Abstract
The recognition and management of transfusion reactions (TRs) are critical to ensure patient safety during and after a blood transfusion. Transfusion reactions are classified into acute transfusion reactions (ATRs) or delayed transfusion reactions, and each category includes different subtypes. Different ATRs share common signs and symptoms which can make categorisation difficult at the beginning of the reaction. Moreover, TRs are often under-recognised and under-reported. To ensure uniform practice and safety, it is necessary to implement a national haemovigilance system and a set of national guidelines establishing policies for blood transfusion and for the detection and management of TRs. In Oman, there are currently no local TR guidelines to guide physicians and hospital blood banks. This paper summarises the available literature and provides consensus guidelines to be used in the recognition, management and reporting of ATRs.
Keywords: Blood Transfusion, Blood Safety, Transfusion Reactions, Health Planning Guidelines, Oman
A key aspect of patient safety during blood transfusions is to ensure that patients are monitored by staff trained in transfusion medicine and the recognition and management of transfusion reactions (TRs). Each organisation should have a local transfusion programme and guidelines for the administration of blood components and for reporting TRs. The true incidence of TRs is uncertain due to the wide variation in reporting rates.1 Reports from the Serious Hazard of Transfusion (SHOT) haemovigilance scheme in the UK suggest an incidence of serious TRs of 14/100,000 components transfused.2
Transfusion in Oman takes place at the secondary and tertiary healthcare settings. Blood is collected and prepared in the central blood bank and the hospitals. Pre-storage leukoreduction has not yet been universally implemented, although some institutions have introduced pre-storage and bedside leukoreduction. The lack of universal leukoreduction can result in an increased risk of a febrile non-haemolytic transfusion reaction (FNHTR), human leukocyte antigen (HLA) alloimmunisation, platelet refractoriness and possibly transfusion-related acute lung injury (TRALI).3 A uniform TR reporting form was implemented in 2007 in Oman. However, there are no national guidelines, and the incidence of serious TRs remains unknown.
This article aims to provide a guide on the diagnosis, investigation, management and reporting of acute transfusion reactions (ATRs). These guidelines were produced by a working group of experts from four different institutions, including two haematopathologists, one pediatric haematologist, three adult haematologists, and an external reviewer with expertise in the field of transfusion medicine. Available literature and international guidelines on ATRs were reviewed and summarised. These guidelines were then customised to the local processes and to the therapeutic options available in Oman, including available laboratory investigations and medications.
Classification of Transfusion Reactions
The general classification of TRs is based on the time between the transfusion and the onset of the reaction. The UK SHOT group has defined two types of TRs—ATRs and delayed transfusion reactions (DTRs).1 ATRs occur within 24 hours of the commencement of the transfusion while DTRs occur afterwards. TRs have various subtypes and further classification is based on the manifestations and the results of the investigations of the reaction. Table 1 describes the general classification and subtypes of TRs. ATRs will be reviewed in detail in these guidelines.
Table 1:
Classifications and subtypes of transfusion reactions
| Acute | Delayed |
|---|---|
| Febrile non-haemolytic transfusion reactions | Delayed haemolytic transfusion reactions |
| Acute haemolytic transfusion reactions | Post-transfusion purpura |
| Minor allergic reactions | Transfusion-associated graft versus host disease |
| Anaphylactic reactions | Alloimmunisation |
| Septic reactions (bacterial contamination) | Transfusion-transmitted infections |
| Hypotensive reactions* | Immunomodulation |
| Transfusion-related acute lung injury | |
| Transfusion-associated circulatory overload | |
| Transfusion-associated dyspnoea* |
Diagnosis of exclusion.
Recognition of Transfusion Reactions
It is critical to monitor patients throughout a transfusion episode. The recognition of TRs may be improved by asking patients to report any signs and symptoms if these develop during the transfusion. As ATRs can present just hours after completing a transfusion, all patients, including those discharged from hospital, should also be instructed to report any symptoms that develop within the following 24-hour period of completion of the transfusion.4,5
Different ATRs share common manifestations [Table 2], and some features can be obscured by the patient’s underlying condition or treatment. For example, fever is common in ATRs, but can represent febrile neutropaenia in a patient receiving chemotherapy.
