Abstract
Background
Although antineoplastic agents are critical in the treatment of cancer, they can potentially cause hypersensitivity reactions that can have serious consequences. When such a reaction occurs, clinicians can either continue the treatment, at the risk of causing a severe or a potentially fatal anaphylactic reaction, or stop the treatment, although it might be the only one available. The objective of the present work was to evaluate the effectiveness of methods used to prevent and treat hypersensitivity reactions to platinum- or taxane-based chemotherapy and to develop evidence-based recommendations.
Methods
The scientific literature published to December 2013, inclusive, was reviewed.
Results
Premedication with antihistamines, H2 blockers, and corticosteroids is not effective in preventing hypersensitivity reactions to platinum salts. However, premedication significantly reduces the incidence of hypersensitivity to taxanes. A skin test can generally be performed to screen for patients at risk of developing a severe reaction to platinum salts in the presence of grade 1 or 2 reactions, but skin testing does not appear to be useful for taxanes. A desensitization protocol allows for re-administration of either platinum- or taxane-based chemotherapy to some patients without causing severe hypersensitivity reactions.
Conclusions
Several strategies such as premedication, skin testing, and desensitization protocols are available to potentially allow for administration of platinum- or taxane-based chemotherapy to patients who have had a hypersensitivity reaction and for whom no other treatment options are available. Considering the available evidence, the Comité de l’évolution des pratiques en oncologie made recommendations for clinical practice in Quebec.
Keywords: Hypersensitivity reactions, taxanes, platinum salts, skin testing, desensitization, chemotherapy, management
1. INTRODUCTION
Although the incidence of hypersensitivity to anti-neoplastic agents can increase with the number of treatments, reactions are not predictable and often are not associated with the pharmacologic mechanism of action of the medication1. When patients experience hypersensitivity reactions, clinicians can either continue the treatment, at the risk of causing a severe reaction and potentially a fatal anaphylactic shock, or stop the treatment, although it might be the only option available.
The molecular mechanisms of hypersensitivity to anticancer agents are not yet well defined. As in other drug reactions, and based on the results of skin testing, immediate reactions sometimes appear to involve immunoglobulin E. Delayed reactions might be caused by other mechanisms, such as activation of the complement cascade2.
The diagnosis of hypersensitivity is based on the patient’s medical history and, if possible, the results of diagnostic (skin or challenge) tests3. The patient’s clinical history can be difficult to evaluate because cancer patients are often receiving multiple medications that can also trigger hypersensitivity reactions. In addition, the proposed chemotherapy might also trigger immune responses not mediated by immunoglobulin E4. The cancer itself can also occasionally produce clinical symptoms that resemble a hypersensitivity reaction caused by mast cell activation. Epidemiology studies have shown that certain types of cancer are associated with an increased risk of hypersensitivity2.
The incidence of hypersensitivity is significant for certain anticancer agents, including platinum drugs (cisplatin, carboplatin, oxaliplatin), taxanes (paclitaxel, docetaxel), l-asparaginase, epipodophyllotoxins (teniposide, etoposide), monoclonal antibodies, procarbazine, and to a lesser extent, 6-mercaptopurine3–6.
The objectives of the present work were to review the scientific literature evaluating the effectiveness of methods used to prevent and treat hypersensitivity to taxane- or platinum-based chemotherapy and to make clinical recommendations based on the best available evidence.
2. METHODS
This article is an updated adaptation of an original clinical guideline available online (http://www.msss.gouv.qc.ca/sujets/prob_sante/cancer/download.php?f=ec531e187f7e1b161079228273b58a18). To begin, the scientific literature published up to April 2013 was searched in PubMed using the keywords “desensitization,” “skin test,” “platinum salts,” “cisplatin,” “carboplatin,” “oxaliplatin,” “taxane,” “paclitaxel,” “docetaxel,” and “hypersensitivity.” The literature review was subsequently updated to cover the period from April 2013 to December 2013. Only English- and French-language studies were selected. Economic studies were not considered. Clinical guidelines and consensus statements issued by relevant international organizations and cancer agencies were also identified.
The level of evidence of selected studies and the strength of guideline recommendations were evaluated using the American Society of Clinical Oncology and European Society for Medical Oncology grading system (Table i). The original guideline was developed by a subcommittee of the Comité de l’évolution des pratiques en oncologie (cepo), reviewed by independent experts, and finally adopted by the cepo by consensus.
TABLE I.
Levels of evidence and grades of recommendations (adapted from Cook et al., 19927)
| Levels of evidence | Grades of recommendation | ||
|---|---|---|---|
|
|
|
||
| Level | Type of evidence | Grade | Recommendation |
| i | Evidence demonstrated by means of meta-analyses of well-designed controlled trials or large randomized trials with clear-cut results (low false-positive and false-negative errors, high power) | A | Supported by level i evidence or multiple level ii, iii, or iv trials presenting concordant observations |
| ii | Evidence demonstrated by means of small randomized trials with uncertain results (high false-positive and false-negative errors, low power) | B | Supported by level ii, iii, or iv trials presenting generally concordant observations |
| iii | Evidence demonstrated by means of nonrandomized concur- rent cohort comparisons with contemporaneous controls | C | Supported by level ii, iii, or iv trials presenting non-concordant observations |
| iv | Evidence demonstrated by means of nonrandomized historical cohort comparisons | D | Supported by little or no empiric evidence |
| v | Evidence demonstrated by means of case series without controls | ||
3. RESULTS
3.1. Platinum Drugs
3.1.1. Incidence
Widespread use of platinum antineoplastic agents has led to an increased incidence of hypersensitivity reactions (Table ii). Hypersensitivity is rarely reported during the initial course of treatment. A major feature of hypersensitivity to platinum drugs, compared with other types of agents, is that allergic reactions can appear after a significant number of infusions with no prior clinical signs10. Carboplatin-based chemotherapy is often administered over 6 cycles, and hypersensitivity reactions are usually observed during re-treatment, after a period of remission. Hypersensitivity reactions to oxaliplatin are thought to be less frequent, although the growing use of that agent in recent years has led to a reported incidence of approximately 19%11.
TABLE II.
Hypersensitivity reactions to platinum- and taxane-based chemotherapy (adapted from Syrigou et al., 20108 and Makrilia et al., 20109)
| Drug | Overall incidence (%) | Time of initial onset | Reactions | Characteristics or severity |
|---|---|---|---|---|
| Platinum agents | ||||
| Cisplatin | 5–20 | Within minutes or days of infusion start, between 4th and 8th cycles, generally after 6 cycles | Rash, pruritus, fever, dyspnea, bronchospasm, hypotension | Increase with concomitant radiation Variable reactions to moderate, sometimes lethal |
| Carboplatin | 1–44 | Within the first 30 minutes or days from infusion, generally after 7 cycles | Grade 1 or 2: urticaria, itching, erythema (palms and soles) Grade 3 or 4: cutaneous (face swelling, diffuse erythema), gastrointestinal (abdominal cramps, diarrhea), respiratory (dyspnea, bronchospasm), cardiovascular (chest pain, tachycardia, hypotension, hypertension) | <1% during cycles 1–5 6.5% in cycle 6 27% in cycle 7 or subsequent 44% in 3rd-line treatment 60%–70% are grade 1 or 2 |
| Oxaliplatin | 10–19 | Within minutes or days from infusion, generally after 6 cycles | Grade 1 or 2: itching, erythema (palms and soles) Grade 3 or 4: urticaria, face swelling, diffuse erythroderma, bronchospasm in rare instances leading to anaphylactic shock | 1.6% are grades 3 and 4 Severe anaphylaxis in case reports Symptoms can appear during or within hours from infusion In many patients, 1st reaction is moderate and becomes severe with next infusion |
| Taxanes | ||||
| Paclitaxel | 8–45 | Within first minutes of infusion, during cycle 1 or 2 | Dyspnea (with or without bronchospasm), urticaria, hypotension (or sometimes hypertension), erythema, back pain, chest pain, abdominal or pelvic pain | Minor reactions in 40% of patients Severe reactions in 1.3% of patients |
| Docetaxel | 25–50 | Within first minutes of infusion, during cycle 1 or 2 | Dyspnea (with or without bronchospasm), urticaria, hypotension (or sometimes hypertension), erythema, fluid retention syndrome | Severe anaphylactic reactions in 2% of patients |
The incidence of cross-reactivity between platinum drugs has not been fully studied. Allergic cross-reactivity between cisplatin and carboplatin has been reported, but the number of cases is insufficient to establish the true incidence12.
