Abstract
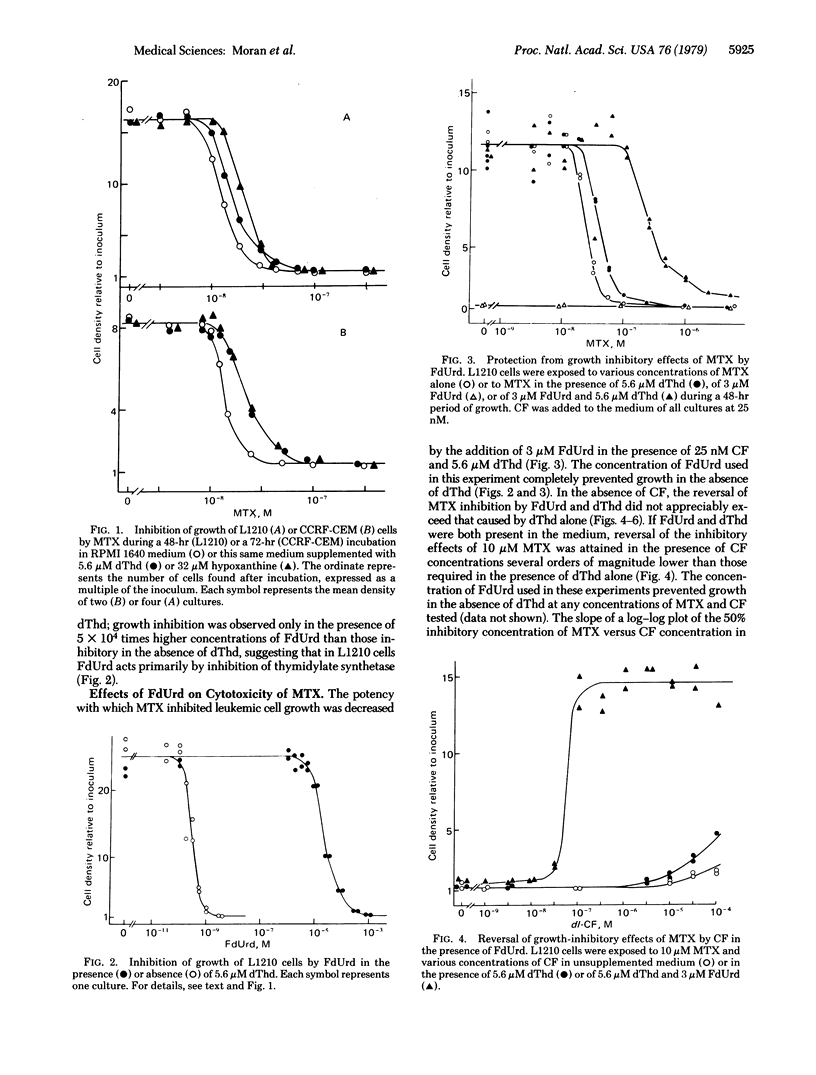
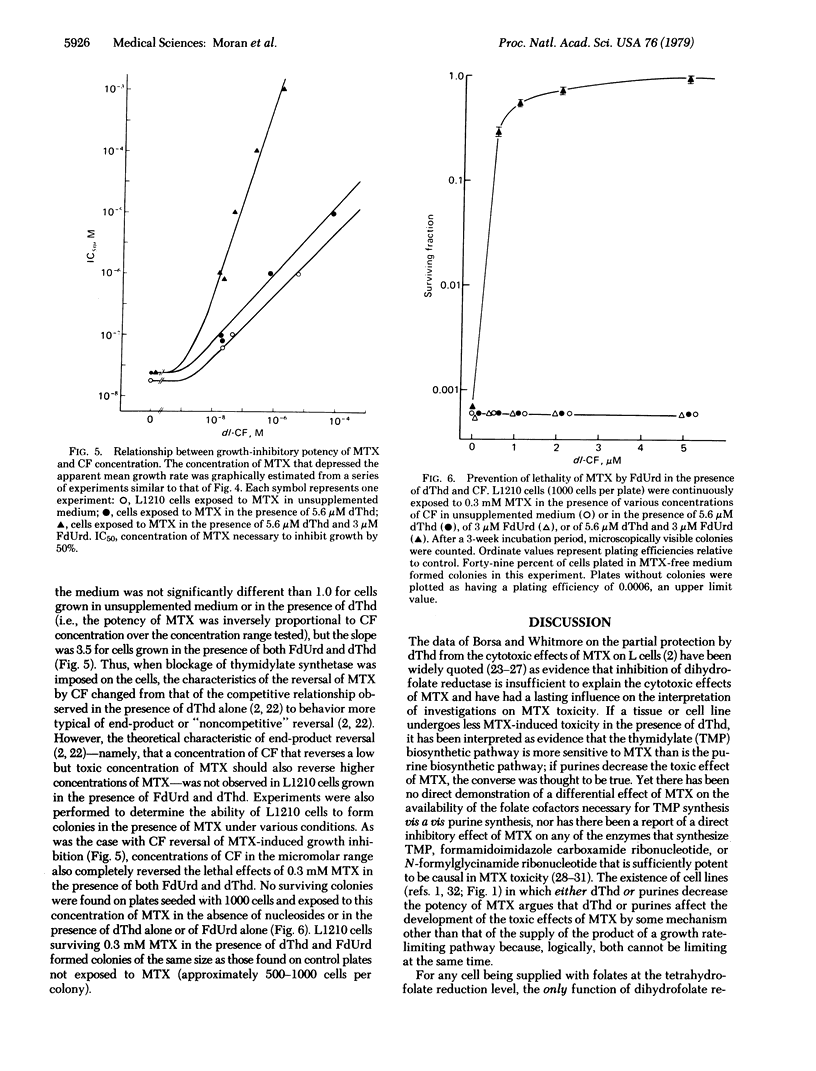
Methotrexate (MTX) inhibition of the growth of mouse or human leukemia cells in culture was partially prevented by either thymidine (dThd) or hypoxanthine. 5-Fluoro-2'-deoxyuridine (FdUrd) also decreased the growth-inhibitory potency of MTX in the presence of small concentrations of 5-formyltetrahydrofolate (citrovorum factor) and sufficient exogenous dThd to support the synthesis of thymidylate nucleotides by salvage mechanisms. In addition, citrovorum factor-induced reversal of MTX was several orders of magnitude more efficient in the presence of both FdUrd and dThd than in the presence of dThd alone or in the absence of both nucleosides. Likewise, the presence of FdUrd (3 microM) and dThd (5.6 microM) completely prevented the lethality of 0.3 mM MTX to L1210 cells in culture medium supplemented with micromolar concentrations of citrovorum factor. We propose that this protection against the cytotoxic effects of MTX by dThd, hypoxanthine, and FdUrd have a common biochemical mechanism--namely, inhibition of the de novo synthesis of thymidylate by either a direct [FdUrd; inhibition of thymidylate synthetase (thymidylate synthase; 5,10-methylenetetrahydrofolate:dUMP C-methyl-transferase, EC 2.1.1.45)] or indirect (dThd and hypoxanthine; feedback inhibition by anabolites on ribonucleotide reductase and deoxycytidylate deaminase) effect. The resultant decreased rate of loss of reduced folates due to de novo thymidylate synthesis would allow a higher degree of inhibition of dihydrofolate reductase to be endured without damage to the cell.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aunio R., Hakala M. T. Tetrahydrofolate-dependent enzymes in sarcoma 180 cells sensitive and resistant to amethopterin. Biochem Pharmacol. 1968 Aug;17(8):1744–1747. doi: 10.1016/0006-2952(68)90239-6. [DOI] [PubMed] [Google Scholar]
- BERTINO J. R., BOOTH B. A., BIEBER A. L., CASHMORE A., SARTORELLI A. C. STUDIES ON THE INHIBITION OF DIHYDROFOLATE REDUCTASE BY THE FOLATE ANTAGONISTS. J Biol Chem. 1964 Feb;239:479–485. [PubMed] [Google Scholar]
- Bertino J. R., Sawicki W. L., Lindquist C. A., Gupta V. S. Schedule-dependent antitumor effects of methotrexate and 5-fluorouracil. Cancer Res. 1977 Jan;37(1):327–328. [PubMed] [Google Scholar]
- Bird O. D., McGlohon V. M., Vaitkus J. W. Naturally occurring folates in the blood and liver of the rat. Anal Biochem. 1965 Jul;12(1):18–35. doi: 10.1016/0003-2697(65)90138-7. [DOI] [PubMed] [Google Scholar]
- Borsa J., Whitmore G. F. Cell killing studies on the mode of action of methotrexate on L-cells in vitro. Cancer Res. 1969 Apr;29(4):737–744. [PubMed] [Google Scholar]
