiveness: thalidomide–dexamethasone compared with bortezomib–thalidomide–dexamethasone and with vbmcp/vbad/bortezomib in newly diagnosed disease; presents data on time to progression | ||
| Rosinol et al., 200973 (abs.) | Interim analysis | |
| Rosinol et al., 201174 (abs.) | Data on response rate and time to progression | |
| panorama1 | San Miguel et al., 201285 (abs.) | |
| Reports a blinded safety analysis from a randomized controlled trial: panobinostat plus bortezomib and dexamethasone compared with placebo plus bortezomib and dexamethasone in relapsed or refractory disease | ||
| San-Miguel et al., 201177 (abs.) | Update on 273 patients | |
| Systematic reviews | ||
| Palumbo | Palumbo et al., 20095 | |
| Systematic review of evidence for an update to a previous guideline for the management of multiple myeloma | ||
| Palumbo | Palumbo and Rajkumar 201012 | |
| Review of novel agents and discussion of the role of asct | ||
| Kumar | Kumar et al., 201110 | |
| Indirect comparison of melphalan–prednisone–thalidomide and melphalan–prednisone–bortezomib | ||
| hta | Picot et al., 201111 | |
| Review the clinical effectiveness and cost-effectiveness of bortezomib and thalidomide in combination regimens with an alkylating agent and a corticosteroid for the first-line treatment of multiple myeloma; includes a systematic review and an economic evaluation | ||
| Piro | Piro et al., 201113 | |
| Systematic review of bortezomib in patients with renal impairment | ||
| Wang | Wang et al., 201214 | |
| Systematic review and meta-analysis of randomized controlled trials of novel agents bortezomib, lenalidomide, and thalidomide in newly diagnosed disease before asct; subgroup analyses were conducted by type of new agent | ||
| Guidelines | ||
| Bird | Bird et al., 20118 | |
| Guidelines for the diagnosis and management of multiple myeloma | ||
| nice technology appraisal guidance 228 | Doss et al., 20119 | |
| Guidelines for the use of bortezomib and thalidomide for first-line treatment of multiple myeloma | ||
| sie, sies, gitmo | Barosi et al., 20127 | |
| Guidelines on the use of thalidomide, bortezomib, and lenalidomide for multiple myeloma | ||
qol = quality of life; abs. = abstract; os = overall survival; asct = autologous stem-cell transplantation; pfs = progression-free survival; vbmcp = vincristine (1.2 mg/m2 intravenously day 1), carmustine (20 mg/m2 intravenously day 1), melphalan 8 mg/m2 orally days 1–4), cyclophosphamide (400 mg/m2 intravenously day 1), prednisone (40 mg/m2 orally days 1–7); vbad= vincristine, carmustine, doxorubicin, and high-dose dexamethasone; nice= U.K. National Institute for Health and Care Excellence.
Seven abstract publications of interim analyses of ongoing trials were also identified79–85. They are presented in Table i, but are not further discussed here.
The publications related to the main studies contributed information about extended follow-up of the original studies33,45,49,53, subgroup analyses34,39–44,48,51,52,55,57,59, health-related qol36,47,56,76, outcome data that were not reported in the original publication35,37,46,49,54,60,61,64,68,74,78, and data on toxicity38,50,62.
3.2. Trial Quality
Two trials reported in abstract form were randomized noncomparative phase ii trials18,65. Because the authors of those trials did not compare the treatment arms within each trial on any outcome, neither trial is further discussed here.
We applied the amstar tool86,87 to measure the quality of the two systematic reviews (see Appendix 2, Table 2, in the Cancer Care Ontario Evidence-Based Series #6–18, available at https://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=34323).
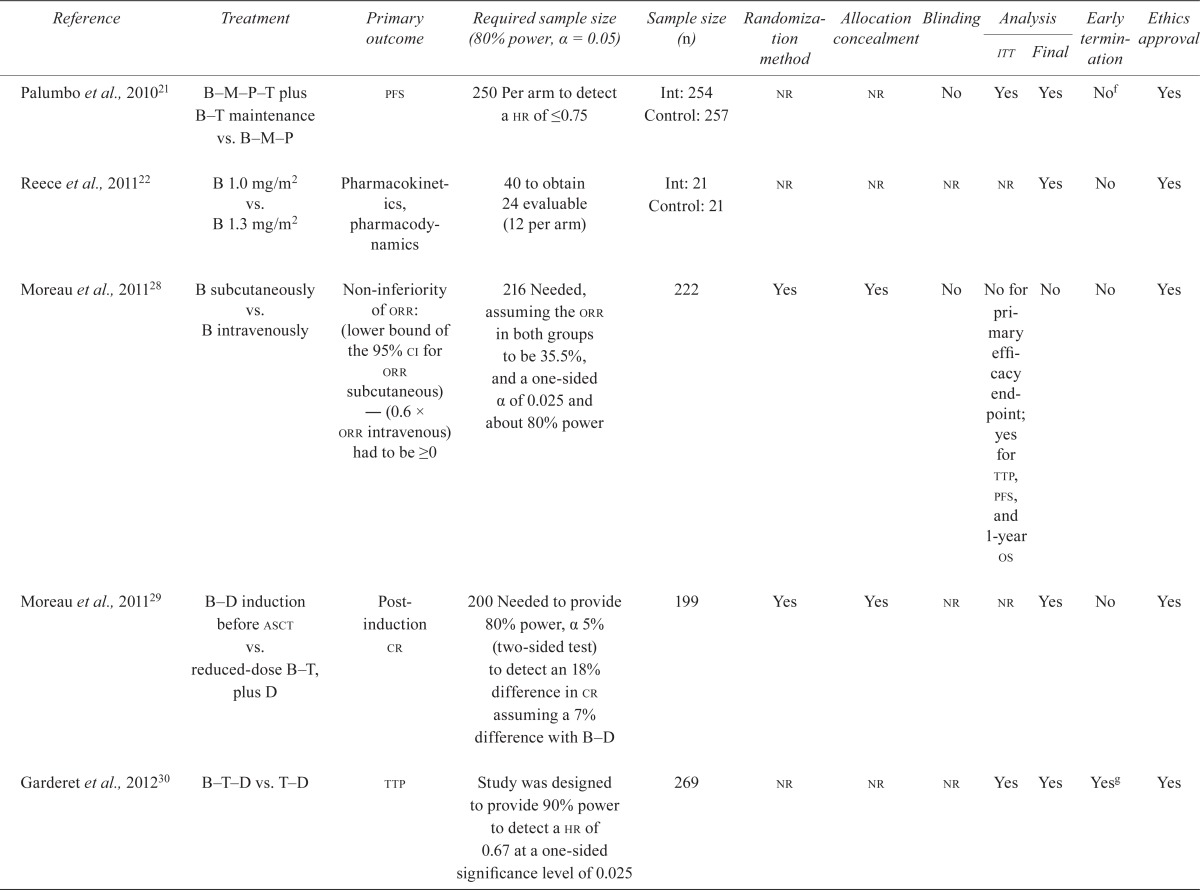
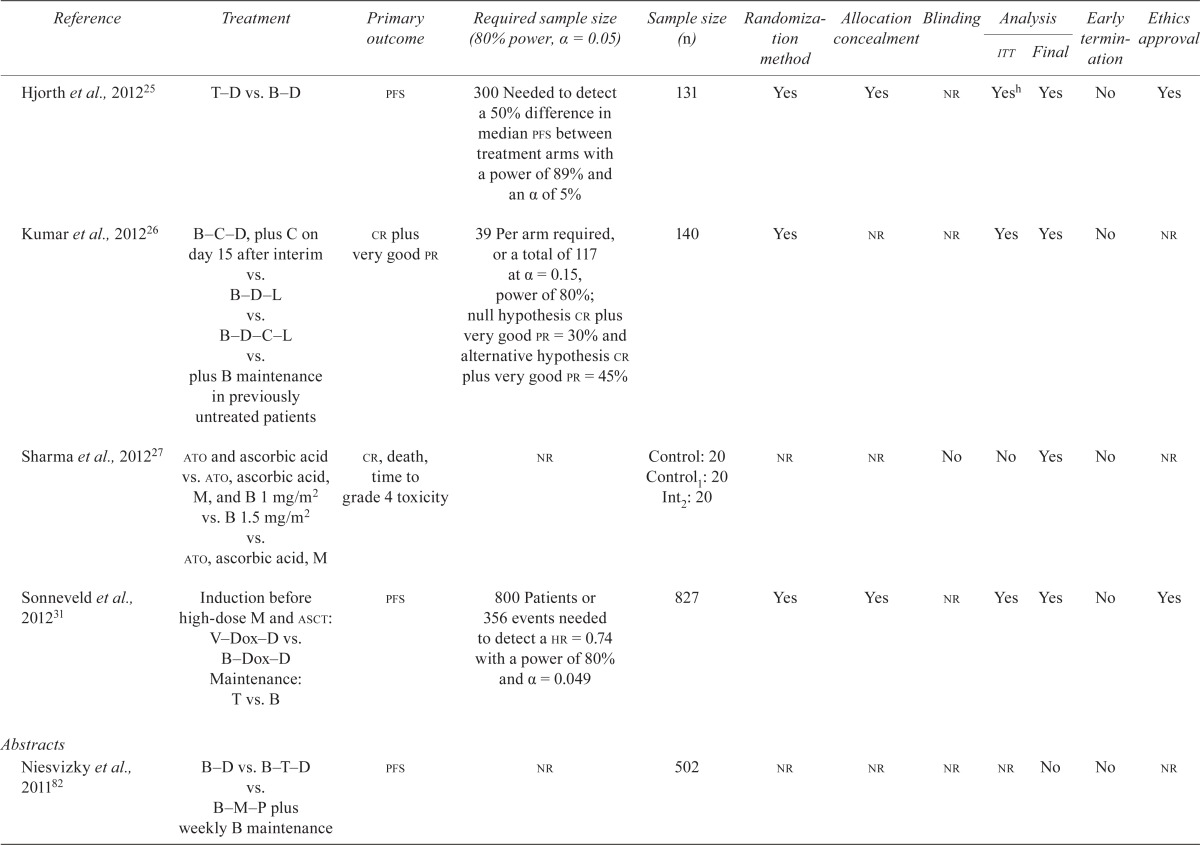
Table ii shows the quality assessment of the remaining rcts.
TABLE II.
Quality characteristics of included randomized controlled trials of bortezomib in multiple myeloma
| Reference | Treatment | Primary outcome | Required sample size (80% power, α = 0.05) | Sample size (n) | Randomization method | Allocation concealment | Blinding |
Analysis
|
Early termination | Ethics approval | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| itt | Final | ||||||||||
| Fully published trials | |||||||||||
| Richardson et al., 200523 | B vs. high-dose D | ttp | 310 Per arm to detect a 30% difference | Int: 333 Control: 336 |
nr | nr | No | Yes | Yes | Yesa | Yes |
| Orlowski et al., 200720 | B vs. peg-Dox plus B | ttp | 460 Events to detect a hr of 1.3 | Int: 322 Control: 324 |
nr | nr | No | Yes | Yesb | Yesc | Yes |
| San Miguel et al., 200824 | B–M–P vs. M–P | ttp | 340 Per arm to detect a 33% improvement | Int: 344 Control: 338 |
nr | nr | No | Yes | Yes | Yesd | Yes |
| Cavo et al., 201015 | B–T–D vs. T–D | cr or near-cr | 225 Per arm to provide 80% power to detect a significant increase in cr plus near-cr from 15% with T–D to 27% with B–T–D | Int: 236 Control: 238 |
Central | Yes | No | Yes | Yes | No | Yes |
| Harousseau et al., 201016 | V–Dox–D vs. V–Dox–D plus D–C–E–Cis consolidation vs. B–D vs. B–D plus D–C–E–Cis consolidation | cr or near-cr | 110 Per arm to detect a 10% difference after induction | Int1: 121 Control1: 121 Int2: 121 Control2: 119 |
Central | Yes | Yese | Yes | Yes | No | Yes |
| Lonial et al., 201017 | Escalating dose of B 24 hours before vs. after high-dose M | Maximum tolerated dose | nr | Int: 19 Control: 20 |
nr | No | No | No | Yes | No | Yes |
| Mateos et al., 201019 | Stage 1: B–M–P vs. B–T–P Stage 2: maintenance B–P vs. B–T |
rr at induction and maintenance | 130 Per arm to detect a 15% difference in cr after induction | Stage 1 – Int: 130 Control: 130 Stage 2 – IntB–T: 44 ControlB–T: 47 IntB–P: 43 ControlB–P: 44 |
Central | Yes | No | Yes | No | No | Yes |
| Palumbo et al., 201021 | B–M–P–T plus B–T maintenance vs. B–M–P | pfs | 250 Per arm to detect a hr of ≤0.75 | Int: 254 Control: 257 |
nr | nr | No | Yes | Yes | Nof | Yes |
| Reece et al., 201122 | B 1.0 mg/m2 vs. B 1.3 mg/m2 | Pharmacokinetics, pharmacodynamics | 40 to obtain 24 evaluable (12 per arm) | Int: 21 Control: 21 |
nr | nr | nr | nr | Yes | No | Yes |
| Moreau et al., 201128 | B subcutaneously vs. B intravenously | Non-inferiority of orr: (lower bound of the 95% ci for orr subcutaneous) — (0.6 × orr intravenous) had to be ≥0 | 216 Needed, assuming the orr in both groups to be 35.5%, and a one-sided α of 0.025 and about 80% power | 222 | Yes | Yes | No | No for primary efficacy end-point; yes for ttp, pfs, and 1-year os | No | No | Yes |
| Moreau et al., 201129 | B–D induction before asct vs. reduced-dose B–T, plus D | Post-induction cr | 200 Needed to provide 80% power, α 5% (two-sided test) to detect an 18% difference in cr assuming a 7% difference with B–D | 199 | Yes | Yes | nr | nr | Yes | No | Yes |
| Garderet et al., 201230 | B–T–D vs. T–D | ttp | Study was designed to provide 90% power to detect a hr of 0.67 at a one-sided significance level of 0.025 | 269 | nr | nr | nr | Yes | Yes | Yesg | Yes |
| Hjorth et al., 201225 | T–D vs. B–D | pfs | 300 Needed to detect a 50% difference in median pfs between treatment arms with a power of 89% and an α of 5% | 131 | Yes | Yes | nr | Yesh | Yes | No | Yes |
| Kumar et al., 201226 | B–C–D, plus C on day15 after interim vs. B–D–L vs. B–D–C–L vs. plus B maintenance in previously untreated patients | cr plus very good pr | 39 Per arm required, or a total of 117 at α = 0.15, power of 80%; null hypothesis cr plus very good pr = 30% and alternative hypothesis cr plus very good pr = 45% | 140 | Yes | nr | nr | Yes | Yes | No | nr |
| Sharma et al., 201227 | ato and ascorbic acid vs. ato, ascorbic acid, M, and B 1 mg/m2 vs. B 1.5 mg/m2 vs. ato, ascorbic acid, M | cr, death, time to grade 4 toxicity | nr | Control: 20 Control1: 20 Int2: 20 |
nr | nr | No | No | Yes | No | nr |
| Sonneveld et al., 201231 | Induction before high-dose M and asct: V–Dox–D vs. B–Dox–D Maintenance: T vs. B |
pfs | 800 Patients or 356 events needed to detect a hr = 0.74 with a power of 80% and α = 0.049 | 827 | Yes | Yes | nr | Yes | Yes | No | Yes |
| Abstracts | |||||||||||
| Niesvizky et al., 201182 | B–D vs. B–T–D vs. B–M–P plus weekly B maintenance | pfs | nr | 502 | nr | nr | nr | nr | No | No | nr |
| Rosinol et al., 201284 | Induction before asct: T–D vs. B–T–D vs. vbmcp/vbad/B Maintenance: T–B vs. T vs. alfa-2b-interferon |
ttp | nr | 266 (maintenance only) | nr | nr | nr | Yes | No | No | nr |
| Orlowski et al., 201283 | Siltuximab plus B vs. placebo plus B | pfs | nr | 286 | nr | nr | nr | nr | No | No | nr |
Trial was terminated early on recommendation from Data and Safety Monitoring Board after a planned interim analysis demonstrated significantly improved ttp in the bortezomib arm compared with dexamethasone. The a priori sample size requirement was met, but follow-up ended at the early termination date, and all patients in the dexamethasone arm were offered bortezomib.