3.1.2. Risk Factors
Risk factors for developing hypersensitivity to platinum drugs, especially cisplatin and oxaliplatin, have to be better identified. An existing hypersensitivity to certain drugs and environmental factors has been associated with an increased risk of developing symptoms from carboplatin13. Among patients receiving carboplatin, the number of treatments and the total lifetime exposure to platinum drugs have been associated with the risk of developing hypersensitivity to carboplatin10.
The interval between the last cycle of the initial course of treatment and the first cycle of the second course is a predictive variable13. In this regard, Schwartz et al.14 showed that the risk of a severe reaction during carboplatin treatment was 47% if the interval between courses was more than 24 months and 6.5% when the interval was less than 12 months (n = 36).
When carboplatin forms part of combination chemotherapy, the incidence of hypersensitivity seems to be affected by the antineoplastic agent with which carboplatin is being combined. The calypso study showed that hypersensitivity occurred more often among patients receiving a carboplatin–paclitaxel combination than among those receiving carboplatin combined with pegylated liposomal doxorubicin (18.8% vs. 5.6%)15. In a Southwest Oncology Group study, Markman et al.16 showed that, compared with single-agent carboplatin, the combination of pegylated liposomal doxorubicin and carboplatin reduced the frequency of hypersensitivity reactions (0% vs. 30%).
Hypersensitivity reactions have been observed for all routes of administration, including intravenous, intraperitoneal, and intravesical17,18.
3.2. Taxanes
3.2.1. Incidence
Hypersensitivity symptoms generally develop within the first 10–15 minutes after infusion (78% within the first 10 minutes)19. In 95% of cases, reactions occur during the first or second infusion20, but reactions can appear during subsequent infusions (3%)19,20. Some patients also develop skin reactions several days or up to a week after infusion. The incidence of paclitaxel and docetaxel hypersensitivity varies between 8% and 50% (Table ii).
Paclitaxel and carboplatin are often administered concomitantly, and so it can be difficult to distinguish which drug has triggered a hypersensitivity reaction. Diagnosis is facilitated by the clinical presentation and the time of appearance because the reactions are different for and specific to each agent.
3.2.2. Risk Factors
In the case of paclitaxel, hypersensitivity reactions occur more often in patients with a history of atopy4. For both docetaxel and paclitaxel, a history of mild skin reactions during earlier treatments; the presence of respiratory dysfunction, overweight, or obesity; and menopausal or postmenopausal status have also been reported as risk factors21–23.
Whether hypersensitivity can be attributed to the taxanes themselves or to their formulation vehicles has not yet been established24–26. Paclitaxel is administered in a solution of ethanol and Kolliphor el (formerly Cremophor el: BASF, Ludwigshafen, Germany), which is also used as a vehicle for other compounds known potentially to cause hypersensitivity (cyclosporine, teniposide, diazepam, propofol, and vitamin K)25,27. However, studies have shown that Kolliphor used without paclitaxel and without premedication does not trigger hypersensitivity reactions28.
The reaction to docetaxel has been attributed to its vehicle, polysorbate 80, which is also used for etoposide29. However, data have shown that the drug itself might be the cause of hypersensitivity30,31.
3.3. Evaluation and Prevention of Hypersensitivity Reactions
3.3.1. Skin Testing
Skin testing was developed to predict immunoglobulin E–mediated allergic reactions, and certain precautions for the use of skin testing have been reported in the literature32. Indeed, patients with cardiovascular disease and elderly patients can be at risk of complications from testing. As with any medication, the patient’s general condition must be considered if complications could occur and require attention. Furthermore, patients presenting with dermographism, urticaria, eczema, or cutaneous mastocytosis can have false-positive skin tests. Patients with improperly controlled asthma and reduced pulmonary function, and those with a history of severe hypersensitivity when exposed to weak doses of antigens, can be at risk of developing an anaphylactic reaction to skin testing.
Certain medications can affect skin-test reactivity32. The time interval between drug discontinuation and skin testing vary with the drug (Table iii). The use of muscle relaxants and antiemetics with antihistaminic properties can reduce skin-test reactivity, as can the use of tricyclic antidepressants and phenothiazines. Because many of those drugs have a long half-life or often cannot be interrupted for clinical reasons (or both), skin testing often proceeds in spite of their presence. If testing triggers a weak or negative reaction, interference can therefore be considered. Use of decongestants, inhaled beta-agonists, or cromolyn has no effect on skin-test results.
TABLE III.
Interval between drug discontinuation and skin testing (adapted from Brockow and Romano33)
| Drug | Interval |
|---|---|
| H1 antihistamines | |
| 1st generation | 1–3 Days |
| 2nd generation | 7 Days |
| H2 antihistamines | 2 Days |
| Imipramines and phenothiazines | 5 Days |
| Beta-adrenergic blockers | 48 Hours or according to their elimination half-life |
| Glucocorticoids (prednisolone equivalent) | |
| Long-term | |
| ≤10 mg | None |
| >10 mg | 3 Weeks |
| Short-term | |
| ≤50 mg | 3 Days |
| >50 mg | 7 Days |
Some authors recommend that all patients receiving carboplatin be tested before each treatment after the 6th4,34. Patients who experience hypersensitivity symptoms and a positive skin-test result should undergo a desensitization protocol. Markman et al.35 reported that 6 of 7 patients with a positive skin-test result for carboplatin developed an anaphylactic reaction during re-treatment with carboplatin.
Skin testing can be used to evaluate the potential for cross-reactions between two platinum drugs36–38. If indicated, patients with negative skin-test results for a particular platinum agent might be able to continue treatment with a different agent without premedication39. Compared with patients having a negative skin-test result, those with a positive result have a greater risk of experiencing cross-reactivity between platinum salts40.
Although used to identify a potential allergic reaction, skin testing can be used in patients who have had symptoms of anaphylaxis2; however, 2–6 weeks should elapse after the anaphylactic reaction and before the test to avoid generating a false-negative result because of mast cell hyporeactivity37,41. If testing occurs earlier, a positive result is always significant.
3.3.2. Infusion Time and Premedication
Infusion Time:
O’Cearbhaill et al.10 noted that administration of a 3-hour carboplatin infusion together with premedication (instead of the standard 30-minute infusion) could reduce the risk of hyper-sensitivity (3.4% vs. 21%).