Table 2:
Signs and symptoms of acute transfusion reactions
| Sign/symptom | Type of reaction | Comments |
|---|---|---|
| Fever* | FNHTR, AHTR, TRALI (with respiratory symptoms) or a septic reaction (bacterial contamination). This could also be unrelated to the blood transfusion. | Can coexist with other symptoms such as chills, rigors, myalgia, nausea or vomiting, dyspnoea, hypotension (≥30 mmHg below the baseline) and tachycardia (heart rate of >40 bpm above the baseline).8 |
| Urticaria, hives or pruritus | Allergic TR or anaphylaxis. | Can be mild and localised or more severe with generalised urticaria. |
| Angioedema | Allergic TR or anaphylaxis. | May be preceded by a tingling sensation around the face and lips. |
| Dyspnoea or hypoxia | TRALI, TACO, TAD or a severe allergic TR. | Severe dyspnoea without shock may occur in TRALI or TACO. TAD is a diagnosis of exclusion. Therefore patients should be assessed for other causes of dyspnoea before making this diagnosis. |
| Stridor or bronchospasms | Allergic TR/anaphylaxis or TACO. | - |
| Pulmonary oedema | TACO or TRALI. | - |
| Hypotension† | AHTR, severe allergic TR, anaphylaxis, septic reaction (bacterial contamination), TRALI, TACO, TAD or a hypotensive reaction (bradykinin-mediated hypotension). This could also be unrelated to the transfusion. | Isolated hypotensive reactions are a diagnosis of exclusion and occur within an hour of transfusion, in the absence of allergic or anaphylactic symptoms. These reactions usually require no or only minor interventions.1 Patients on ACE inhibitors are at risk. The risk is higher with bedside leukofiltration. |
| Pain | FNHTR (generalised aches), AHTR (pain at the infusion site, abdomen, chest or loins) or an anaphylactic reaction (chest pain). | - |
| Severe anxiety or feelings of impending doom | AHTR or a septic reaction (bacterial contamination). | Mild anxiety is common in patients undergoing transfusions, especially for the first time. However, patients should be assessed for any TRs if anxiety develops. |
| Acute onset of bleeding | DIC can be associated with AHTR or a septic reaction (bacterial contamination). | - |
FNHTR = febrile non-haemolytic transfusion reaction; AHTR = acute haemolytic transfusion reaction; TRALI = transfusion-related acute lung injury; TR = transfusion reaction; TACO = transfusion-associated circulatory overload; TAD = transfusion-associated dyspnoea; DIC = disseminated intravascular coagulation.
Defined as a temperature of ≥38 °C and a rise of 1–2 °C from the baseline;1
Defined as a drop in systolic and/or diastolic blood pressure by >30 mmHg and a systolic blood pressure of ≤80 mm.1
Scant data exist regarding the frequency of TRs in children, which may reflect the under-recognition or under-reporting of these reactions.6 Some researchers have observed increased adverse reactions in children over two years of age, who presented with allergic reactions more frequently than adults, while FNHTRs were more common in children aged 1–2 years.7,8 It is necessary to monitor the patients closely and to treat any manifestations (e.g. skin rashes, tachycardia, tachypnoea and irritability) that develop during a transfusion as an alert to a potential TR.