Data on overall survival, a secondary outcome, will continue to be collected by the authors, and a final analysis of that outcome will be conducted when 80% of patients have died.
Trial was terminated early on recommendation from Data and Safety Monitoring Board after a planned interim analysis at 230 events demonstrated significantly improved ttp in the pegylated liposomal doxorubicin plus bortezomib arm compared with the bortezomib-alone arm.
Trial was terminated early on recommendation from Data and Safety Monitoring Board after a planned interim analysis demonstrated significantly improved ttp in the bortezomib plus melphalan–prednisone arm compared with the melphalan–prednisone arm.
Blinded assessors of outcomes.
After the first 139 patients, the protocol was amended to reduce the incidence of peripheral neuropathy; 5-week cycles of 1.3 mg/m2 bortezomib were used instead of 6-week cycles.
Trial stopped at the second interim analysis for B–T–D superiority over T–D after 134 events and a median follow-up of 24 months.
Except time to response and response duration.
itt = intention-to-treat; B = bortezomib; D = dexamethasone; ttp = time to progression; Int = intervention; nr = not reported; peg-Dox = pegylated liposomal doxorubicin; M = melphalan; P = prednisone; T = thalidomide; cr = complete response; V = vincristine; Dox = doxorubicin; C = cyclophosphamide; E = etoposide; Cis = cisplatinum; rr = relative risk; pfs = progression-free survival; hr = hazard ratio; orr = objective response rate; ci = confidence interval; os = overall survival; asct = autologous stem-cell transplantation; L = lenalidomide; pr = partial response; ato = arsenic trioxide; vbmcp = vincristine (1.2 mg/m2 intravenously day 1), carmustine (20 mg/m2 intravenously day 1), melphalan 8 mg/m2 orally days 1–4), cyclophosphamide (400 mg/m2 intravenously day 1), prednisone (40 mg/m2 orally days 1–7); vbad = vincristine, carmustine, doxorubicin, and high-dose dexamethasone.
Nine studies were available as fully published reports15–17,19–24. Eight of the nine fully published rcts reported the a priori sample size required to find a statistically significant difference in the primary endpoints: ttp, progression-free survival (pfs), complete response (cr), pharmacokinetics and pharmacodynamics, and response rate15,16,19–24. Eight of the nine studies presented a final analysis15–17,20–24, and seven of the eight conducted an intention-to-treat analysis15,16,19–21,23,24. Three studies were terminated early because the intervention significantly improved ttp20,23,24. One study conducted a blinded outcomes assessment16, and three studies reported concealment of allocation15,16,19. None of the studies reported a loss to follow up exceeding 8%. The included studies were funded by pharmaceutical companies15,16,23,24, government or philanthropic organizations15,17,19,20, or by a foundation21.
Among the studies reported in abstract form, one study stated that the analysis was final63. The other five69,70,73,75,79 were identified as interim; they are not shown in Table ii and will not be discussed further.
3.3. Study Characteristics
3.3.1. Previously Untreated MM
Indirect Comparison:
The network meta-analysis by Kumar et al.10 indirectly compared bortezomib and thalidomide (both in combination with melphalan and prednisone) in newly diagnosed mm patients. No differences were detected for most outcomes, but benefits in cr [relative risk (rr): 2.34; 95% confidence interval (ci): 1.12 to 4.90] and in grade 3 or 4 adverse events (rr: 0.53; 95% ci: 0.38 to 0.73) were observed in favour of the bortezomib combination.
Direct Comparison:
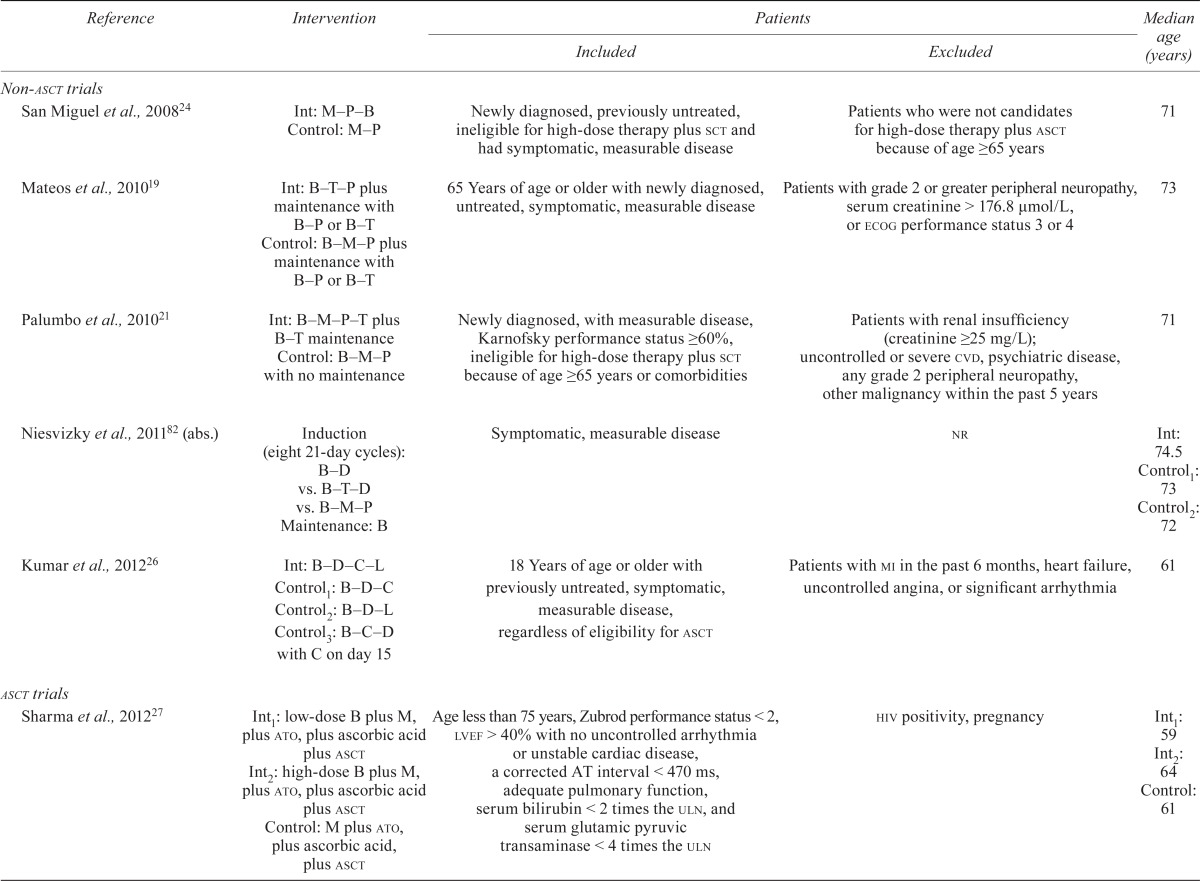
Eleven rcts—nine full-text publications15,16,19,21,24,26–28,31 and two abstracts82,84—examined the use of bortezomib in patients with de novo mm. Table iii details inclusion criteria and intervention details for those trials.
TABLE III.
Patient characteristics in studies of patients with de novo multiple myeloma
| Reference | Intervention |
Patients
|
Median age (years) | |
|---|---|---|---|---|
| Included | Excluded | |||
| Non-asct trials | ||||
| San Miguel et al., 200824 | Int: M–P–B Control: M–P |
Newly diagnosed, previously untreated, ineligible for high-dose therapy plus sct and had symptomatic, measurable disease | Patients who were not candidates for high-dose therapy plus asct because of age ≥65 years | 71 |
| Mateos et al., 201019 | Int: B–T–P plus maintenance with B–P or B–T Control: B–M–P plus maintenance with B–P or B–T |
65 Years of age or older with newly diagnosed, untreated, symptomatic, measurable disease | Patients with grade 2 or greater peripheral neuropathy, serum creatinine > 176.8 μmol/L, or ecog performance status 3 or 4 | 73 |
| Palumbo et al., 201021 | Int: B–M–P–T plus B–T maintenance Control: B–M–P with no maintenance |
Newly diagnosed, with measurable disease, Karnofsky performance status ≥60%, ineligible for high-dose therapy plus sct because of age ≥65 years or comorbidities | Patients with renal insufficiency (creatinine ≥25 mg/L); uncontrolled or severe cvd, psychiatric disease, any grade 2 peripheral neuropathy, other malignancy within the past 5 years | 71 |
| Niesvizky et al., 201182 (abs.) | Induction (eight 21-day cycles): B–D vs. B–T–D vs. B–M–P Maintenance: B |
Symptomatic, measurable disease | nr | Int: 74.5 Control1: 73 Control2: 72 |
| Kumar et al., 201226 | Int: B–D–C–L Control1: B–D–C Control2: B–D–L Control3: B–C–D with C on day 15 |
18 Years of age or older with previously untreated, symptomatic, measurable disease, regardless of eligibility for asct | Patients with mi in the past 6 months, heart failure, uncontrolled angina, or significant arrhythmia | 61 |
| asct trials | ||||
| Sharma et al., 201227 | Int1: low-dose B plus M, plus ato, plus ascorbic acid plus asct Int2: high-dose B plus M, plus ato, plus ascorbic acid plus asct Control: M plus ato, plus ascorbic acid, plus asct |
Age less than 75 years, Zubrod performance status < 2, lvef> 40% with no uncontrolled arrhythmia or unstable cardiac disease, a corrected AT interval < 470 ms, adequate pulmonary function, serum bilirubin < 2 times the uln, and serum glutamic pyruvic transaminase < 4 times the uln | hiv positivity, pregnancy | Int1: 59 Int2: 64 Control: 61 |
| Cavo et al., 201015 | Int: B–T–D plus double asct Control: T–D plus double transplantation |
Less than 65 years of age with newly diagnoseddisease | Grade 2 or greater peripheral neuropathy; history of venous thromboembolism; previous diagnosis of thrombophilic alterations | Int: 58 Control: 57 |
| Harousseau et al., 201016 | Int1: B–D and no consolidation Int2: B–D plus D–C–E–P consolidation Control1: V–Dox–D and no consolidation Control2: V–Dox–D plus D–C–E–P consolidation |
Less than 65 years of age with measurable disease; ecog performance status of ≤2 or less; life expectancy of 2 months or more; and adequate renal, hematologic, and hepatic function | Confirmed amyloidosis, hiv positivity, history of other malignancy, uncontrolled diabetes, and grade 2 or greater peripheral neuropathy | Int: 57.2 Control: 57.1 |
| Moreau et al., 201128 | B–D vs. reduced-dose B–T plus D before asct | 65 Years of age or less, with symptomatic and measurable disease, ecog performance status ≤ 2, and adequate renal function | Confirmed amyloidosis, hiv positivity, history of other malignancy, uncontrolled diabetes, and grade 2 or greater peripheral neuropathy | Int: 57 Control: 58 |
| Rosinol et al., 201284 (abs.) | Maintenance: T–B vs. T vs. alfa-2b-interferon | Newly diagnosed symptomatic disease and asct | nr | nr |
| Sonneveld et al., 201231 | Induction: B–Dox–D plus high-dose M, plus asct Maintenance: B 1.3 mg/m2 every 2 weeks |
18–65 Years of age with newly diagnosed disease stage ii–iii, who performance status 0–2 or 3 if caused by the multiple myeloma | Systemic amyloid chain amyloidosis, nonsecretory disease, neuropathy grade 2 or greater, active malignancy during the past 5 years, hiv positivity, serum aminotransferases ≥ 30 μmol/L or ≥ 2.5 times normal | 57 |
asct = autologous stem-cell transplantation; Int = intervention group; M = melphalan; P = prednisone; B = bortezomib; sct = stem-cell transplantation; T = thalidomide; ecog = Eastern Cooperative Oncology Group; cvd = cardiovascular disease; abs. = abstract; D = dexamethasone; nr = not reported; L = lenalidomide; C = cyclophosphamide; mi = myocardial infarction; ato = arsenic trioxide; lvef = left ventricular ejection fraction; uln = upper limit of normal; hiv = human immunodeficiency virus; C = cyclophosphamide; V = vincristine; Dox = doxorubicin.
Non-transplantation Therapy: Five trials enrolled newly diagnosed patients who were not candidates for autologous stem-cell transplantation (asct) either because of older age (≥65 years) or because of other coexisting conditions19,21,24,26,82.
Transplantation Therapy: Six rcts enrolled younger untreated mm patients who were candidates for asct15,16,27,28,31,84.