A meta-analysis evaluated the impact of paclitaxel infusion time and showed no difference in the risk of developing hypersensitivity when treatment was administered over 3 or 24 hours (risk ratio: 1.86; 95% confidence interval: 0.63 to 5.52)42. Furthermore, Hainsworth et al.43 noted no difference in activity between 1-day and 3-day paclitaxel schedules in which each dose was administered by 1-hour infusion.
Premedication:
Routine premedication with anti-histamines and steroids is not recommended before commencement of platinum-based therapy8,12,44,45. A small retrospective study showed that premedication (diphenhydramine, dexamethasone, granisetron, and famotidine vs. dexamethasone and granisetron) reduced hypersensitivity reactions associated with modified folfox6 (leucovorin, 5-fluorouracil, oxaliplatin)46. In the case of a grade 1 or 2 hypersensitivity reaction, it is possible to continue oxaliplatin treatment without reducing the infusion rate if the patient is premedicated with dexamethasone47. Administration of premedication with corticosteroids and a histamine receptor antagonist has allowed some patients to be re-treated with platinum drugs without desensitization9; however, there is always a risk of recurrence of the reaction.
Routine premedication with glucocorticoids can diminish the incidence of hypersensitivity during paclitaxel or docetaxel therapy from 30% to 3%20,48. With premedication, the incidence of hypersensitivity reactions to paclitaxel is between 1% and 3% regardless of infusion time (1, 3, or 24 hours). Kwon et al.49 showed that, compared with a single administration 30 minutes before treatment, administration of dexamethasone 12 and 6 hours before infusion of paclitaxel led to fewer hypersensitivity reactions; another study showed no difference50. Premedication with dexamethasone (8 mg for 3 days starting the day before infusion) can reduce the incidence of grade 3 or 4 hypersensitivity reactions to docetaxel to about 2%51,52. As a result, the median cumulative dose administered before the incidence of severe symptoms increased (from 490 mg/m2 to 790 mg/m2)53–55. Chouhan et al.56 showed that a single dose of dexamethasone 30 minutes before docetaxel infusion was sufficient to prevent hypersensitivity reactions.
3.3.3. Treatment of Hypersensitivity Symptoms
Early recognition of hypersensitivity reactions is essential for the patient’s well-being and can, in some cases, save lives. Patients must be informed of adverse events so that they can contact medical personnel as soon as possible57.
Treatment must be interrupted immediately when a patient develops a hypersensitivity reaction. Depending on the symptoms, it might be necessary to administer medication. Antihistamines, corticosteroids, and if necessary, epinephrine and bronchodilators should be administered, and oxygen should be readily available57. In the case of a severe reaction, treatment should not be continued. However, with a mild reaction, treatment can be resumed the same day57–59. In many cases, a mild-to-moderate reaction will resolve after a brief interruption of treatment and administration of appropriate medication60. Re-treatment has the potential to have serious consequences, and close monitoring is therefore essential during the next cycle.
3.3.4. Substitution of Therapy
A new formulation of nanoparticle albumin-bound paclitaxel (nab-paclitaxel) avoids the use of Kolliphor as a vehicle, and thus premedication is not required61,62. No hypersensitivity reactions were reported in several phase i, ii, and iii studies that used this agent28,62–64, and administration of nab-paclitaxel was well tolerated in 5 patients who had previously experienced hypersensitivity reactions to paclitaxel65. However, one phase iii study indicated that nab-paclitaxel can cause hypersensitivity reactions28.
3.3.5. Desensitization Protocols
Desensitization is indicated when no alternative therapy exists or when the available alternative is less effective than the treatment being used. Desensitization protocols involve inducing a temporary tolerance to a treatment by gradually reintroducing a small amount of the antigen over a relatively short period of time until the total scheduled dose has been administered41,66,67. Because a desensitization protocol induces only a temporary tolerance, it must be carried out every time the patient receives the treatment1,41,66.
A 12-step desensitization protocol has been developed by the Dana–Farber Cancer Institute and the Brigham and Women’s Hospital41,57,67,68. Unlike most of the other protocols, it is the only one to have been successfully used during several hundred desensitizations for multiple antineoplastic agents. The protocol involves the preparation of three different solutions with escalating concentrations of the drug (Table iv). The infusion rate changes every 15 minutes, and the volume infused is approximately double that of the preceding step. As reported in three publications, use of this 12-step protocol resulted either in no hypersensitivity reaction or in a less-severe reaction than the reaction that originally led to the need for desensitization (Table v).
TABLE IV.
General principles of the 12-step desensitization protocol (adapted from Feldweg et al.57)
| Step | Solution | Rate (mL/h) | Time (minutes) | Volume infused per step (mL) |
|---|---|---|---|---|
| 1 | 1:100 dilution of the final target concentration | 2 | 15 | 0.5 |
| 2 | 5 | 15 | 1.25 | |
| 3 | 10 | 15 | 2.5 | |
| 4 | 20 | 15 | 5 | |
| 5 | 1:10 dilution of the final target concentration | 5 | 15 | 1.25 |
| 6 | 10 | 15 | 2.5 | |
| 7 | 20 | 15 | 5 | |
| 8 | 40 | 15 | 10 | |
| 9 | Usual concentration; cumulative dose administered in steps 1–8 | 10 | 15 | 2.5 |
| 10 | 20 | 15 | 5 | |
| 11 | 40 | 15 | 10 | |
| 12 | 75 | Prolonged until complete dose | 232.5 |
TABLE V.
Studies using the 12-step desensitization protocol
| Study | Drug | Patients (desensitizations) [N (n)] | Premedication | Steps | Duration | Success rate |
|---|---|---|---|---|---|---|
| Feldweg et al., 200557 | Paclitaxel and docetaxel | 17 (77) | Oral dexamethasone 20 mg 12 and 6 hours before infusion, intravenous diphenhydramine 50 mg and ranitidine 50 mg 30 minutes before infusion | From 1:100 to 1:1 in 12 steps | 5.8 Hours | 100% |
| Lee et al., 200567 | Carboplatin Paclitaxel Docetaxel |
31 (127) 22 (114) 1 (14) |
Intravenous diphenhydramine 25 mg, intravenous famotidine 20 mg, and lorazepam 1 mg as needed for anxiety | From 1:100 to 1:1 in 12 steps (high concentration for outpatients) | 5.8 Hours (inpatients); 3.8 hours (outpatients) | 85% of treatments without symptoms (complete initial dose with treatment) |
| Castells et al., 200841 | Cisplatin Carboplatin Oxaliplatin Paclitaxel |
3 (12) 60 (212) 1 (1) 28 (140) |
Oral or intravenous diphenhydramine or hydroxyzine 25 mg, intravenous famotidine 20 mg or ranitidine 50 mg, and oral or intravenous lorazepam 0.5–1 mg as needed for anxiety 20 minutes before infusion, and oral dexamethasone 20 mg night before and morning of paclitaxel infusion | From 1:100 to 1:1 in 12 steps | 5.8 Hours | 100% |
In 2005, Feldweg et al.57 reported the results of paclitaxel and docetaxel desensitization for patients admitted to a desensitization protocol because of severe hypersensitivity reactions (dyspnea, laryngeal edema, bronchospasm, oxygen desaturation, chest pain, significant change in blood pressure, or loss of consciousness) during prior treatment. During desensitization, 4 patients developed a reaction. The reactions were less severe than the original ones (palmar–plantar erythema, abdominal pain, burning sensation in the chest, and moderate flushing). Treatment was interrupted, and the patients received diphenhydramine. One patient had complications warranting readmission to hospital. That patient opted for a change in treatment; the other three underwent subsequent desensitizations using the standard or a modified protocol without any reactions.