Initial Clinical Assessment and Management
The main aim of the initial clinical assessment of ATRs is to identify patients with serious or life-threatening ATRs so as to provide timely and appropriate management. The management of ATRs should be guided by the manifestation and severity of the reaction, and be based on the most probable reaction subtype.1
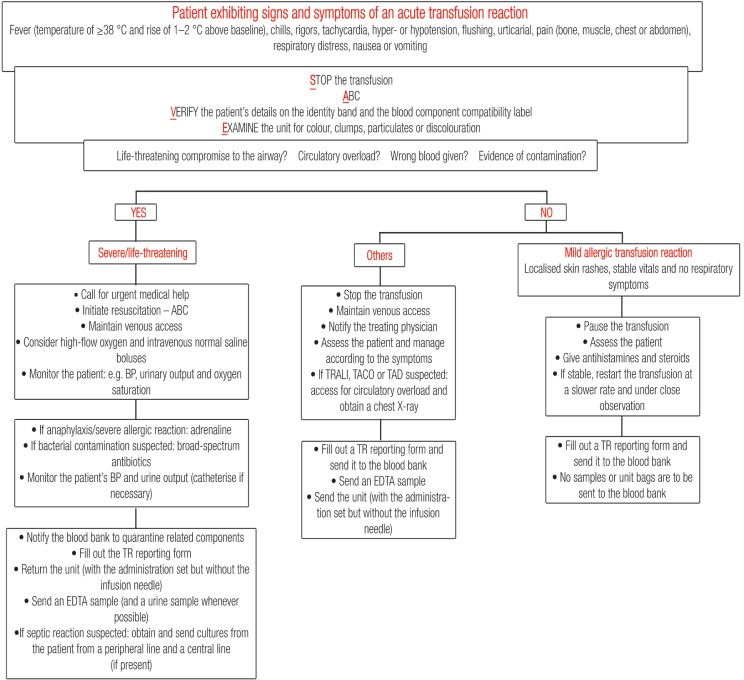
The first step to approaching any ATR is to stop the transfusion. The venous access needs to be maintained with normal saline while the patient is being assessed. The patient’s airway, breathing and circulation (ABC) need to be assessed next. If dyspnoea is present, the patient’s airway patency needs to be ensured and high-flow oxygen should be started. If the patient is wheezing without an upper airway obstruction being present, a physician should be informed immediately and the patient needs to be given a short-acting beta-2 agonist (e.g. salbutamol). If the patient is hypotensive, they should be positioned in a recumbent position with their legs elevated. The patient’s details on their identity band should then be verified to ensure that they correspond exactly with those on the blood component compatibility label. Finally, the unit undergoing transfusion needs to be examined for its colour and for the presence of any unusual clumps, particulate matter or discolouration suggestive of bacterial contamination. After these steps have been undertaken, an initial assessment is made. The SAVE acronym summarises these common steps taken in the initial assessment and management of any TR [Figure 1].
Figure 1:
Approach to acute transfusion reactions.
ABC = airway, breathing and circulation; BP = blood pressure; TRALI = transfusion-related acute lung injury; TACO = transfusion-associated circulatory overload; TAD = transfusion-associated dyspnoea; TR = transfusion reaction; EDTA = ethylene-diamine-tetra-acetic acid.
Subsequent decisions follow based on the severity of the ATR. It is very important to determine the severity of the reaction upon its onset in order to call for immediate medical help. In addition, any suspicion of ABO blood group incompatibility or bacterial contamination upon visual assessment of the unit or by the patient’s symptoms and manifestations should prompt an immediate call to the hospital blood bank. This step is undertaken so as to quarantine and possibly recall related components that may pose a risk to other recipients.
Once initial measures have been undertaken, additional therapies may be given and a decision needs to be made on whether to resume or discontinue the transfusion. Patients with mild febrile and allergic reactions, with no or limited changes in their vital signs and who are lacking other clinical symptoms, can have their transfusion resumed at a slower rate and under direct observation. However, if symptoms recur, the transfusion must be discontinued. Transfusions should not be resumed in patients with other types of ATR. Subsequent management depends on the patient’s signs and symptoms.
All TRs must be reported to the hospital blood bank regardless of the severity. A TR form should be completed and sent to the blood bank along with the implicated unit and post-transfusion samples. Units that were transfused within 24 hours prior to the reaction can be associated with the reaction, and should be retained if possible and sent to the blood bank. If there is suspicion of a septic reaction, bacterial cultures should be requested. Figure 1 summarises the steps to be undertaken in the event of an ATR and Table 3 details the steps to be undertaken after the initial assessment is performed.