3.3.2. Relapsed or Refractory MM
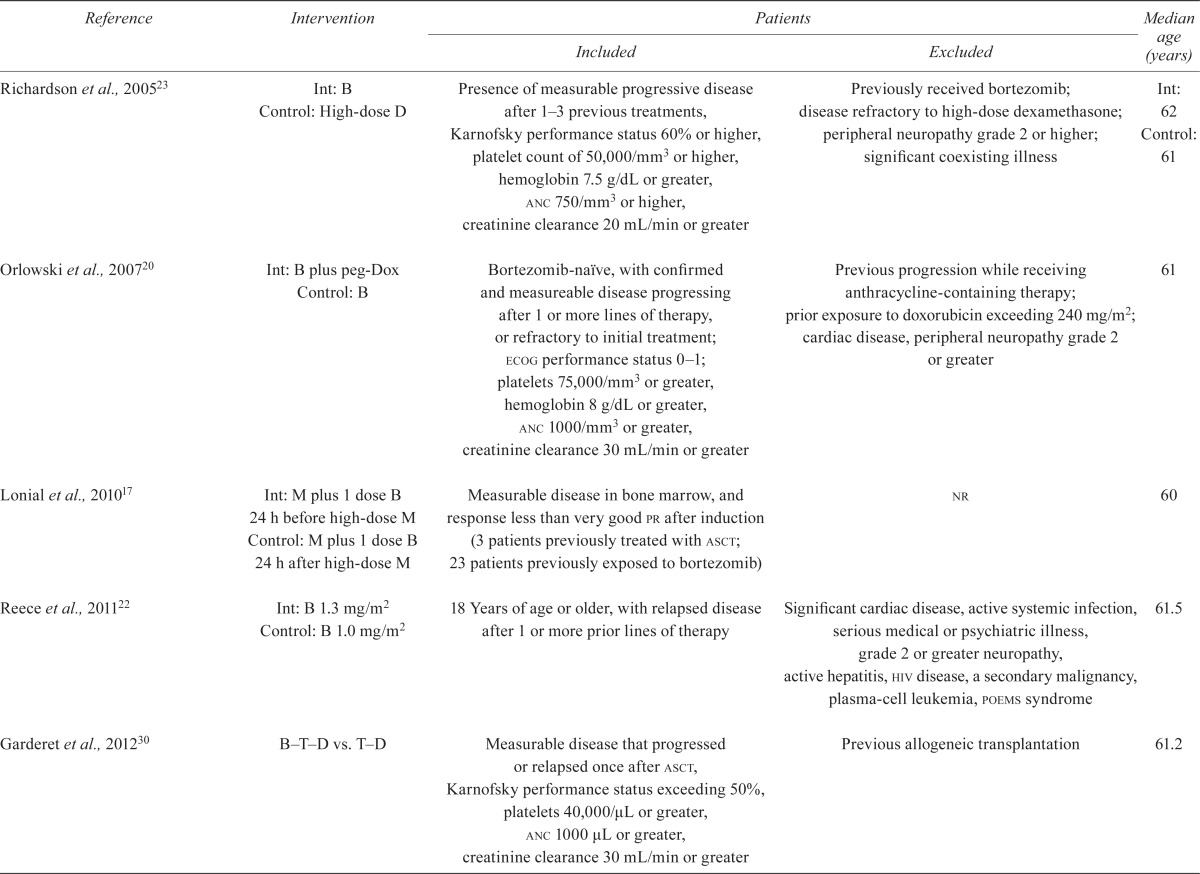
Seven rcts examined the use of bortezomib in patients with relapsed or refractory mm. All studies but one were fully published reports. Three included only bortezomib-naïve patients20,23,25; the remaining four17,22,30,83 included patients who had previously received treatment for mm, including bortezomib. Table iv details the characteristics of the study patients.
TABLE IV.
Patient characteristics in studies of patients with relapsed or refractory multiple myeloma
| Reference | Intervention |
Patients
|
Median age (years) | |
|---|---|---|---|---|
| Included | Excluded | |||
| Richardson et al., 200523 | Int: B Control: High-dose D |
Presence of measurable progressive disease after 1–3 previous treatments, Karnofsky performance status 60% or higher, platelet count of 50,000/mm3 or higher, hemoglobin 7.5 g/dL or greater, anc 750/mm3 or higher, creatinine clearance 20 mL/min or greater | Previously received bortezomib; disease refractory to high-dose dexamethasone; peripheral neuropathy grade 2 or higher; significant coexisting illness | Int: 62 Control: 61 |
| Orlowski et al., 200720 | Int: B plus peg-Dox Control: B |
Bortezomib-naive, with confirmed and measureable disease progressing after 1 or more lines of therapy, or refractory to initial treatment; ecog performance status 0–1; platelets 75,000/mm3 or greater, hemoglobin 8 g/dL or greater, anc 1000/mm3 or greater, creatinine clearance 30 mL/min or greater | Previous progression while receiving anthracycline-containing therapy; prior exposure to doxorubicin exceeding 240 mg/m2; cardiac disease, peripheral neuropathy grade 2 or greater | 61 |
| Lonial et al., 201017 | Int: M plus 1 dose B 24 h before high-dose M Control: M plus 1 dose B 24 h after high-dose M |
Measurable disease in bone marrow, and response less than very good pr after induction (3 patients previously treated with asct; 23 patients previously exposed to bortezomib) | nr | 60 |
| Reece et al., 201122 | Int: B 1.3 mg/m2 Control: B 1.0 mg/m2 |
18 Years of age or older, with relapsed disease after 1 or more prior lines of therapy | Significant cardiac disease, active systemic infection, serious medical or psychiatric illness, grade 2 or greater neuropathy, active hepatitis, hiv disease, a secondary malignancy, plasma-cell leukemia, poems syndrome | 61.5 |
| Garderet et al., 201230 | B–T–D vs. T–D | Measurable disease that progressed or relapsed once after asct, Karnofsky performance status exceeding 50%, platelets 40,000/μL or greater, anc 1000 μL or greater, creatinine clearance 30 mL/min or greater | Previous allogeneic transplantation | 61.2 |
| Hjorth et al., 201225 | B–D vs. T–D | Any age, with symptomatic disease refractory to melphalan | Previous treatment with thalidomide, bortezomib or lenalidomide; sensory neuropathy grade 3 or greater; platelet count less than 25×109/L; severe comorbidity; transformation to plasma-cell leukemia or aggressive lymphoma; nonsecreting disease without abnormal free light-chain ratio | 71 |
| Orlowski et al., 201283 (abs.) | Siltuximab + B vs. placebo plus B | nr | nr | Int: 64 Control: 61 |
Int= intervention group; B= bortezomib; D= dexamethasone; anc= absolute neutrophil count; peg-Dox= pegylated liposomal doxorubicin; ecog= Eastern Cooperative Oncology Group; M = melphalan; pr= partial response; asct= autologous stem-cell transplantation; nr= not reported; poems = polyneuropathy, organomegaly, endocrinopathy, plasma-cell leukemia; T = thalidomide. significantly
The primary outcomes in the studies of patients with relapsed or refractory mm were ttp21,23,30, pfs25,83, and toxicity17. Reece et al.22 reported on pharmacodynamic and pharmacokinetic parameters, response, and the toxicity of bortezomib.
3.4. Endpoints
Question:
What is the efficacy of bortezomib alone or in combination?
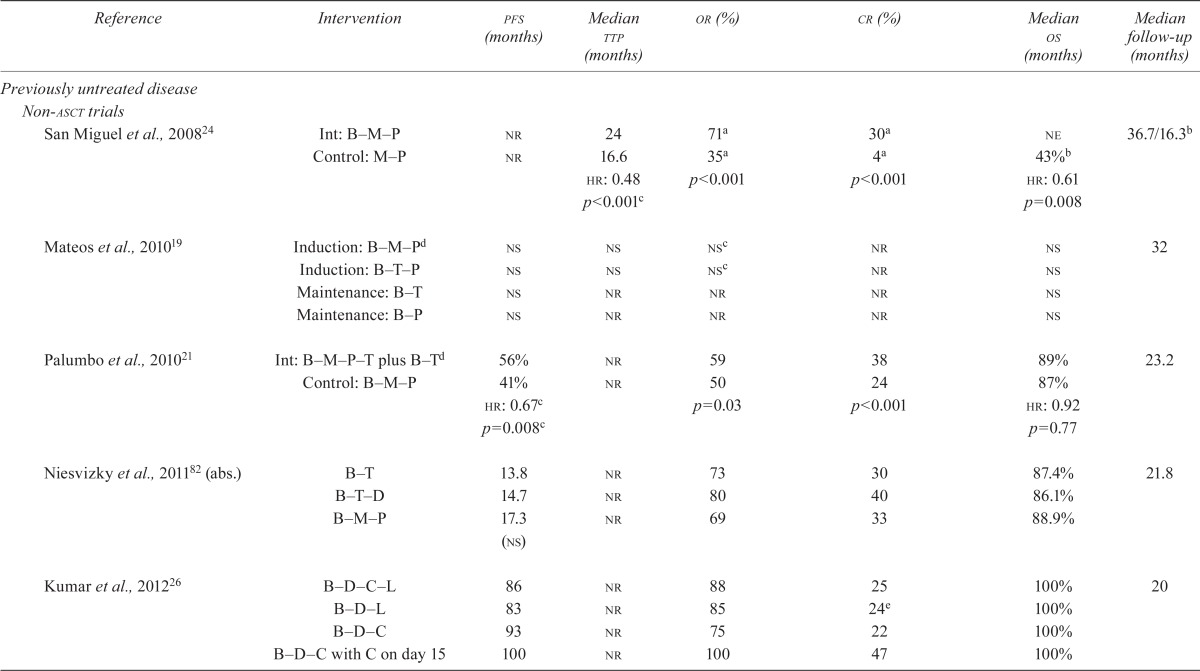
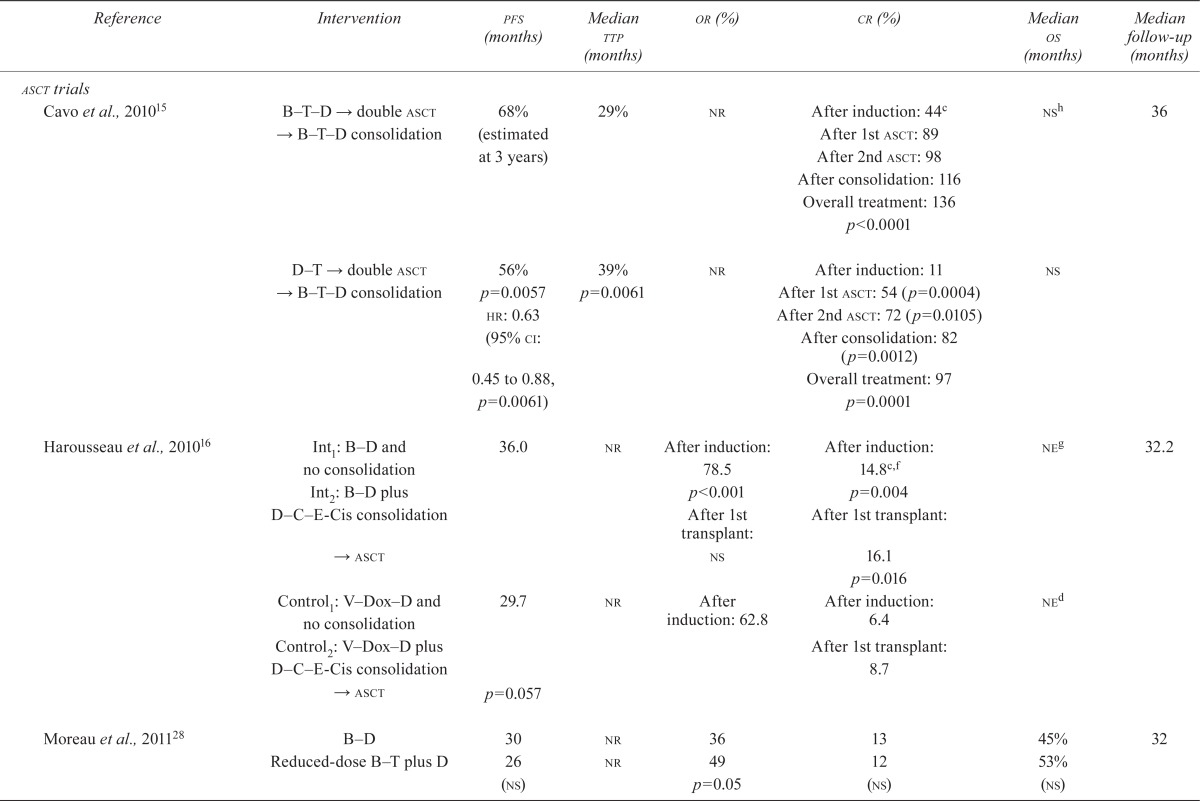
The subsections that follow summarize the results of the included trials. Detailed efficacy data can be found in Table v.
TABLE V.