In 2005, Lee et al.67 reported the results of desensitization to carboplatin, paclitaxel, and four other agents (docetaxel, trastuzumab, doxorubicin, and mesna). To be eligible, patients had to have experienced a hypersensitivity reaction to chemo-therapy less than 6 hours after infusion, with the need to continue on the same agent. All patients completed their desensitization protocols. A total of 225 desensitizations (88.2%) were completed without any symptoms, and in 18 patients (30 desensitizations), symptoms were less severe than the initial ones during the first (n = 13), second (n = 3), third (n = 1), or fourth (n = 1) desensitization. Skin reactions in those 18 patients were treated with antihistamines (n = 8), and chest pain and shortness of breath were treated with antihistamines either alone or combined with oxygen. In 6 patients, treatments given in addition to antihistamines included albuterol for dyspnea; corticosteroids for symptoms persisting more than 5 minutes; a beta-blocker for chest pain, tachycardia, and hypertension; and intramuscular epinephrine for throat tightness and hypotension. Once the hypersensitivity symptoms were managed, all patients completed their treatment and received subsequent desensitizations on a modified protocol.
In 2008 and 2010, Castells et al.41 and Limsuwan and Castells68 published the results of desensitizations for carboplatin, paclitaxel, cisplatin, oxaliplatin, and other treatments (rituximab, liposomal doxorubicin, and doxorubicin). To be eligible, patients had to have experienced a hypersensitivity reaction during or within 48 hours after infusion. Of all the desensitizations, 94% produced a mild reaction or no reaction. All reactions were controlled by interrupting treatment and administering appropriate medication, with epinephrine needed for 1 patient. Overall, 7% of reactions occurred during steps 1–4, 18% during steps 5–8, and 75% during steps 9–12, with 51% of reactions occurring during the last step of the desensitization protocol. All patients received their treatment at full dose.
Other multi-step desensitization protocols of 4–13 steps have been suggested40,69–71. Most patients completed them with few or no hypersensitivity symptoms.
4. DISCUSSION
Hypersensitivity reactions to chemotherapy are unpredictable adverse events with potentially lethal consequences. Among the agents most likely to cause this type of reaction are platinum drugs and taxanes. Literature on this subject often comes from case studies and populations consisting of a limited number of patients. Further research is needed to validate the reported information.
According to the U.S. National Cancer Institute grading system, grade 1 hypersensitivity reactions allow treatment to be continued without any modification, and grade 2 reactions might require modification. Depending on the drug being administered, modifications such as substituting therapy, using steroid and antihistamine premedication, and reducing the infusion rate are a possibility. Optimal treatment is determined using clinical judgment based on the presenting symptoms. In the case of grade 3 or 4 reactions, patients should not be given the same treatment without modifications. In the event of mild-to-moderate reactions, any reintroduction of the drug could lead to a severe or even lethal reaction72. In such cases, a desensitization protocol should be planned2.
Premedication is ineffective with platinum drugs, but using premedication with taxanes can diminish the risk of hypersensitivity reactions8,12,44.
Modifying the infusion time seems to have an effect on the risk of having a reaction to some agents, but additional research is needed to clarify this issue42,43,73,74.
The utility of skin testing in clinical practice remains a contentious issue9. However, its use could help to diagnose or predict a hypersensitivity reaction or to evaluate the potential for cross-reactions between two platinum drugs. It is important to note that even patients with a negative result are not immune to hypersensitivity reactions. Experts often underscore the fact that skin testing can be useful when the risk for hypersensitivity is high9. Indeed, some reports have shown that, unlike reactions to taxanes, for which skin testing is unreliable, hyper-sensitivity reactions to platinum agents can be identified with such testing2,75. In the case of carboplatin, close monitoring is needed, particularly after the 6th cycle2,25,75–77. However, the recommendation to systematically conduct skin testing after a predetermined number of cycles varies depending on the source. In the presence of a hypersensitivity reaction, it is recommended that an allergist, medical oncologist, or any medical specialist with desensitization experience be consulted to determine the most appropriate course of treatment.
Skin testing must be carried out by skilled personnel specially trained to perform the technique, interpret the results, and manage the allergic reactions that could arise during the test itself. The use of appropriate controls allows for the results to be properly interpreted. Additional research is needed to clarify the role of skin testing and to establish guidelines. On the other hand, a positive result should be considered significant and must be taken into account.
The various desensitization protocols reported in the literature have often been used in a limited number of cases and a limited number of desensitizations. In most cases, efficacy and safety results are nonexistent or minimal. The desensitization protocols for platinum drugs and taxanes discussed earlier in this article were administered to 15 or more patients40,41,57,67,70,71 and are based on administering treatment in numerous dilutions and gradual steps. The associated success rate is nearly 100%, with no severe hypersensitivity reactions in most cases. The large-scale studies reported by the Dana–Farber Cancer Institute and Brigham and Women’s Hospital describe a 12-step protocol tested on a larger group of patients undergoing a significant number of desensitizations involving platinum drugs and taxanes41,57,67,68. In every case, the planned therapy was successfully administered, with no hypersensitivity reactions or with just mild reactions, all of which were less severe than the initial ones. Premedication was administered before desensitization for both platinum drugs and taxanes. When hypersensitivity reactions occurred during the desensitization protocol, administration of antihistamines and interruption of treatment successfully controlled the symptoms in most cases and allowed the protocol to be completed. During subsequent desensitizations, a modified protocol allowed for all patients to receive the scheduled number of treatment cycles.
It is important to emphasize that successful application of a desensitization protocol depends on the experience of the team in charge of its administration and the team’s ability to accurately assess the risks41,57,67,68. All personnel involved must be trained to recognize hypersensitivity reactions and to respond immediately. Emergency medications—including epinephrine, antihistamines, bronchodilators, and oxygen—must be at the patient’s bedside to ensure quick administration if needed. Patients must be educated to recognize early symptoms and to immediately inform the medical staff.
5. CONCLUSIONS AND RECOMMENDATIONS
When a hypersensitivity reaction occurs, the dilemma is whether treatment should be stopped, continued, or modified for the patient’s well-being. In many cases, implementing effective preventive measures can ensure that the patient is offered the best treatment available. Considering the evidence available to date, the cepo makes these recommendations:
- With respect to platinum drugs (cisplatin, carboplatin, oxaliplatin):
- Routine premedication with antihistamines and steroids to prevent hypersensitivity reactions is not recommended (grade B recommendation).
- Whenever possible, skin testing (prick and intradermal) should be considered for patients with suspected hypersensitivity to platinum drugs (grade B recommendation). Skin testing must take place at least 2–6 weeks after a hypersensitivity reaction.
- In the case of a positive skin test, a desensitization protocol should be considered when treatment cannot be substituted or interrupted (grade B recommendation).
- In the case of a negative skin test, a risk of reaction remains; the decision to continue treatment or to administer a desensitization protocol is based on clinical judgment (grade D recommendation).
- Skin testing should be performed by a multidisciplinary team specially trained to perform the technique, interpret the results, and manage the rare allergic reactions that can occur during the test itself (grade D recommendation).