Table 3:
Subsequent actions and procedures to be followed with acute transfusion reactions
| Action | Procedure |
|---|---|
| Notification |
|
| Specific management |
|
| Decision to resume or discontinue the transfusion |
|
| Reporting* |
|
ATR = acute transfusion reaction; TR = transfusion reaction; EDTA = ethylene-diamine-tetra-acetic acid.
All TRs must be reported to the hospital blood bank, regardless of severity.
Subtypes of Acute Transfusion Reactions
Febrile Non-Haemolytic Transfusion Reactions
FNHTRs result from the patient’s reaction to donor white blood cells or to biological response modifiers in the component.9 Pre-storage leukoreduction reduces the rate of FNHTRs.10 Patients may present with either mild, moderate or severe reactions.1,11 Mild FNHTRs are characterised by an oral temperature ≥38 °C and a rise of ≥1 °C from the baseline without systemic symptoms. On the other hand, moderate/severe reactions are defined by an oral temperature ≥39 °C or a rise of ≥2 °C from the baseline and/or any rise in temperature accompanied with systemic signs or symptoms (e.g. chills, rigors, myalgia, nausea or vomiting). If the patient has anxiety, tachycardia, dyspnoea, back/chest pain, haemoglobinuria, oliguria or bleeding from the intravenous (IV) site, other differential diagnoses should be considered, including bacterial contamination, an acute haemolytic transfusion reaction (AHTR) and TRALI.
Management
Classic FNHTRs are transient, resolving with symptomatic intervention including the administration of antipyretics (such as paracetamol/acetaminophen) or non-steroidal anti-inflammatory drugs (NSAIDs). Pethidine may be effective for patients with severe rigors.
If the FNHTR is mild, the transfusion can be resumed after management and under direct observation of the patient.1,12 The TR needs to be reported to the blood bank, but post-transfusion samples and the implicated unit do not need to be sent. If the symptoms recur after restarting the transfusion in a mild FNHTR, or if the FNHTR is moderate to severe, the transfusion has to be discontinued. The TR must be reported to the blood bank by sending the TR reporting form along with the post-transfusion samples and the implicated unit(s).
Prevention
Patients with significant and recurrent FNHTRs may benefit from oral paracetamol (acetaminophen) given one hour prior to subsequent transfusions.1 However, there is no firm evidence supporting this practice. If the fever persists despite premedication, leukoreduced red blood cells (RBCs) should be considered for future transfusions, if the implicated unit was not leukoreduced and if leukoreduction is locally available. If the fever persists or leukoreduction is unavailable, washed RBCs may be considered.1
Allergic Transfusion Reactions
Allergic TRs are more frequently seen with platelets and plasma than with RBCs.2 The cause of the majority of allergic reactions remains unexplained and it is not easy to identify the allergens causing the reaction. Evidence suggests that a history of allergies may predispose a patient to an allergic TR. However, both immunoglobulin A (IgA) and haptoglobin (Hp) deficiency should be suspected in the event of recurrent and/or severe allergic TRs. IgA-deficient patients have a tendency to develop anti-IgA antibodies, which may cause severe and potentially fatal anaphylactic reactions if high titres of anti-IgA antibodies are present. These reactions occur early and can be fatal if not managed appropriately.13 Hp-deficient individuals may experience anaphylaxis upon exposure to Hp in transfused products. There are data suggesting racial differences in the incidence of IgA- and Hp-deficiency.14 However, the incidence in Oman is unknown.
Allergic reactions are subclassified as mild or moderate to severe. Mild reactions are defined as localised mild skin rashes/urticaria in the absence of other symptoms. Moderate to severe reactions are defined as severe urticarial reactions with widespread lesions and/or bronchospasms, angioedema or respiratory compromise. Another possible sign is hypotension which, if present, should suggest the possibility of impending anaphylactic shock. Urticarial rashes may also be associated with pruritus, erythema, flushing, nausea/vomiting, abdominal cramps or diarrhoea.