Results of studies of patients with multiple myeloma
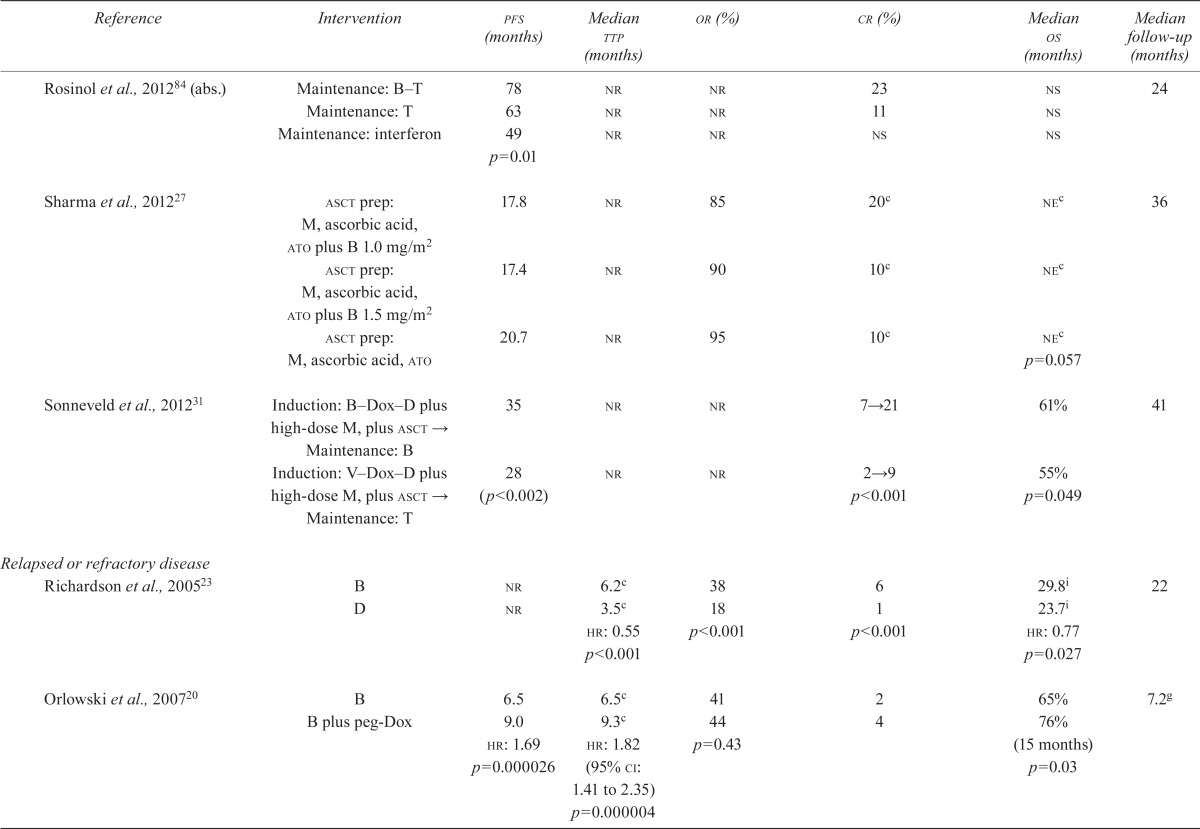
| Reference | Intervention | pfs (months) | Median ttp (months) | or (%) | cr (%) | Median os (months) | Median follow-up (months) |
|---|---|---|---|---|---|---|---|
| Previously untreated disease | |||||||
| Non-asct trials | |||||||
| San Miguel et al., 200824 | Int: B–M–P | nr | 24 | 71a | 30a | ne | 36.7/16.3b |
| Control: M–P | nr | 16.6 | 35a | 4a | 43%b | ||
| hr: 0.48 | p<0.001 | p<0.001 | hr: 0.61 | ||||
| p<0.001c | p=0.008 | ||||||
| Mateos et al., 201019 | Induction: B–M–Pd | ns | ns | nsc | nr | ns | 32 |
| Induction: B–T–P | ns | ns | nsc | nr | ns | ||
| Maintenance: B–T | ns | nr | nr | nr | ns | ||
| Maintenance: B–P | ns | nr | nr | nr | ns | ||
| Palumbo et al., 201021 | Int: B–M–P–T plus B–Td | 56% | nr | 59 | 38 | 89% | 23.2 |
| Control: B–M–P | 41% | nr | 50 | 24 | 87% | ||
| hr: 0.67c | p=0.03 | p<0.001 | hr: 0.92 | ||||
| p=0.008c | p=0.77 | ||||||
| Niesvizky et al., 201182 (abs.) | B–T | 13.8 | nr | 73 | 30 | 87.4% | 21.8 |
| B–T–D | 14.7 | nr | 80 | 40 | 86.1% | ||
| B–M–P | 17.3 (ns) | nr | 69 | 33 | 88.9% | ||
| Kumar et al., 201226 | B–D–C–L | 86 | nr | 88 | 25 | 100% | 20 |
| B–D–L | 83 | nr | 85 | 24e | 100% | ||
| B–D–C | 93 | nr | 75 | 22 | 100% | ||
| B–D–C with C on day 15 | 100 | nr | 100 | 47 | 100% | ||
| asct trials | |||||||
| Cavo et al., 201015 | B T D → double asct → B T D consolidation | 68% (estimated at 3 years) | 29% | nr | After induction: 44c | nsh | 36 |
| After 1st asct: 89 | |||||||
| After 2nd asct: 98 | |||||||
| After consolidation: 116 | |||||||
| Overall treatment: 136 | |||||||
| p<0.0001 | |||||||
| D T → double asct → B T D consolidation | 56% | 39% | nr | After induction: 11 | ns | ||
| p=0.0057 | p=0.0061 | After 1st asct: 54 (p=0.0004) | |||||
| hr: 0.63 | After 2nd asct: 72 (p=0.0105) | ||||||
| (95% ci: 0.45 to 0.88, p=0.0061) | After consolidation: 82 | ||||||
| (p=0.0012) | |||||||
| Overall treatment: 97 | |||||||
| p=0.0001 | |||||||
| Harousseau et al., 201016 | Int1: B–D and no consolidation | 36.0 | nr | After induction: 78.5 | After induction: 14.8c,f | neg | 32.2 |
| Int2: B–D plus D–C–E-Cis consolidation | p<0.001 | p=0.004 | |||||
| → asct | After 1st transplant: ns | After 1st transplant: 16.1 | |||||
| p=0.016 | |||||||
| Control1: V–Dox–D and no consolidation | 29.7 | nr | After induction: 62.8 | After induction: 6.4 | ned | ||
| Control2: V–Dox–D plus D–C–E-Cis consolidation | After 1st transplant: 8.7 | ||||||
| → asct | p=0.057 | ||||||
| Moreau et al., 201128 | B–D | 30 | nr | 36 | 13 | 45% | 32 |
| Reduced-dose B–T plus D | 26 (ns) | nr | 49 p=0.05 | 12 (ns) | 53% (ns) | ||
| Rosinol et al., 201284 (abs.) | Maintenance: B–T | 78 | nr | nr | 23 | ns | 24 |
| Maintenance: T | 63 | nr | nr | 11 | ns | ||
| Maintenance: interferon | 49 | nr | nr | ns | ns | ||
| p=0.01 | |||||||
| Sharma et al., 201227 | asct prep: M, ascorbic acid, ato plus B 1.0 mg/m2 | 17.8 | nr | 85 | 20c | nec | 36 |
| asct prep: M, ascorbic acid, ato plus B 1.5 mg/m2 | 17.4 | nr | 90 | 10c | nec | ||
| asct prep: M, ascorbic acid, ato | 20.7 | nr | 95 | 10c | nec | ||
| p=0.057 | |||||||
| Sonneveld et al., 201231 | Induction: B–Dox–D plus high-dose M, plus asct → | 35 | nr | nr | 7→21 | 61% | 41 |
| Maintenance: B Induction: V–Dox–D plus high-dose M, plus asct → | 28 | nr | nr | 2→9 | 55% | ||
| Maintenance: T | (p<0.002) | p<0.001 | p=0.049 | ||||
| Relapsed or refractory disease | |||||||
| Richardson et al., 200523 | B | nr | 6.2c | 38 | 6 | 29.8i | 22 |
| D | nr | 3.5c | 18 | 1 | 23.7i | ||
| hr: 0.55 | p<0.001 | p<0.001 | hr: 0.77 | ||||
| p<0.001 | p=0.027 | ||||||
| Orlowski et al., 200720 | B | 6.5 | 6.5c | 41 | 2 | 65% | 7.2g |
| B plus peg-Dox | 9.0 | 9.3c | 44 | 4 | 76% | ||
| hr: 1.69 | hr: 1.82 (95% ci: 1.41 to 2.35) | p=0.43 | (15 months) | ||||
| p=0.000026 | p=0.000004 | p=0.03 | |||||
| Lonial et al., 201017 | B (escalating doses of 1.0, 1.3, and 1.6 mg/m 2) 24 hours before M | ns | nr | 47 | 11 | ns | 17.3 |
| B (escalating doses of 1, 1.3, and 1.6 mg/m2) 24 hours after M | ns | nr | 55 | 30 | ns | ||
| Reece et al., 201122 | B 1.3 mg/m2 | nr | nr | 52 | 5 | nr | nr |
| B 1.0 mg/m2 | nr | nr | 48 | 10 | nr | ||
| Garderet et al., 201230 | B–T–D | 19.3 | 19.5 | 45 | 45 | 71% | 30 |
| T–D | 13.6 | 13.8 | 25 | 25 | 65% | ||
| p<0.001 | p<0.001 | p<0.001 | p<0.001 | (ns) | |||
| Hjorth et al., 201225 | B–D | 7.2 | 1.6 | 63 | nr | 19 | 3.5 on B, |
| T–D | 9 (ns) | 3.0 | 55 (ns) | nr | 22.8 | 5.1 on Tj | |
| p<0.005 | |||||||
| Orlowski et al., 201283 (abs.) | Siltuximab plus B | 8.1 | nr | 55 | 11 | 30.8 | 24.5 |
| B | 7.6 (ns) | nr | 47 (ns) | 7 (ns) | 36.9 (ns) | ||
Results in evaluable population (B–M–P: n = 337; M–P: n = 331).
Median follow-up was 36.7 months for overall survival and 16.3 months for time to progression in the original publication. An update55 confirmed a statistically significant survival benefit for B–M–P compared with M–P at a median follow-up of 25.9 months (hr: 0.64; p = 0.003). In a further publication59, 3-year rates of overall survival were estimated at 68.5% for B–M–P compared with 54% with M–P.
Results for primary outcome.
Bortezomib on a weekly schedule.
The original data cut-off was April 28, 2006, at which time the median follow-up was 7.2 months; survival and time to event data were reanalyzed with a data cut-off of November 28, 2006, upon request by the U.S. Food and Drug Administration. Median follow-up for overall survival and time to progression were not reported.
Results in the evaluable population (B–D: n = 223 after induction, n = 197 after first transplant; V–Dox–D: n = 218 after induction, n = 184 after first transplant).
Median overall has not been reached in either group. Overall survival rates were 81.4% with B–D and 81.4% with V–Dox–D.
The estimated 3-year overall survival was 86% compared with 84% (p = 0.30).
Data from an additional publication41.
Terminated early because of low accrual.
pfs = progression-free survival; ttp = time to progression; or = odds ratio; cr = complete response; os = overall survival; asct = autologous stem-cell transplantation; Int = intervention group; B = bortezomib; M = melphalan; P = prednisone; nr = not reported; hr = hazard ratio; ne = not reached or not estimable; T = thalidomide; ns = statistically nonsignificant; D = dexamethasone; C = cyclophosphamide; L = lenalidomide; abs. = abstract; E = etoposide; Cis = cisplatinum; V = vincristine; Dox = doxorubicin; ato = arsenic trioxide; peg-Dox = pegylated liposomal doxorubicin; ci = confidence interval.
3.4.1. Previously Untreated MM
TTP:
Among the studies of patients who were not candidates for asct, the vista study24 found a significant difference in ttp after induction with bortezomib compared with a non-bortezomib-containing regimen (hr: 0.48; p < 0.001). The other studies either did not report results for this endpoint21,26,82 or did not find a significant difference when comparing bortezomib in two different combination regimens19. None of the studies involving patients who were candidates for asct reported on this endpoint.
Overall Survival:
Among studies of patients who were not candidates for asct, the vista trial24,56,57 reported a statistically significant difference in overall survival (os) when bortezomib was compared with a non-bortezomib-containing regimen (hr: 0.65; p < 0.001). In the studies of transplant patients, Sonneveld et al.31 demonstrated a statistically significant difference in os (hr: 0.77; 95% ci: 0.60 to 1.00; p = 0.049); in the other studies, median os was not significantly different for the control groups or was not estimable (see Table v).
PFS:
Among studies of patients who were not candidates for asct, Palumbo et al.21 found a statistically significant difference in pfs favouring bortezomib in a 4-drug combination induction regimen plus bortezomib-containing maintenance compared with bortezomib in a 3-drug combination induction alone (hr: 0.67; p = 0.008). In a related abstract publication, Niesvitzky et al. found no significant difference in pfs between the treatment arms82.
Among the studies of patients who were candidates for asct, Sonneveld et al.31 found a significantly longer pfs in patients allocated to bortezomib, doxorubicin, and dexamethasone than in patients allocated to vincristine, doxorubicin, and dexamethasone (hr: 0.74; 95% ci: 0.62 to 0.89; p < 0.001). Cavo et al.15 suggested a significantly better pfs, projected to be 36 months, for bortezomib, dexamethasone, and thalidomide compared with dexamethasone and thalidomide (hr: 0.63; 95% ci: 0.45 to 0.88; p < 0.006). Harousseau et al.16 compared a bortezomib–dexamethasone combination with a vincristine–doxorubicin–dexamethasone combination, but pfs did not reach statistical significance in favour of the bortezomib arm (p = 0.057). In an abstract publication, Rosinol et al.84 found that pfs was statistically significantly longer in the bortezomib–thalidomide arm than in the thalidomide-alone or interferon arms (pfs at 2 years: 78% vs. 63% vs. 49%; p = 0.01).
QOL:
Health-related qol was measured using various domains of the European Organisation for Research on Treatment of Cancer Quality of Life Questionnaire–Core (qlq-C30)88 in two studies47,76. In a subanalysis of the vista trial24, Dhawan et al.47 showed that newly diagnosed mm patients treated with bortezomib, melphalan, and prednisone had a higher sustained rate of improvement in health-related qol than did patients treated with melphalan and prednisone (14 of 15 domains). They also reported a statistically significant improvement in 3 domains: Nausea/Vomiting (p = 0.0095), Appetite Loss (p = 0.0170), and Diarrhea (p = 0.0082)47. Niesvitsky et al.76 found no statistically significant differences between arms.
Response Rate:
In patients who were not candidates for asct, cr and overall response (or) were found to be statistically significantly different for a bortezomib compared with a non-bortezomib-containing regimen (cr: 30% vs. 4%, p < 0.001; or: 71% vs. 35%, p < 0.001)24 and for a 4-drug–plus– maintenance combination compared with a 3-drug combination (cr: 38% vs. 24%, p < 0.001; or: 59% vs. 50%, p = 0.03)21. No statistically significant difference was found when comparing two 3-drug combinations containing bortezomib19.
Among patients who were candidates for transplantation, Harousseau et al.16 found a statistically significant difference in cr in favour of a bortezomib–dexamethasone combination both after induction and after a first transplant (induction: 14.8% vs. 6.4%, p = 0.004; first transplant: 16.1% vs. 8.7%, p = 0.016). Sonneveld et al.31 found a statistically significant difference in cr in favour of bortezomib at induction and at maintenance (7% vs. 2% and 21% vs. 9% respectively, p < 0.001). For or after first transplant, no statistically significant difference was detected16. Cavo et al.15 reported a significant difference in cr in favour of bortezomib–dexamethasone–thalidomide compared with thalidomide–dexamethasone at induction, after first transplantation, at second transplantation after consolidation, and overall (p values shown in Table iv). Moreau et al.28 found no statistically significant difference in cr and objective response rate between study arms.
3.4.2. Relapsed and Refractory MM
TTP:
Bortezomib monotherapy improved ttp statistically significantly more than did dexamethasone alone (hr: 0.55; p < 0.001)23. Compared with bortezomib monotherapy, the combination of bortezomib with pegylated liposomal doxorubicin (pld) significantly improved ttp (hr: 1.82; p = 0.000004)20. As well, bortezomib–dexamethasone or bortezomib–thalidomide–dexamethasone were more effective than thalidomide–dexamethasone in improving ttp (p < 0.005 and p < 0.001, Table iv)25,30.
OS:
Bortezomib in combination with pld was found to significantly improve os (65% vs. 76%, p = 0.03)20. Bortezomib monotherapy improved os significantly more than did dexamethasone (hr: 0.77, p = 0.003; and hr: 0.67, p = 0.47)23,33. No significant difference was seen with the administration of bortezomib before or after melphalan17 or in combination with thalidomide, dexamethasone, or siltuximab25,30,83.
PFS:
Bortezomib in combination with pld (compared with bortezomib alone) and bortezomib–thalidomide–dexamethasone (compared with thalidomide–dexamethasone) were found to significantly improve pfs (hr: 1.69, p = 0.000026, and hr: 0.61, p < 0.001, respectively)20,30.