- With respect to taxanes (paclitaxel, docetaxel):
- Premedication with glucocorticoids and H1 and H2 antagonists should routinely be administered to reduce the risk of hypersensitivity reactions (grade A recommendation).
- Skin testing is not recommended for identifying hypersensitivity reactions to taxanes (grade B recommendation).
- With respect to desensitization:
- A multi-step desensitization protocol can be used in the case of a positive skin test for platinum drugs or a type i hypersensitivity reaction if treatment cannot be substituted and if stopping treatment would affect the patient’s survival (grade B recommendation). The 12-step protocol developed by Castells et al.41 is supported by the best evidence.
- The desensitization protocol must be carried out under appropriate supervision and closely monitored, with all necessary equipment at hand (grade D recommendation):
- ○ Medical personnel in attendance must have been trained to recognize hypersensitivity reactions and must be prepared to respond immediately.
- ○ Emergency medications (epinephrine, H1 and H2 antihistamines, bronchodilators, and oxygen) must be at the patient’s bedside to ensure quick administration as needed.
- Patients should be educated to recognize the initial symptoms of hypersensitivity and to immediately inform attending personnel (grade D recommendation).
- Premedication must be administered before any desensitization protocol (grade D recommendation).
- If a hypersensitivity reaction should occur during the protocol (grade D recommendation), then
- ○ the infusion must be interrupted and the symptoms controlled by appropriate medication.
- ○ the protocol must be adjusted such that the full dose can be administered if possible.
- ○ the protocol must be modified for subsequent desensitizations.
- ○ a desensitization protocol must be conducted every time the patient subsequently receives treatment.
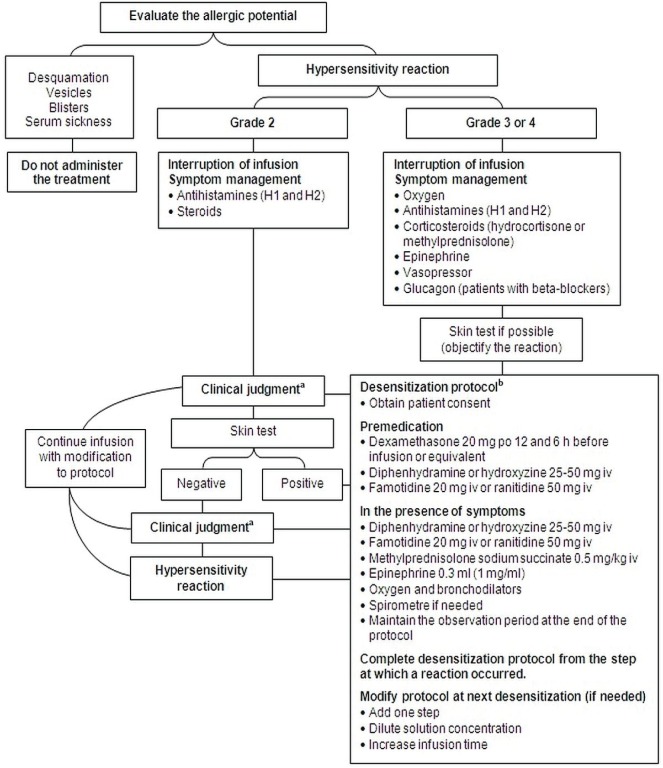
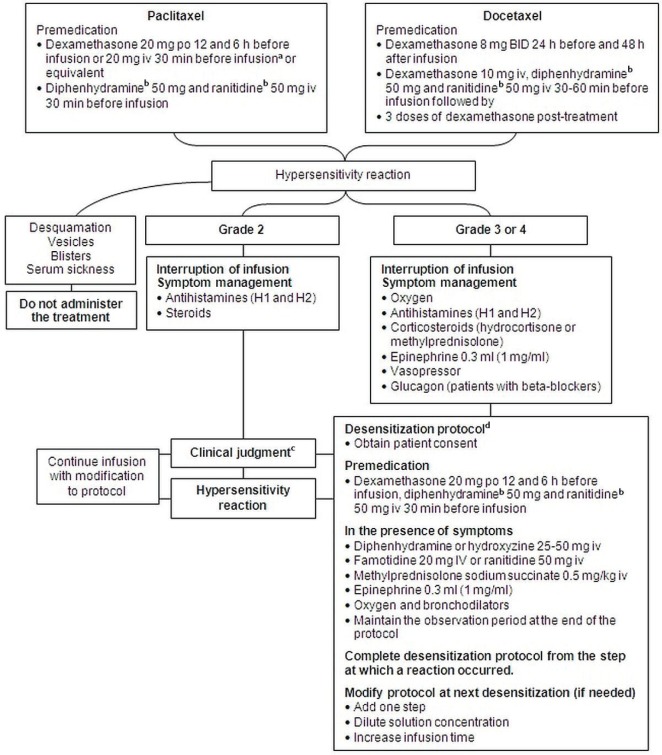
Based on the foregoing recommendations, the cepo proposed two algorithms for the management of hypersensitivity reactions to platinum drugs and taxanes (Figures 1 and 2).
Figure 1.
Management algorithm for hypersensitivity reactions to platinum agents. aApply clinical judgment according to observed symptoms. Consider consultation with an allergist, a medical oncologist, or any specialist with desensitization experience; an increase in the infusion time; use or avoidance of premedication; and use of a desensitization protocol. bDesensitization must be conducted under adequate supervision and with the necessary equipment present. po = orally; iv = intravenously.
Figure 2.
Management algorithm for hypersensitivity reactions to paclitaxel and docetaxel. aUse protocol with oral administration when possible. bThis product or equivalent. cApply clinical judgment according to observed symptoms. Consider consultation with an allergist, a medical oncologist, or any specialist with desensitization experience; an increase in the infusion time; use or avoidance of premedication; and use of a desensitization protocol. dDesensitization must be conducted under adequate supervision and with the necessary equipment present. po = orally; iv = intravenously; bid = twice daily.
6. ACKNOWLEDGMENTS
The cepo is a group of specialists in oncology that reports to the Institut national d’excellence en santé et en services sociaux (inesss). The inesss provided financial support for the present manuscript, which is an adaptation of the original cepo clinical practice guideline, published in July 2013 and freely available in its French version at http://www.msss.gouv.qc.ca/sujets/prob_sante/cancer/download.php?f=ec531e187f7e1b161079228273b58a18. The Direction québécoise de cancérologie of the Ministère de la Santé et des Services sociaux du Québec provided financial support for the original guideline.
The cepo thanks Paul Bessett md, Hôpital Fleurimont (chus); Jean-Luc Dionne md, Hôpital Maisonneuve–Rosemont; Andrew David Moore md, Hôpital Charles–LeMoyne (CSSS Champlain–Charles–LeMoyne); Mireille Poirier bpharm msc bcop, Hôtel-Dieu de Québec (chu de Québec); and Fanny Silviu–Dan md, Montreal General Hospital (muhc) as external reviewers of the original clinical practice guideline.
7. CONFLICT OF INTEREST DISCLOSURES
The authors have no financial conflicts of interest to declare.