- Borsa J., Whitmore G. F. Studies relating to the mode of action of methotrexate. 3. Inhibition of thymidylate synthetase in tissue culture cells and in cell-free systems. Mol Pharmacol. 1969 Jul;5(4):318–332. [PubMed] [Google Scholar]
- Borsa J., Whitmore G. F. Studies relating to the mode of action of methotrexate. II. Studies on sites of action in L-cells in vitro. Mol Pharmacol. 1969 Jul;5(4):303–317. [PubMed] [Google Scholar]
- Bowen D., White J. C., Goldman I. D. A basis for fluoropyrimidine-induced antagonism to methotrexate in Ehrlich ascites tumor cells in vitro. Cancer Res. 1978 Jan;38(1):219–222. [PubMed] [Google Scholar]
- Dolnick B. J., Cheng Y. c. Human thymidylate synthetase derived from blast cells of patients with acture myelocytic leukemia. Purification and chracterization. J Biol Chem. 1977 Nov 10;252(21):7697–7703. [PubMed] [Google Scholar]
- Dubin H. V., Harrell E. R. Liver disease associated with methotrexate treatment of psoriatic patients. Arch Dermatol. 1970 Nov;102(5):498–503. [PubMed] [Google Scholar]
- Ensminger W. D., Frei E., 3rd The prevention of methotrexate toxicity by thymidine infusions in humans. Cancer Res. 1977 Jun;37(6):1857–1863. [PubMed] [Google Scholar]
- FOLEY G. E., LAZARUS H., FARBER S., UZMAN B. G., BOONE B. A., MCCARTHY R. E. CONTINUOUS CULTURE OF HUMAN LYMPHOBLASTS FROM PERIPHERAL BLOOD OF A CHILD WITH ACUTE LEUKEMIA. Cancer. 1965 Apr;18:522–529. doi: 10.1002/1097-0142(196504)18:4<522::aid-cncr2820180418>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Grindey G. B., Moran R. G. Effects of allopurinol on the therapeutic efficacy of methotrexate. Cancer Res. 1975 Jul;35(7):1702–1705. [PubMed] [Google Scholar]
- Grindey G. B., Nichol C. A. Interaction of drugs inhibiting different steps in the synthesis of DNA. Cancer Res. 1972 Mar;32(3):527–531. [PubMed] [Google Scholar]
- HAKALA M. T. Prevention of toxicity of amethopterin for sarcoma-180 cells in tissue culture. Science. 1957 Aug 9;126(3267):255–255. doi: 10.1126/science.126.3267.255. [DOI] [PubMed] [Google Scholar]
- HAKALA M. T., TAYLOR E. The ability of purine and thymine derivatives and of glycine to support the growth of mammalian cells in culture. J Biol Chem. 1959 Jan;234(1):126–128. [PubMed] [Google Scholar]
- HAKALA M. T., ZAKRZEWSKI S. F., NICHOL C. A. Relation of folic acid reductase to amethopterin resistance in cultured mammalian cells. J Biol Chem. 1961 Mar;236:952–958. [PubMed] [Google Scholar]
- HERBERT V., LARRABEE A. R., BUCHANAN J. M. Studies on the identification of a folate compound of human serum. J Clin Invest. 1962 May;41:1134–1138. doi: 10.1172/JCI104565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hryniuk W. M., Bertino J. R. Growth rate and cell kill. Ann N Y Acad Sci. 1971 Nov 30;186:330–342. [PubMed] [Google Scholar]