Management
The management of allergic TRs depends on the severity of the reaction. As for all TRs, the first step is to stop the transfusion. For mild allergic TRs, practitioners should consider systemic antihistamines such as chlorphenamine.1 If symptoms improve with these initial measures, and in the absence of other symptoms, the transfusion may be resumed at a lower infusion rate after consultation with the physician and under direct observation.1,11 If the symptoms persist or recur despite the above measures, the transfusion must be terminated. The TR should still be reported to the blood bank, whether the transfusion was continued or terminated. However, post-transfusion samples and the implicated unit do not need to be sent.
In cases of moderate to severe allergic TRs, the transfusion should be halted immediately and a physician notified urgently. The patient’s ABC should be assessed and, if necessary, interventions should commence. Adrenaline (1:1000 dilution) should be administered immediately. Although both intramuscular (IM) and IV injections can be given, IM injections are more effective and circumvent any delay in establishing venous access. Adrenaline should not be withheld in thrombocytopenic patients or patients with coagulopathy. The dose can be repeated if needed at 5-min intervals depending on the patient’s blood pressure, pulse and respiratory status.
After administration of the adrenaline, the patient should be placed in a recumbent position with the lower extremities elevated. Oxygen (O2) should be administered via facemask at 8–10 L/min. Two large-bore IV cannulae should be inserted (14–16 gauges for adults and 20–22 gauges for paediatric patients). Patients should be given chlorphenamine via IM or slow IV injection.
If the patient is hypotensive, a normal saline bolus of 10–20 mL/kg (500–1,000 mL in adults) should be administered over 15 mins. Repeated boluses may be required to maintain the blood pressure. If the symptoms are severe, methylprednisolone or prednisolone should be considered.14 Finally, a bronchodilator or nebulised beta-2 agonist (e.g. salbutamol) should be considered if bronchospasm develops.
If a blood transfusion is still urgently needed and cannot be postponed, a different unit should be requested and infused as slowly as possible.
Patients with moderate/severe or recurrent allergic TRs should be tested for IgA defiency. IgA deficiency is defined as the selective deficiency of IgA (<0.05 mg/dL) in patients older than four years of age with other causes of hypogammaglobulinaemia having been excluded.15 The results should be interpreted with caution if a post-transfusion sample is tested. Isolated low IgA levels warrant testing for IgA antibodies.
Prevention
There is no evidence to support routine prophylaxis with antihistamines in patients with a previous history of mild allergic TRs and no studies have assessed the use of steroids.16 Patients with a previous mild allergic TR may receive further transfusions without premedication, and subsequent mild reactions can be managed by using systemic antihistamines and reducing the rate of the transfusion.1 However, it might be justifiable to premedicate patients with moderate to severe reactions with antihistamines. Moreover, subsequent transfusions must be performed in settings where patients can be directly observed by trained staff and where resuscitation facilities are available.1
Washed RBCs are indicated in patients with severe or recurrent allergic TRs. However, washing platelet units has its own limitations and is not available in Oman.13 IgA-deficient patients with IgA antibodies and a history of TRs should receive RBC units donated by IgA-deficient donors as the first choice of treatment, or thoroughly washed RBCs as a second choice whenever feasible. Plasma and platelets should also be from IgA-deficient donors.1,17 In view of the lack of an IgA-deficient donor database in Oman, thoroughly washed components should be requested as the most feasible option. However, urgent transfusions should not be withheld if washed components are not immediately available. That being said, the facilities and skills to manage severe allergic TRs must be present.1
Anaphylaxis
Anaphylaxis is a severe life-threatening systemic hypersensitivity characterised by rapidly developing airway, breathing and/or circulation compromises, usually associated with skin and mucosal changes.1 Patients may have bronchospasms, stridor, angioedema, urticaria, hypotension or other symptoms. This reaction shares a common pathophysiology with allergic TRs. Anaphylactic shock has rarely been reported in complement component 4 (C4)-deficient patients after blood transfusions.18,19
Management
The management of anaphylactic reactions is the same as for moderate to severe allergic reactions as outlined above.