8. REFERENCES
- 1.Castells M, Sancho–Serra MD, Simarro M. Hypersensitivity to antineoplastic agents: mechanisms and treatment with rapid desensitization. Cancer Immunol Immunother. 2012:1575–84. doi: 10.1007/s00262-012-1273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pagani M. The complex clinical picture of presumably allergic side effects to cytostatic drugs: symptoms, pathomechanism, reexposure, and desensitization. Med Clin North Am. 2010;94:835–52. xiii. doi: 10.1016/j.mcna.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Demoly P, Bousquet J. Drug allergy diagnosis work up. Allergy. 2002;57(suppl 72):37–40. doi: 10.1034/j.1398-9995.57.s72.7.x. [DOI] [PubMed] [Google Scholar]
- 4.Shepherd GM. Hypersensitivity reactions to chemotherapeutic drugs. Clin Rev Allergy Immunol. 2003;24:253–62. doi: 10.1385/CRIAI:24:3:253. [DOI] [PubMed] [Google Scholar]
- 5.Chung CH. Managing premedications and the risk for reactions to infusional monoclonal antibody therapy. Oncologist. 2008;13:725–32. doi: 10.1634/theoncologist.2008-0012. [DOI] [PubMed] [Google Scholar]
- 6.Ozols RF, Bundy BN, Greer BE, et al. Phase iii trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage iii ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194–200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 7.Cook DJ, Guyatt GH, Laupacis A, Sackett DL. Rules of evidence and clinical recommendations on the use of anti-thrombotic agents. Chest. 1992;102(suppl):305S–11S. [PubMed] [Google Scholar]
- 8.Syrigou E, Triantafyllou O, Makrilia N, et al. Acute hyper-sensitivity reactions to chemotherapy agents: an overview. Inflamm Allergy Drug Targets. 2010;9:206–13. doi: 10.2174/187152810792231887. [DOI] [PubMed] [Google Scholar]
- 9.Makrilia N, Syrigou E, Kaklamanos I, Manolopoulos L, Saif MW. Hypersensitivity reactions associated with platinum antineoplastic agents: a systematic review. Met Based Drugs. 2010;2010 doi: 10.1155/2010/207084. pii:207084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Cearbhaill R, Zhou Q, Iasonos A, et al. The prophylactic conversion to an extended infusion schedule and use of premedication to prevent hypersensitivity reactions in ovarian cancer patients during carboplatin retreatment. Gynecol Oncol. 2010;116:326–31. doi: 10.1016/j.ygyno.2009.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bautista MA, Stevens WT, Chen CS, Curtis BR, Aster RH, Hsueh CT. Hypersensitivity reaction and acute immune-mediated thrombocytopenia from oxaliplatin: two case reports and a review of the literature. J Hematol Oncol. 2010;3:12. doi: 10.1186/1756-8722-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dizon DS, Sabbatini PJ, Aghajanian C, Hensley ML, Spriggs DR. Analysis of patients with epithelial ovarian cancer or fallopian tube carcinoma retreated with cisplatin after the development of a carboplatin allergy. Gynecol Oncol. 2002;84:378–82. doi: 10.1006/gyno.2001.6519. [DOI] [PubMed] [Google Scholar]
- 13.Gadducci A, Tana R, Teti G, Zanca G, Fanucchi A, Genazzani AR. Analysis of the pattern of hypersensitivity reactions in patients receiving carboplatin retreatment for recurrent ovarian cancer. Int J Gynecol Cancer. 2008;18:615–20. doi: 10.1111/j.1525-1438.2007.01063.x. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz JR, Bandera C, Bradley A, et al. Does the platinum-free interval predict the incidence or severity of hypersensitivity reactions to carboplatin? The experience from Women and Infants’ Hospital. Gynecol Oncol. 2007;105:81–3. doi: 10.1016/j.ygyno.2006.10.047. [DOI] [PubMed] [Google Scholar]
- 15.Pujade–Lauraine E, Wagner U, Aavall–Lundqvist E, et al. Pegylated liposomal doxorubicin and carboplatin compared with paclitaxel and carboplatin for patients with platinum-sensitive ovarian cancer in late relapse. J Clin Oncol. 2010;28:3323–9. doi: 10.1200/JCO.2009.25.7519. [DOI] [PubMed] [Google Scholar]
- 16.Markman M, Moon J, Wilczynski S, et al. Single agent carboplatin versus carboplatin plus pegylated liposomal doxorubicin in recurrent ovarian cancer: final survival results of a swog (S0200) phase 3 randomized trial. Gynecol Oncol. 2010;116:323–5. doi: 10.1016/j.ygyno.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gobel BH. Chemotherapy-induced hypersensitivity reactions. Oncol Nurs Forum. 2005;32:1027–35. doi: 10.1188/05.ONF.1027-1035. [DOI] [PubMed] [Google Scholar]
- 18.Shukunami K, Kurokawa T, Kawakami Y, Kubo M, Kotsuji F. Hypersensitivity reactions to intraperitoneal administration of carboplatin in ovarian cancer: the first report of a case. Gynecol Oncol. 1999;72:431–2. doi: 10.1006/gyno.1998.5273. [DOI] [PubMed] [Google Scholar]
- 19.Markman M, Kennedy A, Webster K, Kulp B, Peterson G, Belinson J. Paclitaxel-associated hypersensitivity reactions: experience of the gynecologic oncology program of the Cleveland Clinic Cancer Center. J Clin Oncol. 2000;18:102–5. doi: 10.1200/JCO.2000.18.1.102. [DOI] [PubMed] [Google Scholar]
- 20.Weiss RB. Hypersensitivity reactions. Semin Oncol. 1992;19:458–77. [PubMed] [Google Scholar]
- 21.Sendo T, Sakai N, Itoh Y, et al. Incidence and risk factors for paclitaxel hypersensitivity during ovarian cancer chemotherapy. Cancer Chemother Pharmacol. 2005;56:91–6. doi: 10.1007/s00280-004-0924-9. [DOI] [PubMed] [Google Scholar]
- 22.Kloover JS, den Bakker MA, Gelderblom H, van Meerbeeck JP. Fatal outcome of a hypersensitivity reaction to paclitaxel: a critical review of premedication regimens. Br J Cancer. 2004;90:304–5. doi: 10.1038/sj.bjc.6601303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moisidis C, Mobus V. Erythema multiforme major following docetaxel. Arch Gynecol Obstet. 2005;271:267–9. doi: 10.1007/s00404-004-0643-9. [DOI] [PubMed] [Google Scholar]
- 24.Goldberg A, Confino–Cohen R, Fishman A, Beyth Y, Altaras M. A modified, prolonged desensitization protocol in carboplatin allergy. J Allergy Clin Immunol. 1996;98:841–3. doi: 10.1016/S0091-6749(96)70134-3. [DOI] [PubMed] [Google Scholar]
- 25.Szebeni J, Muggia FM, Alving CR. Complement activation by Cremophor el as a possible contributor to hypersensitivity to paclitaxel: an in vitro study. J Natl Cancer Inst. 1998;90:300–6. doi: 10.1093/jnci/90.4.300. [DOI] [PubMed] [Google Scholar]