Response Rate:
No significant difference in or or cr was detected between bortezomib monotherapy and bortezomib plus pld20. Bortezomib monotherapy was significantly better than dexamethasone for cr and or (p < 0.001)33. Bortezomib in a 3-drug combination with thalidomide and dexamethasone was better than thalidomide and dexamethasone in improving or and cr (45% vs. 25%, p < 0.001, and 45% vs. 11%, p < 0.001)30.
QOL:
The qlq-C30 was used in two studies25,36 to measure qol. Lee et al.36 reported qol for patients in the vista trial (originally reported by Richardson et al.23). The authors assessed health-related qol using the qlq-C3089 and adverse events with neurotoxicity symptoms using the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group–Neurotoxicity subscale88,90. Quality of life was assessed at baseline and every 6 weeks thereafter up to 42 weeks from baseline. A statistically significant difference in Global Health Status favouring bortezomib over dexamethasone during the 42 weeks of the study (p = 0.001) was reported. In addition, the authors reported a statistically significant difference in overall Functional Assessment of Cancer Therapy/Gynecologic Oncology Group–Neurotoxicity score in favour of bortezomib (p = 0.02). On the other hand, Hjorth et al.25 found no statistically significant difference between study arms comparing bortezomib with thalidomide (both combined with dexamethasone).
3.5. Toxicity
Question:
What is the toxicity associated with the use of bortezomib?
3.5.1. Previously Untreated MM
Studies in the previously untreated population showed a significant increase in peripheral neuropathy in the bortezomib group when a drug combination including bortezomib was compared with a nonbortezomib-containing regimen15,16,24,31. In addition, San Miguel et al.24 reported higher incidences of diarrhea and nausea in their bortezomib group than in their control group (8% vs. 1% and 4% vs. <1% respectively, p not reported). Table vi presents detailed data on adverse events.
TABLE VI.
Randomized trials of patients with multiple myeloma: adverse events
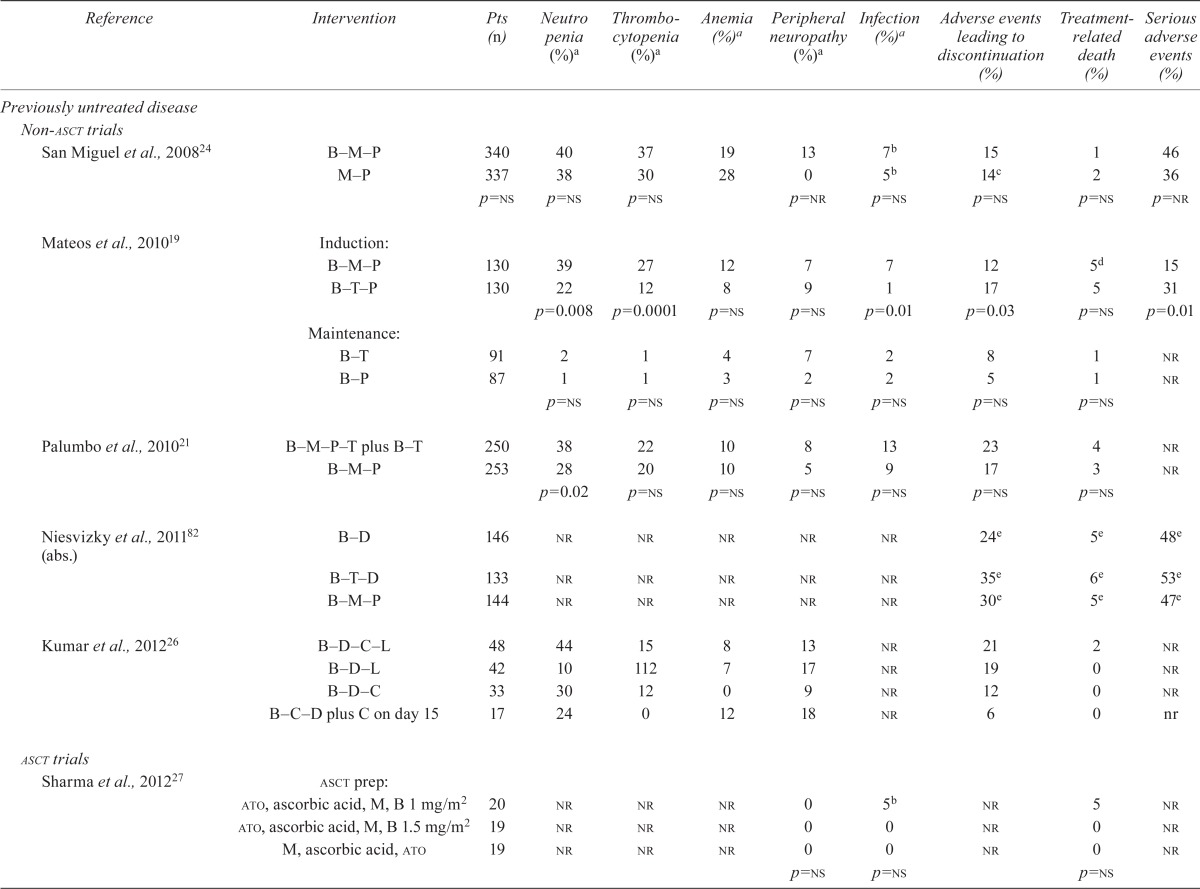
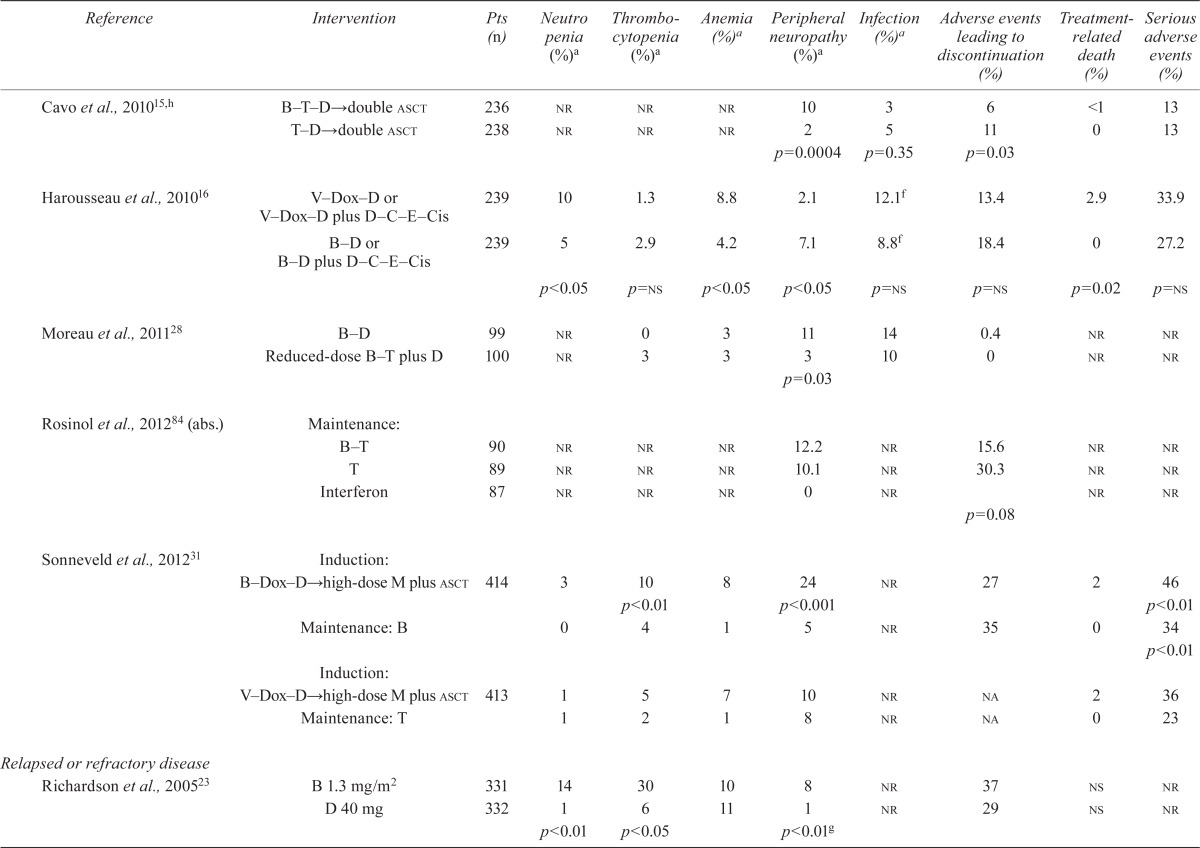
| Reference | Intervention | Pts (n) | Neutro penia (%)a | Thrombocytopenia (%)a | Anemia (%)a | Peripheral neuropathy (%)a | Infection (%)a | Adverse events leading to discontinuation (%) | Treatment-related death (%) | Serious adverse events (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Previously untreated disease | ||||||||||
| Non-asct trials | ||||||||||
| San Miguel et al., 200824 | B–M–P | 340 | 40 | 37 | 19 | 13 | 7b | 15 | 1 | 46 |
| M–P | 337 | 38 | 30 | 28 | 0 | 5b | 14c | 2 | 36 | |
| p=ns | p=ns | p=ns | p=nr | p=ns | p=ns | p=ns | p=nr | |||
| Mateos et al., 201019 | Induction: B–M–P | 130 | 39 | 27 | 12 | 7 | 7 | 12 | 5d | 15 |
| B–T–P | 130 | 22 | 12 | 8 | 9 | 1 | 17 | 5 | 31 | |
| p=0.008 | p=0.0001 | p=ns | p=ns | p=0.01 | p=0.03 | p=ns | p=0.01 | |||
| Maintenance: B–T | 91 | 2 | 1 | 4 | 7 | 2 | 8 | 1 | nr | |
| B–P | 87 | 1 | 1 | 3 | 2 | 2 | 5 | 1 | nr | |
| p=ns | p=ns | p=ns | p=ns | p=ns | p=ns | p=ns | ||||
| Palumbo et al., 201021 | B–M–P–T plus B–T | 250 | 38 | 22 | 10 | 8 | 13 | 23 | 4 | nr |
| B–M–P | 253 | 28 | 20 | 10 | 5 | 9 | 17 | 3 | nr | |
| p=0.02 | p=ns | p=ns | p=ns | p=ns | p=ns | p=ns | ||||
| Niesvizky et al., 201182 (abs.) | B–D | 146 | nr | nr | nr | nr | nr | 24e | 5e | 48e |
| B–T–D | 133 | nr | nr | nr | nr | nr | 35e | 6e | 53e | |
| B–M–P | 144 | nr | nr | nr | nr | nr | 30e | 5e | 47e | |
| Kumar et al., 201226 | B–D–C–L | 48 | 44 | 15 | 8 | 13 | nr | 21 | 2 | nr |
| B–D–L | 42 | 10 | 112 | 7 | 17 | nr | 19 | 0 | nr | |
| B–D–C | 33 | 30 | 12 | 0 | 9 | nr | 12 | 0 | nr | |
| B–C–D plus C on day 15 | 17 | 24 | 0 | 12 | 18 | nr | 6 | 0 | nr | |
| asct trials | ||||||||||
| Sharma et al., 201227 | asct prep:ato, ascorbic acid, M, B 1 mg/m2 | 20 | nr | nr | nr | 0 | 5b | nr | 5 | nr |
| ato, ascorbic acid, M, B 1.5 mg/m2 | 19 | nr | nr | nr | 0 | 0 | nr | 0 | nr | |
| M, ascorbic acid, ato | 19 | nr | nr | nr | 0 | 0 | nr | 0 | nr | |
| p=ns | p=ns | p=ns | ||||||||
| Cavo et al., 201015,h | B T D→double asct | 236 | nr | nr | nr | 10 | 3 | 6 | <1 | 13 |
| T D→double asct | 238 | nr | nr | nr | 2 | 5 | 11 | 0 | 13 | |
| p=0.0004 | p=0.35 | p=0.03 | ||||||||
| Harousseau et al., 201016 | V–Dox–D or V–Dox–D plus D–C–E–Cis | 239 | 10 | 1.3 | 8.8 | 2.1 | 12.1f | 13.4 | 2.9 | 33.9 |
| B–D or B–D plus D–C–E–Cis | 239 | 5 | 2.9 | 4.2 | 7.1 | 8.8f | 18.4 | 0 | 27.2 | |
| p<0.05 | p=ns | p<0.05 | p<0.05 | p=ns | p=ns | p=0.02 | p=ns | |||
| Moreau et al., 201128 | B–D | 99 | nr | 0 | 3 | 11 | 14 | 0.4 | nr | nr |
| Reduced-dose B–T plus D | 100 | nr | 3 | 3 | 3 | 10 | 0 | nr | nr | |
| p=0.03 | ||||||||||
| Rosinol et al., 201284 (abs.) | Maintenance: B–T | 90 | nr | nr | nr | 12.2 | nr | 15.6 | nr | nr |
| T | 89 | nr | nr | nr | 10.1 | nr | 30.3 | nr | nr | |
| Interferon | 87 | nr | nr | nr | 0 | nr | nr | nr | ||
| p=0.08 | ||||||||||
| Sonneveld et al., 201231 | Induction: B Dox D→high-dose M plus asct | 414 | 3 | 10 | 8 | 24 | nr | 27 | 2 | 46 |
| p<0.01 | p<0.001 | p<0.01 | ||||||||
| Maintenance: B | 0 | 4 | 1 | 5 | nr | 35 | 0 | 34 | ||
| p<0.01 | ||||||||||
| Induction:V Dox D→high-dose M plus asct | 413 | 1 | 5 | 7 | 10 | nr | na | 2 | 36 | |
| Maintenance: T | 1 | 2 | 1 | 8 | nr | na | 0 | 23 | ||
| Relapsed or refractory disease | ||||||||||
| Richardson et al., 200523 | B 1.3 mg/m2 | 331 | 14 | 30 | 10 | 8 | nr | 37 | ns | nr |
| D 40 mg | 332 | 1 | 6 | 11 | 1 | nr | 29 | ns | nr | |
| p<0.01 | p<0.05 | p<0.01g | ||||||||
| Orlowski et al., 200720 | B 1.3 mg/m2 | 318 | 15 | 16 | 9 | 9 | nr | nr | 4d | nr |
| B 1.3 mg/m2 plus peg-Dox 30 mg/m2 | 318 | 29 | 23 | 9 | 4 | nr | 5 | 3d | nr | |
| p<0.001 | p=ns | p=ns | p=ns | |||||||
| Lonial et al., 201017 | Escalating dose (B 1.0, 1.3, or 1.6 mg/m2): | |||||||||
| 24 Hours before high-dose M | 19 | 45 | nr | nr | nr | 16 | nr | nr | nr | |
| 24 Hours after high-dose M | 20 | 65 | nr | nr | nr | 10 | nr | nr | nr | |
| p=ns | ||||||||||
| Reece et al., 201122 | B 1.3 mg/m2 | 21 | nr | 33 | 10 | 24 | nr | 33 | nr | nr |
| B 1.0 mg/m2 | 21 | nr | 10 | 10 | 24 | nr | 24 | nr | nr | |
| Garderet et al., 201230 | B–T–D | 135 | 11 | 17 | 8 | 16 | 14 | nr | nr | nr |
| T–D | 134 | 16 | 7 | 5 | 12 | 7 | nr | nr | nr | |
| p=0.02 | ||||||||||
| Hjorth et al., 201225 | B–D | 64 | 17 | 34 | nr | 19 | 33 | nr | nr | nr |
| T–D | 67 | 13 | 6 | nr | 7 | 24 | nr | nr | nr | |
| p=nr | p=nr | p=nr | ||||||||
| Orlowski et al., 201283 (abs.) | Siltuximab plus B | 142 | 49 | 48 | nr | nr | nr | nr | 8 | 29 |
| B | 144 | nr | nr | nr | nr | nr | nr | 5 | 24 | |
Grade 3 or 4 adverse events.