- Hryniuk W. M. Purineless death as a link between growth rate and cytotoxicity by methotrexate. Cancer Res. 1972 Jul;32(7):1506–1511. [PubMed] [Google Scholar]
- Jackson R. C., Hart L. I., Harrap K. R. Intrinsic resistance to methotrexate of cultured mammalian cells in relation to the inhibition kinetics of their dihydrololate reductases. Cancer Res. 1976 Jun;36(6):1991–1997. [PubMed] [Google Scholar]
- Jackson R. C., Niethammer D., Hart L. I. Reactivation of dihydrofolate reductase inhibted by methotrexate or aminopterin. Arch Biochem Biophys. 1977 Aug;182(2):646–656. doi: 10.1016/0003-9861(77)90545-8. [DOI] [PubMed] [Google Scholar]
- Jackson R. C. The regulation of thymidylate biosynthesis in Novikoff hepatoma cells and the effects of amethopterin, 5-fluorodeoxyuridine, and 3-deazauridine. J Biol Chem. 1978 Oct 25;253(20):7440–7446. [PubMed] [Google Scholar]
- Lorenson M. Y., Maley G. F., Maley F. The purification and properties of thymidylate synthetase from chick embryo extracts. J Biol Chem. 1967 Jul 25;242(14):3332–3344. [PubMed] [Google Scholar]
- Lowe J. K., Grindey G. B. Inhibition of growth rate and deoxynucleoside triphosphate concentrations in cultured leukemia L1210 cells. Mol Pharmacol. 1976 Jan;12(1):177–184. [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- MALEY F., MALEY G. F. On the nature of a sparing effect by thymidine on the utilization of deoxycytidine. Biochemistry. 1962 Sep;1:847–851. doi: 10.1021/bi00911a017. [DOI] [PubMed] [Google Scholar]
- McBurney M. W., Whitmore G. F. Mechanism of growth inhibition by methotrexate. Cancer Res. 1975 Mar;35(3):586–590. [PubMed] [Google Scholar]
- Moore E. C., Hurlbert R. B. Regulation of mammalian deoxyribonucleotide biosynthesis by nucleotides as activators and inhibitors. J Biol Chem. 1966 Oct 25;241(20):4802–4809. [PubMed] [Google Scholar]
- Moran R. G., Spears C. P., Heidelberger C. Biochemical determinants of tumor sensitivity to 5-fluorouracil: ultrasensitive methods for the determination of 5-fluoro-2'-deoxyuridylate, 2'-deoxyuridylate, and thymidylate synthetase. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1456–1460. doi: 10.1073/pnas.76.3.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran R. G., Werkheiser W. C., Zakrzewski S. F. Folate metabolism in mammalian cells in culture. I Partial characterization of the folate derivatives present in L1210 mouse leukemia cells. J Biol Chem. 1976 Jun 25;251(12):3569–3575. [PubMed] [Google Scholar]