- 26.Weiss RB, Donehower RC, Wiernik PH, et al. Hypersensitivity reactions from taxol. J Clin Oncol. 1990;8:1263–8. doi: 10.1200/JCO.1990.8.7.1263. [DOI] [PubMed] [Google Scholar]
- 27.O’Dwyer PJ, King SA, Fortner CL, Leyland–Jones B. Hypersensitivity reactions to teniposide (VM-26): an analysis. J Clin Oncol. 1986;4:1262–9. doi: 10.1200/JCO.1986.4.8.1262. [DOI] [PubMed] [Google Scholar]
- 28.Gradishar WJ, Tjulandin S, Davidson N, et al. Phase iii trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23:7794–803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 29.Fossella FV, Lee JS, Murphy WK, et al. Phase ii study of docetaxel for recurrent or metastatic non-small-cell lung cancer. J Clin Oncol. 1994;12:1238–44. doi: 10.1200/JCO.1994.12.6.1238. [DOI] [PubMed] [Google Scholar]
- 30.Essayan DM, Kagey–Sobotka A, Colarusso PJ, Lichtenstein LM, Ozols RF, King ED. Successful parenteral desensitization to paclitaxel. J Allergy Clin Immunol. 1996;97:42–6. doi: 10.1016/S0091-6749(96)70281-6. [DOI] [PubMed] [Google Scholar]
- 31.Prieto Garcia A, Pineda de la Losa F. Immunoglobulin E– mediated severe anaphylaxis to paclitaxel. J Investig Allergol Clin Immunol. 2010;20:170–1. [PubMed] [Google Scholar]
- 32.Nolte H, Kowal K, Lawrence DuBuske. Overview of skin testing for allergic disease [Web resource] Boston, MA: Up-ToDate, Wolters Kluwer Health; n.d. [Current version available online at: http://www.uptodate.com/contents/overview-of-skin-testing-for-allergic-disease; cited July 31, 2012] [Google Scholar]
- 33.Brockow K, Romano A. Skin tests in the diagnosis of drug hypersensitivity reactions. Curr Pharm Des. 2008;14:2778–91. doi: 10.2174/138161208786369821. [DOI] [PubMed] [Google Scholar]
- 34.Tan J, Mehr S. Anaphylaxis to an ondansetron wafer. J Paediatr Child Health. 2012;48:543–4. doi: 10.1111/j.1440-1754.2011.02124.x. [DOI] [PubMed] [Google Scholar]
- 35.Markman M, Zanotti K, Peterson G, Kulp B, Webster K, Belinson J. Expanded experience with an intradermal skin test to predict for the presence or absence of carboplatin hypersensitivity. J Clin Oncol. 2003;21:4611–14. doi: 10.1200/JCO.2003.05.539. [DOI] [PubMed] [Google Scholar]
- 36.Elligers KT, Davies M, Sanchis D, Ferencz T, Saif MW. Re-challenge with cisplatin in a patient with pancreatic cancer who developed a hypersensitivity reaction to oxaliplatin. Is skin test useful in this setting? JOP. 2008;9:197–202. [PubMed] [Google Scholar]
- 37.Leguy–Seguin V, Jolimoy G, Coudert B, et al. Diagnostic and predictive value of skin testing in platinum salt hypersensitivity. J Allergy Clin Immunol. 2007;119:726–30. doi: 10.1016/j.jaci.2006.11.640. [DOI] [PubMed] [Google Scholar]
- 38.Syrigou E, Makrilia N, Vassias A, et al. Administration of cisplatin in three patients with carboplatin hypersensitivity: is skin testing useful? Anticancer Drugs. 2010;21:333–8. doi: 10.1097/CAD.0b013e32833418c0. [DOI] [PubMed] [Google Scholar]
- 39.Robinson JB, Singh D, Bodurka–Bevers DC, Wharton JT, Gershenson DM, Wolf JK. Hypersensitivity reactions and the utility of oral and intravenous desensitization in patients with gynecologic malignancies. Gynecol Oncol. 2001;82:550–8. doi: 10.1006/gyno.2001.6331. [DOI] [PubMed] [Google Scholar]
- 40.Hesterberg PE, Banerji A, Oren E, et al. Risk stratification for desensitization of patients with carboplatin hypersensitivity: clinical presentation and management. J Allergy Clin Immunol. 2009;123:1262–7.e1. doi: 10.1016/j.jaci.2009.02.042. [DOI] [PubMed] [Google Scholar]
- 41.Castells MC, Tennant NM, Sloane DE, et al. Hypersensitivity reactions to chemotherapy: outcomes and safety of rapid desensitization in 413 cases. J Allergy Clin Immunol. 2008;122:574–80. doi: 10.1016/j.jaci.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 42.Williams C, Bryant A. Short versus long duration infusions of paclitaxel for any advanced adenocarcinoma. Cochrane Database Syst Rev. 2011;5:CD003911. doi: 10.1002/14651858.CD003911.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hainsworth JD, Thompson DS, Greco FA. Paclitaxel by 1-hour infusion: an active drug in metastatic non-small-cell lung cancer. J Clin Oncol. 1995;13:1609–14. doi: 10.1200/JCO.1995.13.7.1609. [DOI] [PubMed] [Google Scholar]
- 44.Polyzos A, Tsavaris N, Kosmas C, et al. Hypersensitivity reactions to carboplatin administration are common but not always severe: a 10-year experience. Oncology. 2001;61:129–33. doi: 10.1159/000055363. [DOI] [PubMed] [Google Scholar]
- 45.Bhargava P, Gammon D, McCormick MJ. Hypersensitivity and idiosyncratic reactions to oxaliplatin. Cancer. 2004;100:211–12. doi: 10.1002/cncr.11901. [DOI] [PubMed] [Google Scholar]
- 46.Kidera Y, Satoh T, Ueda S, et al. High-dose dexamethasone plus antihistamine prevents colorectal cancer patients treated with modified folfox6 from hypersensitivity reactions induced by oxaliplatin. Int J Clin Oncol. 2011;16:244–9. doi: 10.1007/s10147-010-0170-6. [DOI] [PubMed] [Google Scholar]
- 47.BC Cancer Agency (bcca) BCCA Protocol Summary for Palliative Combination Chemotherapy for Metastatic Pancreatic Adenocarcinoma Using Irinotecan, Oxaliplatin, Fluorouracil and Folinic Acid (Leucovorin) Vancouver, BC: BCCA; 2012. [Google Scholar]
- 48.Trudeau ME, Eisenhauer EA, Higgins BP, et al. Docetaxel in patients with metastatic breast cancer: a phase ii study of the National Cancer Institute of Canada-Clinical Trials Group. J Clin Oncol. 1996;14:422–8. doi: 10.1200/JCO.1996.14.2.422. [DOI] [PubMed] [Google Scholar]
- 49.Kwon JS, Elit L, Finn M, et al. A comparison of two prophylactic regimens for hypersensitivity reactions to paclitaxel. Gynecol Oncol. 2002;84:420–5. doi: 10.1006/gyno.2001.6546. [DOI] [PubMed] [Google Scholar]
- 50.Rosenberg P, Andersson H, Boman K, et al. Randomized trial of single agent paclitaxel given weekly versus every three weeks and with peroral versus intravenous steroid premedication to patients with ovarian cancer previously treated with platinum. Acta Oncol. 2002;41:418–24. doi: 10.1080/028418602320404998. [DOI] [PubMed] [Google Scholar]
- 51.Syrigou E, Dannos I, Kotteas E, et al. Hypersensitivity reactions to docetaxel: retrospective evaluation and development of a desensitization protocol. Int Arch Allergy Immunol. 2011;156:320–4. doi: 10.1159/000324454. [DOI] [PubMed] [Google Scholar]