Patients with pneumonia.
In addition, bortezomib alone was discontinued in another 19% of patients.
Deaths within 30 days after the last study medication.
Adverse events reported during induction.
A significant difference was reported for between-groups grades 1–4 herpes zoster (p < 0.05).
Grade 3 adverse event.
Adverse events reported during induction.
Pts = patients; asct= autologous stem-cell transplantation; B = bortezomib; M = melphalan; P = prednisone; ns = statistically nonsignificant; nr = not reported; abs.= abstract; T = thalidomide; D = dexamethasone; C = cyclophosphamide; L = lenalidomide; ato = arsenic trioxide; V = vincristine; Dox = doxorubicin; E = etoposide; Cis = cisplatinum; peg-Dox = pegylated liposomal doxorubicin.
The studies that compared various drug combinations containing bortezomib showed a higher incidence of neutropenia (38% vs. 28%, p = 0.02)21 and peripheral neuropathy (10% vs. 2%, p < 0.0004)15 with a 4-drug combination than with a 3-drug combination. A higher incidence of peripheral neuropathy was found when drug combinations with higher bortezomib doses were used (11% vs. 3%, p < 0.003)28. The incidence of neutropenia was also higher with a 3-drug combination containing an alkylating agent than with a 3-drug combination containing an immunomodulatory agent (39% vs. 22%, p = 0.008)19. Sonneveld et al.31 reported a higher incidence of peripheral neuropathy in a bortezomib-containing combination (24% vs. 10%, p < 0.01). Palumbo et al.21 also reported a higher incidence of cardiac events and thromboembolism with a 4-drug combination than with a 3-drug combination (10% vs. 5%, p = 0.04, and 5% vs. 2%, p = 0.05, respectively). Mateos et al.19 showed that, compared with bortezomib combined with melphalan and an alkylating agent, a 3-drug combination including bortezomib and an immunomodulatory agent was favourable for a significantly lower incidence of thrombocytopenia, neutropenia, and infections (Table vi). However, the same study found a significant difference favouring the 3-drug combination including bortezomib and an alkylating agent for adverse events necessitating discontinuation of the drug and for overall serious adverse events (Table vi). The incidence of cardiac events was also significantly higher in the group that received bortezomib combined with an immunomodulatory agent than in the group that received a combination of bortezomib and an alkylating agent (8% vs. 0%, p = 0.001)19. Kumar et al.26 reported a higher incidence of treatment-related deaths from renal failure in the 4-drug combination that included an immunomodulatory and an alkylating agent (Table vi).
3.5.2. Relapsed and Refractory MM
Our review found a higher incidence of hematologic events (Table vi) and peripheral neuropathy, and a significantly higher incidence of diarrhea and nausea (7% vs. 2% and 2% vs. 0% respectively, p < 0.01), in patients with relapsed or refractory mm who received bortezomib than in those who received dexamethasone (control)23. Orlowski et al.20 also showed an increased incidence of neutropenia (Table vi), diarrhea, and nausea in a bortezomib–pld group than in a bortezomib-alone group (7% vs. 4%, p = 0.034, and 2% vs. <1%, p = 0.0241, respectively). A higher incidence of peripheral neuropathy and thrombocytopenia was observed by Sonneveld et al.31 in patients who received bortezomib in combination with other drugs than in those who received bortezomib alone or a non-bortezomib-containing drug combination (24% vs. 10%, p < 0.001, and 10% vs. 5%, p < 0.01). When various doses of bortezomib were compared, Moreau et al.28 also showed a higher incidence of peripheral neuropathy with higher doses of bortezomib (11% with full dose vs. 3% with reduced dose, p = 0.03).
Adverse events leading to discontinuation of treatment occurred in 37% of a bortezomib group compared with 29% of a dexamethasone group (reported by Richardson et al.23). A corollary report from the apex study23 by Chanan–Khan35 examined the incidence of herpes zoster events in patients treated with bortezomib. The authors found that a significantly higher incidence of herpes zoster was associated with bortezomib than with the control dexamethasone treatment (13% vs. 5%, p = 0.0002).
3.6. Subgroups
Question:
Which patients are more or less likely to benefit from treatment with bortezomib?
3.6.1. Previously Untreated MM
The combination of melphalan–prednisone–bortezomib produced better results than did melphalan– prednisone24 in these subgroups:
Patients 75 years of age and older: ttp was identical in the younger and older groups; cr was 26% in the older group and 32% in the younger group, p = 0.29; os, p = 0.17
Patients with impaired renal function: cr, ttp, and os did not differ for 159 patients with normal renal function and for 185 patients with a creatinine clearance less than 60 mL/min
Also, although cytogenetic studies were not available in all participants, the 26 patients with a high-risk cytogenetic profile [t(4;14), t(14;16), and del17p] did not differ from the 142 patients with a standard profile in cr (both groups: 28%), ttp (p = 0.55), and os (p = 0.99)24.
In the study by Mateos et al.19, patients with cytogenetic abnormalities [t(4;14), t(14;16), and del17p (44 vs. 187)] in both treatment groups did not differ for cr, but experienced shorter pfs and os at induction (hr: 0.6, p = 0.01, and hr: 0.5, p = 0.01, respectively) and maintenance (p = 0.01 and p < 0.0001 respectively). In the study by Neben et al.71, a companion study of the hovon-65 study31, patients with all chromosomal aberrations treated with bortezomib– doxorubicin–dexamethasone experienced a pfs and os similar or superior to those of patients treated with vincristine–doxorubicin–dexamethasone. The patients that seemed to benefit more from bortezomib treatment had del(17p13): their pfs duration was 26.2 months compared with 12 months in peers not receiving bortezomib (p = 0.024); the associated 3-year os rates were 69% and 17% (p = 0.028).
3.6.2. Relapsed or Refractory MM
Extensive subset analyses have been performed using data from the apex trial23 of bortezomib compared with dexamethasone for relapsed or refractory myeloma32–39. Bortezomib was consistently superior to dexamethasone in patients 65 years of age and older (response rate: p = 0.0004; ttp: p = 0.002); in patients with International Staging System stage ii and iii disease (response rate: p < 0.0004; ttp: p = 0.0002); in patients refractory to the most recent therapy and in those who had previously received more than one line of therapy (both subgroups—response rate: p < 0.0001; and ttp: p < 0.0001)32; and in patients with renal impairment34. Similarly, bortezomib–pld was more efficacious than bortezomib alone in most subgroups analyzed, including patients of any age; patients with refractory disease; patients with elevated β2-microglobulin; and patients previously exposed to asct, anthracyclines, and immunomodulatory drugs (thalidomide or lenalidomide)20,41. An advantage for bortezomib–pld compared with bortezomib alone was also observed in patients with cytogenetic abnormalities except for deletion 13q20.
4. DISCUSSION
Introduction of the melphalan–prednisone–bortezomib combination in newly diagnosed mm patients significantly improved outcome in patients who are not candidates for asct24. Eligible patients include those more than 65–70 years of age and those with concomitant medical conditions felt to increase the risks of asct. Compared with melphalan and prednisone alone, melphalan–prednisone–bortezomib in a finite course (9 cycles) improved ttp and os and resulted in better or and cr rates. Surprisingly, hematologic toxicity was not increased, and other toxicity rates were similar to those observed in various series using bortezomib. Melphalan–prednisone–bortezomib was superior in all patient subgroups and might have particular benefit in patients with poor prognostic factors in whom melphalan–prednisone has limited efficacy, such as patients with a high β2-microglobulin level and adverse cytogenetics.
Making a direct comparison to initial therapy with melphalan–prednisone–thalidomide is difficult. A previously reported systematic review91 that forms the evidence base of an earlier practice guideline (evidence-based series report #6–21: Thalidomide in Multiple Myeloma)92 indicated that melphalan– prednisone–thalidomide is the preferred treatment option for patients with mm who are not eligible for asct. However, given the lack of actual comparative evidence and the recognition that thalidomide can be difficult to obtain or to tolerate, physicians and patients might choose to initiate therapy with bortezomib-containing therapy—a choice that is currently supported by a network meta-analysis that showed no difference for all outcomes and a significant benefit for cr (rr: 2.34; 95% ci: 1.12 to 4.90) and for grades 3 and 4 adverse events (rr: 0.53; 95% ci: 0.38 to 0.73) in favour of bortezomib10. In particular, bortezomib-based therapy might be preferred in patients with disease-related renal dysfunction or cytogenetic abnormalities. Studies testing lenalidomide and dexamethasone as an upfront option are still ongoing, with no results yet available (search for NCT01554852 at http://clinicaltrials.gov/).
In mm, most studies have indicated that patients who achieve a cr, a near-cr (same as cr, but residual monoclonal protein by immunofixation only), or in some instances, a very good partial remission (defined as >90%), particularly after asct, have superior rates of pfs and os compared with lesser degrees of response. Many phase ii studies of combination regimens containing novel agents such as bortezomib as first-line therapy have reported higher rates of cr, near-cr, or very good partial remission before asct compared with the rates observed with older regimens such as vincristine –doxorubicin–dexamethasone or dexamethasone alone. One approach to improving the results of asct therefore involves using novel agents upfront so that patients will go into transplantation with a greater depth of remission (on the hypothesis that rates of cr or near-cr, and hence survival, will be improved after asct). Several phase iii randomized trials comparing bortezomib-containing induction regimens were designed to test that hypothesis15,16. Those trials found a statistically significant better cr and a favourable pfs in the bortezomib arm. In addition, two studies further supported the use of bortezomib before asct. In the study by Sonneveld et al.31, an os benefit was suggested for a bortezomib-based combination before asct given with bortezomib-based consolidation after asct. The dsg is also aware of a study-level meta-analysis, so far published only in abstract form and therefore excluded from this systematic review, which supports a survival benefit with the use of bortezomib before asct93. Given the benefits and the recognized toxicities associated with earlier chemotherapy-based regimens, the dsg considers bortezomib-based induction to be a recommended option before asct.
Despite effective first-line therapy, nearly all mm patients eventually relapse and require further therapy. Options for the management of recurrent mm include reinstitution of the initial treatment if the duration of response was prolonged, a second asct as salvage therapy, alkylating agents with corticosteroids, high-dose dexamethasone, or thalidomide alone or in combination with corticosteroids. Lenalidomide is now approved by Health Canada for use with dexamethasone in the treatment of mm that has progressed after at least 1 prior treatment regimen. Many phase i–ii trials have combined novel agents, particularly bortezomib, with conventional cytotoxic agents or other novel agents as first-line therapy. Evidence fr om rcts supports the use of bortezomib in combination with pld in patients with relapsed or refractory mm20. In patients who cannot tolerate that therapy, the use of bortezomib alone for relapsed or refractory disease is recommended by the dsg.
The Hematology dsg has already recommended bortezomib monotherapy for patients with mm refractory to or relapsing within 1 year of the conclusion of initial or subsequent treatments and who are candidates for further therapy94. That recommendation was made based on the benefit in os and ttp observed in the apex trial23. The extended follow-up of apex reported by Richardson et al.33 indicates that the benefit still exists. In relapsed and refractory mm, bortezomib monotherapy and combination therapy with pld are both effective approaches. However, compared with bortezomib alone, the combination with pld improves ttp, pfs, and os significantly20. The magnitude of the benefit for the combination of bortezomib and pld is identical to that seen for bortezomib alone compared with dexamethasone in the original pivotal trial of bortezomib, the apex trial23,33. However, whether the benefit applies to all patients with mm is unknown, because the authors excluded patients who had previously received more than 240 mg/m2 or an equivalent dose of doxorubicin20. Particular advantages of the pld–bortezomib combination are its avoidance of the use of corticosteroids (which are required in most of the other anti-mm regimens), its efficacy in high-risk groups, and its effectiveness after prior exposure to immunomodulatory derivatives. However, the combination is associated with more toxicity—specifically, myelo-suppression, gastrointestinal toxicity, and hand–foot syndrome. Bortezomib monotherapy might therefore be preferable in patients with coexisting medical conditions or in frail patients.
5. CONCLUSIONS
In patients with previously untreated mm who are not candidates for asct, bortezomib combined with melphalan and prednisone is the preferred first-line therapy. In patients who are eligible for asct, bortezomib-based induction before transplantation is a recommended option.
In patients with relapsed or refractory mm, the combination of pld plus bortezomib is a more effective treatment option than is bortezomib alone. The combination can be considered for use in patients with a cumulative doxorubicin dose less than 240 mg/m2 (or the equivalent). In patients with poor steroid tolerance, with brittle bones, or with diabetes mellitus, this combination is particularly useful. For individuals who cannot access or tolerate this therapy, treatment with bortezomib alone is recommended. Consideration should be given to the use of antiviral prophylaxis against herpes zoster (shingles), because that condition is now recognized to occur more frequently during bortezomib therapy23,35.
For specific details related to the administration of bortezomib therapy, the authors suggest that clinicians refer to the protocols used in the major trials and to the product monograph. Most toxicities are reversible if dose modification guidelines are followed.
6. ACKNOWLEDGMENTS
The Program in Evidence-Based Care is supported by the Ontario Ministry of Health and Long-Term Care through Cancer Care Ontario. All work produced by the Program in Evidence-Based Care is editorially independent from its funding source.
7. CONFLICT OF INTEREST DISCLOSURES
The authors of this report disclosed potential conflicts. One author (DER) was the principal investigator or the local investigator and received research funding for four trials. DER was also a consultant for the manufacturer of bortezomib, an advisory board participant for a future trial, and received honoraria. One other author (TCK) received honoraria while acting as a consultant for the manufacturer of bortezomib and was an advisory board participant. All other authors declared no financial conflicts of interest.