- Myers C. E., Lippman M. E., Elliot H. M., Chabner B. A. Competitive protein binding assay for methotrexate. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3683–3686. doi: 10.1073/pnas.72.9.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORONHA J. M., ABOOBAKER V. S. Studies on the folate compounds of human blood. Arch Biochem Biophys. 1963 Jun;101:445–447. doi: 10.1016/0003-9861(63)90501-0. [DOI] [PubMed] [Google Scholar]
- Niethammer D., Jackson R. C. Changes of molecular properties associated with the development of resistance against methotrexate in human lymphoblastoid cells. Eur J Cancer. 1975 Dec;11(12):845–854. doi: 10.1016/0014-2964(75)90083-3. [DOI] [PubMed] [Google Scholar]
- Nixon P. F., Slutsky G., Nahas A., Bertino J. R. The turnover of folate coenzymes in murine lymphoma cells. J Biol Chem. 1973 Sep 10;248(17):5932–5936. [PubMed] [Google Scholar]
- O'Donovan G. A., Neuhard J. Pyrimidine metabolism in microorganisms. Bacteriol Rev. 1970 Sep;34(3):278–343. doi: 10.1128/br.34.3.278-343.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinedo H. M., Zaharko D. S., Bull J. M., Chabner B. A. The reversal of methotrexate cytotoxicity to mouse bone marrow cells by leucovorin and nucleosides. Cancer Res. 1976 Dec;36(12):4418–4424. [PubMed] [Google Scholar]
- Roberts D. In search of a key. Natl Cancer Inst Monogr. 1971 Dec;34:118–130. [PubMed] [Google Scholar]
- Roberts D., Warmath E. V. Methotrexate inhibition of CCRF-CEM cultures of human lymphoblasts. Eur J Cancer. 1975 Nov;11(11):771–782. doi: 10.1016/0014-2964(75)90170-x. [DOI] [PubMed] [Google Scholar]
- Rosenfelt F. Methotrexate and the need for continued research. Yale J Biol Med. 1975 May;48(2):97–103. [PMC free article] [PubMed] [Google Scholar]
- Rossi M., Geraci G., Scarano E. Deoxycytidylate aminohydrolase. 3. Modifications of the substrate sites caused by allosteric effectors. Biochemistry. 1967 Dec;6(12):3640–3645. doi: 10.1021/bi00864a003. [DOI] [PubMed] [Google Scholar]
- Scarano E., Geraci G., Rossi M. Deoxycytidylate aminohydrolase. II. Kinetic properties. The activatory effect of deoxycytidine triphosphate and the inhibitory effect of deoxythymidine triphosphate. Biochemistry. 1967 Jan;6(1):192–201. doi: 10.1021/bi00853a031. [DOI] [PubMed] [Google Scholar]
- Semon J. H., Grindey G. B. Potentiation of the antitumor activity of methotrexate by concurrent infusion of thymidine. Cancer Res. 1978 Sep;38(9):2905–2911. [PubMed] [Google Scholar]
- Tattersall M. H., Brown B., Frei E., 3rd The reversal of methotrexate toxicity by thymidine with maintenance of antitumour effects. Nature. 1975 Jan 17;253(5488):198–200. doi: 10.1038/253198a0. [DOI] [PubMed] [Google Scholar]
- Tattersall M. H., Jackson R. C., Connors T. A., Harrap K. R. Combination chemotherapy: the interaction of methotrexate and 5-fluorouracil. Eur J Cancer. 1973 Oct;9(10):733–739. doi: 10.1016/0014-2964(73)90064-9. [DOI] [PubMed] [Google Scholar]