Prevention
Premedication with steroids and antihistamines should be considered to prevent recurrence in patients with a history of anaphylactic reactions. Subsequent transfusions should only be carried out where patients can be observed directly and where staff trained in managing anaphylaxis are available.1
Septic Reactions (Bacterial Contamination)
Bacterial contamination of a blood component can occur either at the time of donation (due to subclinical donor bacteremia or non-adherence to aseptic techniques during the phlebotomy) or during blood component preparation, storage or handling. This can cause a septic ATR when the component is tranfused to a recipient. Both Gram-negative and -positive bacteria have been implicated.12 Although these reactions can occur with any blood component, platelets are the most commonly implicated since they are stored at room temperature which allows faster bacterial growth.4 When the implicated unit is transfused, the patient rapidly develops acute manifestations.20
Bacterial contamination should be considered if the patient presents with a moderate or severe fever defined as a temperature ≥39 °C or a temperature rise of ≥2 °C from baseline. Bacterial contamination should also be suspected if any fever is accompanied with systemic symptoms such as chills, rigors, severe hypotension, shock, dyspnoea, myalgia, nausea or vomiting.1 Bacterial contamination should also be considered if the fever is persistent or unresponsive to symptomatic measures.
Patients may also present with isolated hypotension. Other symptoms, such as pain in the chest, back, abdomen or transfusion site, may confuse this reaction with an AHTR.21 Symptoms may develop rapidly or within four hours of the transfusion.21 The severity of the reaction is influenced by the type of bacteria involved, bacterial load and the recipient’s clinical status. Shock and disseminated intravascular coagulation (DIC) can occur if a Gram-negative bacterially infected unit with high levels of endotoxin was transfused. There should be increased caution in patients receiving blood transfusions while under anaesthesia (where manifestations may be masked) in patients with underlying fevers and in those who have been pre-medicated with antipyretics, as they may not develop a high-grade temperature as a warning sign.
Management
The management of patients suspected of a septic reaction begins with halting the transfusion. The implicated unit should be inspected for any discolouration, abnormal particles, clumps or signs of leakage. If any of these are present, the hospital blood bank must be notified immediately so its staff may recall and quarantine any other components from the implicated donation. A TR form must be sent to the blood bank along with the patient’s post-transfusion samples and the implicated unit(s). The implicated unit should be cultured for aerobic and anaerobic organisms. In addition, blood cultures should be drawn from the patient and broad-spectrum IV antibiotics should be started. Blood cultures from the implicated unit should be examined for aerobic and anaerobic organisms. If the cultures are positive, all related components must be discarded and further antibiotic coverage should be determined based on the organism identified.
Prevention
Future transfusions do not require any precautions. However, if there is a high frequency of these reactions, then a critical review of the blood collection process and storage facilities is indicated. The routine visual inspection of any unit at the time of laboratory processing and at the bedside prior to blood transfusion is crucial. All personnel must be trained to perform these visual assessments. The normal appearance of a unit does not exclude bacterial contamination.1 Therefore, if this type of reaction is clinically suspected, action must be undertaken as detailed above.
Transfusion-Related Acute Lung Injury
TRALI is defined as noncardiogenic pulmonary oedema following blood transfusion. Although all blood components have been implicated in TRALI, frozen plasma is the most common.22 However, small volumes of plasma (e.g. in RBC units) can cause TRALI if there are high levels of HLA or human neutrophil antigen (HNA) antibodies in the implicated unit and the patient has susceptible risk factors.