8. REFERENCES
- 1.Yang H, Zonder JA, Dou QP. Clinical development of novel proteasome inhibitors for cancer treatment. Expert Opin Investig Drugs. 2009;18:957–71. doi: 10.1517/13543780903002074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Health Canada . Approval of Velcade with conditions [letter to health professionals] Ottawa, ON: Health Canada; 2006. [Available online at: http://www.hc-sc.gc.ca/dhp-mps/alt_formats/hpfb-dgpsa/pdf/prodpharma/velcade_dhcpl_lapds_2006_098077-eng.pdf; cited January 5, 2011] [Google Scholar]
- 3.Engelhardt M, Kleber M, Udi J, et al. Consensus statement from European experts on the diagnosis, management, and treatment of multiple myeloma: from standard therapy to novel approaches. Leuk Lymphoma. 2010;51:1424–43. doi: 10.3109/10428194.2010.487959. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network (nccn) NCCN Clinical Practice Guidelines in Oncology Multiple Myeloma. Fort Washington, PA: NCCN; 2011. Ver. 1.2011. [Current version available online at: http://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf (free registration required); cited January 5, 2011] [DOI] [PubMed] [Google Scholar]
- 5.Palumbo A, Sezer O, Kyle R, et al. International Myeloma Working Group guidelines for the management of multiple myeloma patients ineligible for standard high-dose chemotherapy with autologous stem cell transplantation. Leukemia. 2009;23:1716–30. doi: 10.1038/leu.2009.122. [DOI] [PubMed] [Google Scholar]
- 6.Reece D, Imrie K, Stevens A, Smith CA, on behalf of the Hematology Disease Site Group of Cancer Care Ontario’s Program in Evidence-Based Care Bortezomib in multiple myeloma and lymphoma: a systematic review and clinical practice guideline. Curr Oncol. 2006;13:162–72. doi: 10.3747/co.v13i5.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barosi G, Merlini G, Billio A, et al. sie, sies, gitmo evidence-based guidelines on novel agents (thalidomide, bortezomib, and lenalidomide) in the treatment of multiple myeloma. Ann Hematol. 2012;91:875–88. doi: 10.1007/s00277-012-1445-y. [DOI] [PubMed] [Google Scholar]
- 8.Bird JM, Owen RG, D’Sa S, et al. on behalf of the Haematooncology Task Force of British Committee for Standards in Haematology (bcsh) and UK Myeloma Forum Guidelines for the diagnosis and management of multiple myeloma 2011. Br J Haematol. 2011;154:32–75. doi: 10.1111/j.1365-2141.2011.08573.x. [DOI] [PubMed] [Google Scholar]
- 9.Doss S, Hay N, Sutcliffe F. nice guidance on bortezomib and thalidomide for first-line treatment of multiple myeloma. Lancet Oncol. 2011;12:837–8. doi: 10.1016/S1470-2045(11)70202-9. [DOI] [PubMed] [Google Scholar]
- 10.Kumar A, Hozo I, Wheatley K, Djulbegovic B. Thalidomide versus bortezomib based regimens as first-line therapy for patients with multiple myeloma: a systematic review. Am J Hematol. 2011;86:18–24. doi: 10.1002/ajh.21904. [DOI] [PubMed] [Google Scholar]
- 11.Picot J, Cooper K, Bryant J, Clegg AJ. The clinical effectiveness and cost-effectiveness of bortezomib and thalidomide in combination regimens with an alkylating agent and a corticosteroid for the first-line treatment of multiple myeloma: a systematic review and economic evaluation. Health Technol Assess. 2011;15:1–204. doi: 10.3310/hta15410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palumbo A, Rajkumar SV. Multiple myeloma: chemotherapy or transplantation in the era of new drugs. Eur J Haematol. 2010;84:379–90. doi: 10.1111/j.1600-0609.2010.01431.x. [DOI] [PubMed] [Google Scholar]
- 13.Piro E, Molica S. A systematic review on the use of bortezomib in multiple myeloma patients with renal impairment: what is the published evidence? Acta Haematol. 2011;126:163–8. doi: 10.1159/000328417. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Ran X, Wang B, Sheng Z, Liu L. Novel agents-based regimens as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: a meta-analysis of randomized controlled trials. Hematol Oncol. 2012;30:57–61. doi: 10.1002/hon.1007. [DOI] [PubMed] [Google Scholar]
- 15.Cavo M, Tacchetti P, Patriarca F, et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet. 2010;376:2075–85. doi: 10.1016/S0140-6736(10)61424-9. [DOI] [PubMed] [Google Scholar]
- 16.Harousseau JL, Attal M, Avet–Loiseau H, et al. Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: results of the ifm 2005-01 phase iii trial. J Clin Oncol. 2010;28:4621–9. doi: 10.1200/JCO.2009.27.9158. [DOI] [PubMed] [Google Scholar]
- 17.Lonial S, Kaufman J, Tighiouart M, et al. A phase i/ii trial combining high-dose melphalan and autologous transplant with bortezomib for multiple myeloma: a dose- and schedule-finding study. Clin Cancer Res. 2010;16:5079–86. doi: 10.1158/1078-0432.CCR-10-1662. [DOI] [PubMed] [Google Scholar]
- 18.Ludwig H, Viterbo L, Greil R, et al. Bortezomib, thalidomide, and dexamethasone (vtd) versus vtd plus cyclophosphamide as induction therapy in previously untreated multiple myeloma patients eligible for hdt-asct: a randomized phase 2 trial [abstract 2312] Blood. 2009;114 [Google Scholar]
- 19.Mateos MV, Oriol A, Martinez–Lopez J, et al. Bortezomib, melphalan, and prednisone versus bortezomib, thalidomide, and prednisone as induction therapy followed by maintenance treatment with bortezomib and thalidomide versus bortezomib and prednisone in elderly patients with untreated multiple myeloma: a randomised trial. Lancet Oncol. 2010;11:934–41. doi: 10.1016/S1470-2045(10)70187-X. [DOI] [PubMed] [Google Scholar]
- 20.Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized phase iii study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol. 2007;25:3892–901. doi: 10.1200/JCO.2006.10.5460. [DOI] [PubMed] [Google Scholar]
- 21.Palumbo A, Bringhen S, Rossi D, et al. Bortezomib–melphalan–prednisone–thalidomide followed by maintenance with bortezomib–thalidomide compared with bortezomib–melphalan–prednisone for initial treatment of multiple myeloma: a randomized controlled trial. J Clin Oncol. 2010;28:5101–9. doi: 10.1200/JCO.2010.29.8216. [DOI] [PubMed] [Google Scholar]
- 22.Reece DE, Sullivan D, Lonial S, et al. Pharmacokinetic and pharmacodynamic study of two doses of bortezomib in patients with relapsed multiple myeloma. Cancer Chemother Pharmacol. 2011;67:57–67. doi: 10.1007/s00280-010-1283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–98. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 24.San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–17. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 25.Hjorth M, Hjertner O, Knudsen LM, et al. on behalf of the Nordic Myeloma Study Group (nmsg) Thalidomide and dexamethasone vs. bortezomib and dexamethasone for melphalan refractory myeloma: a randomized study. Eur J Haematol. 2012;88:485–96. doi: 10.1111/j.1600-0609.2012.01775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar S, Flinn I, Richardson PG, et al. Randomized, multicenter, phase 2 study (evolution) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood. 2012;119:4375–82. doi: 10.1182/blood-2011-11-395749. [DOI] [PubMed] [Google Scholar]
- 27.Sharma M, Khan H, Thall PF, et al. A randomized phase 2 trial of a preparative regimen of bortezomib, high-dose melphalan, arsenic trioxide, and ascorbic acid. Cancer. 2012;118:2507–15. doi: 10.1002/cncr.26517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moreau P, Avet–Loiseau H, Facon T, et al. Bortezomib plus dexamethasone versus reduced-dose bortezomib, thalidomide plus dexamethasone as induction treatment before autologous stem cell transplantation in newly diagnosed multiple myeloma. Blood. 2011;118:5752–8. 5982. doi: 10.1182/blood-2011-05-355081. [DOI] [PubMed] [Google Scholar]
- 29.Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12:431–40. doi: 10.1016/S1470-2045(11)70081-X. [Erratum in: Lancet Oncol 2011;12:522] [DOI] [PubMed] [Google Scholar]
- 30.Garderet L, Iacobelli S, Moreau P, et al. Superiority of the triple combination of bortezomib–thalidomide–dexamethasone over the dual combination of thalidomide–dexamethasone in patients with multiple myeloma progressing or relapsing after autologous transplantation: The mmvar/ifm 2005-04 randomized phase iii trial from the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2012;30:2475–82. doi: 10.1200/JCO.2011.37.4918. [DOI] [PubMed] [Google Scholar]
- 31.Sonneveld P, Schmidt–Wolf IG, van der Holt B, et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase iii hovon-65/gmmg-hd4 trial. J Clin Oncol. 2012;30:2946–55. doi: 10.1200/JCO.2011.39.6820. [DOI] [PubMed] [Google Scholar]
- 32.Richardson PG, Sonneveld P, Schuster MW, et al. Safety and efficacy of bortezomib in high-risk and elderly patients with relapsed multiple myeloma. Br J Haematol. 2007;137:429–35. doi: 10.1111/j.1365-2141.2007.06585.x. [DOI] [PubMed] [Google Scholar]
- 33.Richardson PG, Sonneveld P, Schuster M, et al. Extended follow-up of a phase 3 trial in relapsed multiple myeloma: final time-to-event results of the apex trial. Blood. 2007;110:3557–60. doi: 10.1182/blood-2006-08-036947. [DOI] [PubMed] [Google Scholar]
- 34.San-Miguel JF, Richardson PG, Sonneveld P, et al. Efficacy and safety of bortezomib in patients with renal impairment: results from the apex phase 3 study. Leukemia. 2008;22:842–9. doi: 10.1038/sj.leu.2405087. [DOI] [PubMed] [Google Scholar]
- 35.Chanan–Khan A, Sonneveld P, Schuster MW, et al. Analysis of herpes zoster events among bortezomib-treated patients in the phase iii apex study. J Clin Oncol. 2008;26:4784–90. doi: 10.1200/JCO.2007.14.9641. [DOI] [PubMed] [Google Scholar]
- 36.Lee SJ, Richardson PG, Sonneveld P, et al. Bortezomib is associated with better health-related quality of life than high-dose dexamethasone in patients with relapsed multiple myeloma: results from the apex study. Br J Haematol. 2008;143:511–19. doi: 10.1111/j.1365-2141.2008.07378.x. [DOI] [PubMed] [Google Scholar]
- 37.Niesvizky R, Richardson PG, Rajkumar SV, et al. The relationship between quality of response and clinical benefit for patients treated on the bortezomib arm of the international, randomized, phase 3 apex trial in relapsed multiple myeloma. Br J Haematol. 2008;143:46–53. doi: 10.1111/j.1365-2141.2008.07303.x. [DOI] [PubMed] [Google Scholar]
- 38.Richardson PG, Sonneveld P, Schuster MW, et al. Reversibility of symptomatic peripheral neuropathy with bortezomib in the phase iii apex trial in relapsed multiple myeloma: impact of a dose-modification guideline. Br J Haematol. 2009;144:895–903. doi: 10.1111/j.1365-2141.2008.07573.x. [DOI] [PubMed] [Google Scholar]
- 39.Vogl DT, Stadtmauer EA, Richardson PG, et al. Impact of prior therapies on the relative efficacy of bortezomib compared with dexamethasone in patients with relapsed/refractory multiple myeloma. Br J Haematol. 2009;147:531–4. doi: 10.1111/j.1365-2141.2009.07875.x. [DOI] [PubMed] [Google Scholar]
- 40.San Miguel JF, Richardson PG, Orlowski RZ, et al. Risk of second primary malignancies (spms) following bortezomib (Btz)-based therapy: analysis of four phase 3 randomized controlled trials in previously untreated or relapsed multiple myeloma (mm) [abstract 2933] Blood. 2011;118 [Google Scholar]
- 41.Sonneveld P, Hajek R, Nagler A, et al. Combined pegylated liposomal doxorubicin and bortezomib is highly effective in patients with recurrent or refractory multiple myeloma who received prior thalidomide/lenalidomide therapy. Cancer. 2008;112:1529–37. doi: 10.1002/cncr.23326. [DOI] [PubMed] [Google Scholar]
- 42.Shah J, Blade J, Sonneveld P, et al. The effect of paraprotein heavy chain and free light chain types on the efficacy of pegylated liposomal doxorubicin + bortezomib versus bortezomib alone in patients with relapsed/refractory multiple myeloma [abstract 5190] Blood. 2008;112 [Google Scholar]
- 43.Shah J, Blade J, San Miguel J, et al. The effect of bone marrow involvement on the efficacy of pegylated liposomal doxorubicin + bortezomib vs. bortezomib alone in patients with relapsed/refractory multiple myeloma [abstract 5192] Blood. 2008;112 [Google Scholar]
- 44.Buda G, Ricci D, Cohen N, et al. Polymorphisms in the multiple drug resistance protein 1 and in P-glycoprotein 1 are associated with time to event outcomes in patients with relapsed and/or refractory multiple myeloma treated with bortezomib and pegylated liposomal doxorubicin [abstract 109] Blood. 2009;114 doi: 10.1007/s00277-010-0992-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.San Miguel JF, Schlag R, Khuageva NK, et al. Updated follow-up and results of subsequent therapy in the phase iii vista trial: bortezomib plus melphalan–prednisone versus melphalan–prednisone in newly diagnosed multiple myeloma [abstract 650] Blood. 2008;112 [Google Scholar]
- 46.Harousseau JL, Palumbo A, Richardson P, et al. Superior outcomes associated with complete response: analysis of the phase iii vista study of bortezomib plus melphalan–prednisone versus melphalan–prednisone [abstract 2778] Blood. 2008;112 doi: 10.1182/blood-2010-03-275800. [DOI] [PubMed] [Google Scholar]
- 47.Dhawan R, Robinson D, Jr, Meunier J, et al. Sustained health-related quality of life (hrqol) improvement in newly diagnosed multiple myeloma patients treated with bortezomib/melphalan/prednisone versus melphalan/prednisone: results from the vista trial [abstract 1881] Blood. 2009;114 [Google Scholar]
- 48.Dimopoulos MA, Richardson PG, Schlag R, et al. vmp (bortezomib, melphalan, and prednisone) is active and well tolerated in newly diagnosed patients with multiple myeloma with moderately impaired renal function, and results in reversal of renal impairment: cohort analysis of the phase iii vista study. J Clin Oncol. 2009;27:6086–93. doi: 10.1200/JCO.2009.22.2232. [DOI] [PubMed] [Google Scholar]