- 52.Wang GS, Yang KY, Perng RP. Life-threatening hypersensitivity pneumonitis induced by docetaxel (Taxotere) Br J Cancer. 2001;85:1247–50. doi: 10.1054/bjoc.2001.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burris HA. Optimal use of docetaxel (Taxotere): maximizing its potential. Anticancer Drugs. 1996;7(suppl 2):25–8. doi: 10.1097/00001813-199608002-00007. [DOI] [PubMed] [Google Scholar]
- 54.Burstein HJ, Manola J, Younger J, et al. Docetaxel administered on a weekly basis for metastatic breast cancer. J Clin Oncol. 2000;18:1212–19. doi: 10.1200/JCO.2000.18.6.1212. [DOI] [PubMed] [Google Scholar]
- 55.Piccart MJ, Klijn J, Paridaens R, et al. Corticosteroids significantly delay the onset of docetaxel-induced fluid retention: final results of a randomized study of the European Organization for Research and Treatment of Cancer Investigational Drug Branch for Breast Cancer. J Clin Oncol. 1997;15:3149–55. doi: 10.1200/JCO.1997.15.9.3149. [DOI] [PubMed] [Google Scholar]
- 56.Chouhan JD, Herrington JD. Single premedication dose of dexamethasone 20 mg IV before docetaxel administration. J Oncol Pharm Pract. 2011;17:155–9. doi: 10.1177/1078155210367950. [DOI] [PubMed] [Google Scholar]
- 57.Feldweg AM, Lee CW, Matulonis UA, Castells M. Rapid desensitization for hypersensitivity reactions to paclitaxel and docetaxel: a new standard protocol used in 77 successful treatments. Gynecol Oncol. 2005;96:824–9. doi: 10.1016/j.ygyno.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 58.Ardavanis A, Tryfonopoulos D, Yiotis I, Gerasimidis G, Baziotis N, Rigatos G. Non-allergic nature of docetaxel-induced acute hypersensitivity reactions. Anticancer Drugs. 2004;15:581–5. doi: 10.1097/01.cad.0000131685.06390.b7. [DOI] [PubMed] [Google Scholar]
- 59.Zanotti KM, Markman M. Prevention and management of antineoplastic-induced hypersensitivity reactions. Drug Saf. 2001;24:767–79. doi: 10.2165/00002018-200124100-00005. [DOI] [PubMed] [Google Scholar]
- 60.Lenz HJ. Management and preparedness for infusion and hypersensitivity reactions. Oncologist. 2007;12:601–9. doi: 10.1634/theoncologist.12-5-601. [DOI] [PubMed] [Google Scholar]
- 61.Foote M. Using nanotechnology to improve the characteristics of antineoplastic drugs: improved characteristics of nabpaclitaxel compared with solvent-based paclitaxel. Biotechnol Annu Rev. 2007;13:345–57. doi: 10.1016/S1387-2656(07)13012-X. [DOI] [PubMed] [Google Scholar]
- 62.Montana M, Ducros C, Verhaeghe P, Terme T, Vanelle P, Rathelot P. Albumin-bound paclitaxel: the benefit of this new formulation in the treatment of various cancers. J Chemother. 2011;23:59–66. doi: 10.1179/joc.2011.23.2.59. [DOI] [PubMed] [Google Scholar]
- 63.Ibrahim NK, Desai N, Legha S, et al. Phase i and pharmacokinetic study of ABI-007, a Cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin Cancer Res. 2002;8:1038–44. [PubMed] [Google Scholar]
- 64.Ibrahim NK, Samuels B, Page R, et al. Multicenter phase ii trial of ABI-007, an albumin-bound paclitaxel, in women with metastatic breast cancer. J Clin Oncol. 2005;23:6019–26. doi: 10.1200/JCO.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 65.Fader AN, Rose PG. Abraxane for the treatment of gynecologic cancer patients with severe hypersensitivity reactions to paclitaxel. Int J Gynecol Cancer. 2009;19:1281–3. doi: 10.1111/IGC.0b013e3181a38e2f. [DOI] [PubMed] [Google Scholar]
- 66.Castells M. Desensitization for drug allergy. Curr Opin Allergy Clin Immunol. 2006;6:476–81. doi: 10.1097/ACI.0b013e3280108716. [DOI] [PubMed] [Google Scholar]
- 67.Lee CW, Matulonis UA, Castells MC. Rapid inpatient/outpatient desensitization for chemotherapy hypersensitivity: standard protocol effective in 57 patients for 255 courses. Gynecol Oncol. 2005;99:393–9. doi: 10.1016/j.ygyno.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 68.Limsuwan T, Castells MC. Outcomes and safety of rapid desensitization for chemotherapy hypersensitivity. Expert Opin Drug Saf. 2010;9:39–53. doi: 10.1517/14740330903446936. [DOI] [PubMed] [Google Scholar]
- 69.Confino–Cohen R, Fishman A, Altaras M, Goldberg A. Successful carboplatin desensitization in patients with proven carboplatin allergy. Cancer. 2005;104:640–3. doi: 10.1002/cncr.21168. [DOI] [PubMed] [Google Scholar]
- 70.Cortijo–Cascajares S, Nacle–Lopez I, Garcia–Escobar I, et al. Effectiveness of oxaliplatin desensitization protocols. Clin Transl Oncol. 2013;15:219–25. doi: 10.1007/s12094-012-0909-9. [DOI] [PubMed] [Google Scholar]
- 71.Rose PG, Fusco N, Smrekar M, Mossbruger K, Rodriguez M. Successful administration of carboplatin in patients with clinically documented carboplatin hypersensitivity. Gynecol Oncol. 2003;89:429–33. doi: 10.1016/S0090-8258(03)00178-1. [DOI] [PubMed] [Google Scholar]
- 72.Gammon D, Bhargava P, McCormick MJ. Hypersensitivity reactions to oxaliplatin and the application of a desensitization protocol. Oncologist. 2004;9:546–9. doi: 10.1634/theoncologist.9-5-546. [DOI] [PubMed] [Google Scholar]
- 73.Giacchetti S, Perpoint B, Zidani R, et al. Phase iii multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2000;18:136–47. doi: 10.1200/JCO.2000.18.1.136. [DOI] [PubMed] [Google Scholar]
- 74.Greco FA, Stroup SL, Hainsworth JD. Paclitaxel by 1-hour infusion in combination chemotherapy of stage iii non-small cell lung cancer. Semin Oncol. 1995;22(suppl 9):75–7. [PubMed] [Google Scholar]
- 75.Lee C, Gianos M, Klaustermeyer WB. Diagnosis and management of hypersensitivity reactions related to common cancer chemotherapy agents. Ann Allergy Asthma Immunol. 2009;102:179–87. doi: 10.1016/S1081-1206(10)60078-6. [DOI] [PubMed] [Google Scholar]
- 76.Gomez R, Harter P, Luck HJ, et al. Carboplatin hypersensitivity: does introduction of skin test and desensitization reliably predict and avoid the problem? A prospective single-center study. Int J Gynecol Cancer. 2009;19:1284–7. doi: 10.1111/IGC.0b013e3181a418ff. [DOI] [PubMed] [Google Scholar]
- 77.Zanotti KM, Rybicki LA, Kennedy AW, et al. Carboplatin skin testing: a skin-testing protocol for predicting hypersensitivity to carboplatin chemotherapy. J Clin Oncol. 2001;19:3126–9. doi: 10.1200/JCO.2001.19.12.3126. [DOI] [PubMed] [Google Scholar]