- 49.Mateos MV, Richardson PG, Schlag R, et al. Bortezomib plus melphalan and prednisone compared with melphalan and prednisone in previously untreated multiple myeloma: updated follow-up and impact of subsequent therapy in the phase iii vista trial. J Clin Oncol. 2010;28:2259–66. doi: 10.1200/JCO.2009.26.0638. [DOI] [PubMed] [Google Scholar]
- 50.Dimopoulos MA, Mateos MV, Richardson PG, et al. Risk factors for, and reversibility of, peripheral neuropathy associated with bortezomib–melphalan–prednisone in newly diagnosed patients with multiple myeloma: subanalysis of the phase 3 vista study. Eur J Haematol. 2011;86:23–31. doi: 10.1111/j.1600-0609.2010.01533.x. [DOI] [PubMed] [Google Scholar]
- 51.Favis R, Sun Y, van de Velde H, et al. Genetic variation associated with bortezomib-induced peripheral neuropathy. Pharmacogenet Genomics. 2011;21:121–9. doi: 10.1097/FPC.0b013e3283436b45. [DOI] [PubMed] [Google Scholar]
- 52.Delforge M, Terpos E, Richardson PG, et al. Fewer bone disease events, improvement in bone remodeling, and evidence of bone healing with bortezomib plus melphalan–prednisone vs. melphalan–prednisone in the phase iii vista trial in multiple myeloma. Eur J Haematol. 2011;86:372–84. doi: 10.1111/j.1600-0609.2011.01599.x. [DOI] [PubMed] [Google Scholar]
- 53.San Miguel JF, Schlag R, Khuageva NK, et al. Continued overall survival benefit after 5 years’ follow-up with bortezomib– melphalan–prednisone (vmp) versus melphalan–prednisone (mp) in patients with previously untreated multiple myeloma, and no increased risk of second primary malignancies: final results of the phase 3 vista trial [abstract 476] Blood. 2011;118 [Google Scholar]
- 54.Bringhen S, Larocca A, Rossi D, et al. Efficacy and safety of once-weekly bortezomib in multiple myeloma patients. Blood. 2010;116:4745–53. doi: 10.1182/blood-2010-07-294983. [DOI] [PubMed] [Google Scholar]
- 55.Avet–Loiseau H, Moreau P, Mathiot C, et al. Use of bortezomib to overcome the poor prognosis of t(4;14), but not del(17p), in young patients with newly diagnosed multiple myeloma [abstract 8113] J Clin Oncol. 2010;28 [Available online at: http://meetinglibrary.asco.org/content/48562-74; cited April 14, 2014] [Google Scholar]
- 56.Moreau P, Hulin C, Marit G, et al. on behalf of the ifm group Stem cell collection in patients with de novo multiple myeloma treated with the combination of bortezomib and dexamethasone before autologous stem cell transplantation according to ifm 2005-01 trial. Leukemia. 2010;24:1233–5. doi: 10.1038/leu.2010.82. [DOI] [PubMed] [Google Scholar]
- 57.Moreau P, Attal M, Pegourie B, et al. Achievement of vgpr to induction therapy is an important prognostic factor for longer pfs in the ifm 2005-01 trial. Blood. 2011;117:3041–4. doi: 10.1182/blood-2010-08-300863. [DOI] [PubMed] [Google Scholar]
- 58.Cavo M, Tacchetti P, Patriarca F, et al. A phase iii study of double autotransplantation incorporating bortezomib–thalidomide–dexamethasone (vtd) or thalidomide–dexamethasone (td) for multiple myeloma: superior clinical outcomes with vtd compared to td (abstract 351) Blood. 2009;114 [Google Scholar]
- 59.Brioli A, Perrone G, Volpe S, et al. Autologous peripheral blood stem-cell (pbsc) collection is not impaired by bortezomib–thalidomide–dexamethasone (btd) induction therapy in newly diagnosed multiple myeloma (mm) [abstract 317] Blood. 2011;118 [Google Scholar]
- 60.Cavo M, Pantani L, Patriarca F, et al. Superior complete response rate (cr) and progression-free survival (pfs) with bortezomib–thalidomide–dexamethasone (vtd) versus thalidomide–dexamethasone (td) as consolidation therapy after autologous stem-cell transplantation (asct) in multiple myeloma (mm) [abstract 1871] Blood. 2011;118 doi: 10.1182/blood-2011-07-364315. [DOI] [Google Scholar]
- 61.Cavo M, Pantani L, Petrucci MT, et al. Bortezomib–thalidomide–dexamethasone is superior to thalidomide–dexamethasone as consolidation therapy after autologous hematopoietic stem cell transplantation in patients with newly diagnosed multiple myeloma. Blood. 2012;120:9–19. doi: 10.1182/blood-2012-02-408898. [DOI] [PubMed] [Google Scholar]
- 62.Tacchetti P, Terragna C, Catania G, et al. Bortezomib- and thalidomide-induced peripheral neuropathy (pn) in multiple myeloma (mm): clinical and molecular analysis of 474 patients treated with thalidomide–dexamethasone (td) or bortezomib-td (vtd) [abstract 1821] Blood. 2011;118 [Google Scholar]
- 63.Sharma M, Thall P, Wang X, et al. A randomized phase ii trial of high-dose melphalan, ascorbic acid and arsenic trioxide with or without bortezomib in multiple myeloma [abstract 2309] Blood. 2009;114 doi: 10.1182/blood-2009-03-206482. [DOI] [Google Scholar]
- 64.Mateos MV, Oriol A, Teruel AI, et al. Maintenance therapy with bortezomib plus thalidomide (vt) or bortezomib plus prednisone (vp) in elderly myeloma patients included in the gem2005mas65 Spanish randomized trial [abstract 477] Blood. 2011;118 doi: 10.1182/blood-2011-04-345801. [DOI] [PubMed] [Google Scholar]
- 65.Kumar S, Flinn IW, Hari PN, et al. Novel three- and four-drug combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide, for newly diagnosed multiple myeloma: encouraging results from the multi-center, randomized, phase 2 evolution study [abstract 127] Blood. 2009;114 doi: 10.1182/blood-2009-04-205013. [DOI] [Google Scholar]
- 66.Kumar A, Flinn I, Richardson P, et al. Final results from the multi-center, randomized, phase 2 evolution study of combinations of bortezomib (v), dexamethasone (d), clophosphamide (c), and lenalidomide (r) in previously untreated multiple myeloma (mm) [abstract S100] Haematologica. 2011;96 [Google Scholar]
- 67.Kumar A, Kimlinger T, Morice WG, et al. Minimal residual disease (mrd) assessment by multiparameter flow cytometry (fcm) in a multicenter randomized phase 2 study in newly diagnosed multiple myeloma (mm): feasibility and correlation with response and survival outcomes [abstract S99] Haematologica. 2011;96 [Google Scholar]
- 68.Moreau P, Karamanesht II, Domnikova N, et al. Pharmacokinetics (pk) and pharmacodynamics (pd) of subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: effects of subcutaneous injection site and concentration, and patient characteristics [abstract 1863] Blood. 2011;118 [Google Scholar]
- 69.Moreau P, Facon T, Attal M, et al. Comparison of reduced-dose bortezomib plus thalidomide plus dexamethasone (vtd) to bortezomib plus dexamethasone (vd) as induction treatment prior to asctin de novo multiple myeloma (mm): results of ifm 2007-02 study [abstract 8014] J Clin Oncol. 2010;28 doi: 10.1200/JCO.2009.27.9158. [Available online at: http://meetinglibrary.asco.org/content/43424-74; cited April 14, 2014] [DOI] [PubMed] [Google Scholar]
- 70.Sonneveld P, van der Holt B, Schmidt–Wolf IGH, et al. First analysis of hovon-65/gmmg-hd4 randomized phase iii trial comparing bortezomib, Adriamycine, dexamethasone (pad) vs. vad as induction treatment prior to high dose melphalan (hdm) in patients with newly diagnosed multiple myeloma (mm) [abstract 653] Blood. 2008;112 [Google Scholar]
- 71.Neben K, Lokhorst HM, Jauch A, et al. Administration of bortezomib before and after autologous stem cell transplantation improves outcome in multiple myeloma patients with deletion 17p. Blood. 2012;119:940–8. doi: 10.1182/blood-2011-09-379164. [DOI] [PubMed] [Google Scholar]
- 72.Mellqvist UH, Gimsing P, Hjertner O, et al. Improved progression free survival with bortezomib consolidation after high dose melphalan; results of a randomized phase iii trial [abstract S31] Haematologica. 2011;96 [Google Scholar]
- 73.Rosinol L, Cibeira MT, Martinez J, et al. Thalidomide/dexamethasone (td) vs. bortezomib (Velcade)/thalidomide/dexamethasone (vtd) vs. vbmcp/vbad/Velcade as induction regimens prior autologous stem cell transplantation (asct) in multiple myeloma (mm): results of a phase iii pethema/gem trial [abstract 130] Blood. 2009;114 [Google Scholar]
- 74.Rosinol L, Cibeira MT, Mateos MV, et al. A phase iii pethema/gem randomized trial of postransplant (asct) maintenance in multiple myeloma: superiority of bortezomib/thalidomide compared with thalidomide and alfa-2b interferon [abstract 3962] Blood. 2011;118 [Google Scholar]
- 75.Niesvizky R, Reeves J, Flinn IW, et al. Phase 3b upfront study: interim results from a community practice-based prospective randomized trial evaluating three bortezomib-based regimens in elderly, newly diagnosed multiple myeloma patients [abstract 129] Blood. 2009;114 [Google Scholar]
- 76.Niesvizky R, Flinn IW, Rifkin R, et al. Patient-reported quality of life (qol) in elderly, newly diagnosed multiple myeloma (mm) patients receiving bortezomib-based combinations: results from all randomized patients in the community-based, phase 3b upfront study [abstract 1864] Blood. 2011;118 [Google Scholar]
- 77.San-Miguel JF, De Moraes Hungria VT, Yoon SS, et al. Update on a phase iii study of panobinostat with bortezomib and dexamethasone in patients with relapsed multiple myeloma: panorama 1 [abstract 3976] Blood. 2011;118 [Google Scholar]
- 78.Kaura S, Dranitsaris G. Number needed to treat (nnt) as a measure of drug benefit: lenalidomide versus bortezomib for treatment of relapsed/refractory multiple myeloma (mm) [abstract e18562] J Clin Oncol. 2012;30 [Available online at: http://meetinglibrary.asco.org/content/96962-114; cited April 14, 2014] [Google Scholar]
- 79.Mellqvist UH, Westin J, Gimsing P, et al. Improved response rate with bortezomib consolidation after high dose melphalan: first results of a Nordic Myeloma Study Group randomized phase iii trial [abstract 530] Blood. 2009;114 [Google Scholar]
- 80.Dimopoulos MA, Jagannath S, Yoon SS, et al. Vantage 088: vorinostat in combination with bortezomib in patients with relapsed/refractory multiple myeloma: results of a global, randomized phase 3 trial [abstract 811] Blood. 2011;118 [Google Scholar]
- 81.Dimopoulos MA, Beksac M, Benboubker L, et al. Bortezomib–dexamethasone alone or plus cyclophosphamide or lenalidomide as second-line treatment for patients with multiple myeloma: final results from a phase 2 study [abstract 1861] Blood. 2011;118 [Google Scholar]
- 82.Niesvizky R, Flinn IW, Rifkin R, et al. Efficacy and safety of three bortezomib-based combinations in elderly, newly diagnosed multiple myeloma patients: results from all randomized patients in the community-based, phase 3b upfront study [abstract 478] Blood. 2011;118 [Google Scholar]
- 83.Orlowski RZ, Gercheva L, Williams C, et al. Phase ii, randomized, double blind, placebo-controlled study comparing siltuximab plus bortezomib versus bortezomib alone in pts with relapsed/refractory multiple myeloma [abstract 8018] J Clin Oncol. 2012;30:8018. [Available online at: http://meetinglibrary.asco.org/content/96732-114; cited April 4, 2014] [Google Scholar]
- 84.Rosinol L, Cibeira MT, Mateos MV, et al. A phase iii pethema/gem randomised trial of posttransplant maintenance in multiple myeloma: superiority of bortezomib. Bone Marrow Transplant. 2012;47:S2. [Google Scholar]
- 85.San Miguel JF, Moreau P, Yoon SS, et al. Phase iii study of panobinostat with bortezomib and dexamethasone in patients with relapsed multiple myeloma (panorama 1) [abstract 18572] J Clin Oncol. 2012;30 [Available online at: http://meetinglibrary.asco.org/content/91326-114; cited April 14, 2014] [Google Scholar]
- 86.Shea BJ, Hamel C, Wells GA, et al. amstar is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol. 2009;62:1013–20. doi: 10.1016/j.jclinepi.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 87.Shea BJ, Grimshaw JM, Wells GA, et al. Development of amstar: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10. doi: 10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Calhoun EA, Welshman EE, Chang CH, et al. Psychometric evaluation of the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group–Neurotoxicity (fact/gog-Ntx) questionnaire for patients receiving systemic chemotherapy. Int J Gynecol Cancer. 2003;13:741–8. doi: 10.1111/j.1525-1438.2003.13603.x. [DOI] [PubMed] [Google Scholar]
- 89.Fayers PM, Aaronson N, Bjordal K, Curran D, Groenvold M. The EORTC QLQ-C30 Scoring Manual. 2nd ed. Brussels, Belgium: European Organisation for Research and Treatment of Cancer; 1999. [Google Scholar]
- 90.Calhoun EA, Fishman DA, Roland PY, Lurain JR, Chang C, Cella D. Validity and selective sensitivity of the fact-gog Ntx [abstract 446a] J Clin Oncol. 2000;19 [Google Scholar]
- 91.Hicks LK, Haynes AE, Reece DE, et al. A meta-analysis and systematic review of thalidomide for patients with previously untreated multiple myeloma. Cancer Treat Rev. 2008;34:442–52. doi: 10.1016/j.ctrv.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 92.Hicks LK, Haynes AE, Reece D, et al. on behalf of the Hematology Disease Site Group . Thalidomide in Multiple Myeloma: Guideline Recommendations. Toronto, ON: Cancer Care Ontario; 2010. Evidence-based series 6-21. Section 1. [Google Scholar]
- 93.Nooka AK, Kaufman JL, Behera M, et al. The improved efficacy of bortezomib containing induction regimens (bcir) versus non-bortezomib containing induction regimens (nbcir) in transplant-eligible patients with multiple myeloma (mm): meta-analysis of phase iii randomized controlled trials (rcts) [abstract 3994] Blood. 2011;118 [Google Scholar]
- 94.Kouroukis CT, Fernandez LAV, Crump M, et al. A phase ii study of bortezomib and gemcitabine in relapsed mantle cell lymphoma from the National Cancer Institute of Canada Clinical Trials Group (ind 172) Leuk Lymphoma. 2011;52:394–9. doi: 10.3109/10428194.2010.546015. [Erratum in: Leuk Lymphoma 2011;52:1160] [DOI] [PubMed] [Google Scholar]