The diagnosis of TRALI must fulfill the following criteria: (1) acute respiratory distress during the transfusion or within six hours of completing the transfusion; (2) hypoxaemia, defined by partial pressure of O2 (PaO2) in arterial blood divided by the fraction of inspired O2 (FiO2) ≤300 mmHg, blood oxygen saturation (SPO2) <90% on room air or other clinical evidence of hypoxaemia; (3) bilateral pulmonary infiltrates on a frontal chest X-ray (CXR); (4) no evidence of transfusion-associated circulatory overload (TACO) with pulmonary arterial wedge pressure ≤18 mmHg or central venous pressure ≤15 mmHg; (5) no pre-existing acute lung injury (ALI), and (6) no temporal relationship to an alternative risk factor of ALI, including aspiration, severe sepsis, pneumonia, multiple fractures, pancreatitis, shock, cardiopulmonary bypass, burn injuries, toxic inhalation, lung contusion, drug overdose or near drowning.24 If the first five criteria are met but there is a temporal association with another risk factor for ALI, the case is categorised as possible TRALI.
The primary mechanism of TRALI is the accumulation and activation of neutrophils within the pulmonary vascular endothelium. There are two existing models of TRALI. The two-hit model supports the hypothesis that TRALI occurs due to the interaction of two factors. The first factor is an underlying condition (e.g. critical illness, surgery, mechanical ventilation or severe sepsis) that may result in neutrophil priming and adherence to the pulmonary endothelium.25 The second factor is a mediator within the transfused component which activates the primed neutrophils and endothelial cells, inducing capillary leakage and pulmonary oedema.23,26 This can be caused by donor HLA or HNA antibodies that target recipient antigens, or bioactive lipids and soluble CD40 ligands that accumulate during the storage of cellular components.27 The second model, known as the threshold model, is based on the hypothesis that both the recipient and transfusion factors act together to overcome a particular recipient’s threshold and induce TRALI.24,27
Manifestations of TRALI can occur during the transfusion or in the six hours after completion of the transfusion, although they usually occur within two hours.1,28 Dyspnoea with acute hypoxia usually dominates the clinical picture. Other features include tachycardia, rigors, fever, hypothermia and hypotension.28 TRALI remains a clinical diagnosis.29 Although TACO is in the differential diagnosis, certain features help to differentiate TRALI from TACO [Table 4]. A clinical examination and CXR are crucial to rule out any evidence of cardiac overload. The severity of TRALI can range from mild, requiring supplemental O2, to severe, necessitating mechanical ventilation. With respiratory support, most patients with TRALI recover in 24–72 hours, however 5–25% of cases are fatal.30,31
Table 4:
Helpful features in differentiating transfusion-related acute lung injury from transfusion-associated circulatory overload
| Feature | TRALI | TACO |
|---|---|---|
| Patient characteristics | May occur in any patient but are more frequently reported in haematological and surgical patients | May occur at any age, but characteristically occurs at >70 years |
| Type of component | Usually plasma or platelets | Any |
| Speed of onset | During the transfusion or within six hours of completion, usually within the first two hours | During the transfusion or within six hours of completion of the transfusion |
| Dyspnoea | Present | Present |
| Fever | Present | Usually absent |
| Blood pressure | Usually hypotension | Usually hypertension |
| Oxygen saturation | Reduced | Reduced |
| JVP | Normal | Raised |
| Full blood count | Leukopenia with neutropenia and monocytopenia followed by neutrophil leukocytosis and thrombocytopenia | No specific changes |
| CXR | Pulmonary oedema with normal heart size | Pulmonary oedema with cardiomegaly |
| ECHO | Normal left ventricular function* | Normal or decreased left ventricular function |
| Pulmonary wedge pressure | Low | Raised |
| Other tests | Normal BNP levels | Raised BNP levels |
| Response to fluid load | Improves | Worsens |
| Response to diuretics | Worsens | Improves |
TRALI = transfusion-related acute lung injury; TACO = transfusion-associated circulatory overload; JVP = jugular venous pressure; CXR = chest X-ray; ECHO = echocardiogram; BNP = B-natriuretic peptide.
Decreased left ventricular function does not exclude TRALI.
Adapted from: Tinegate H, Birchall J, Gray A, Haggas R, Massey E, Norfolk D, et al. Guideline on the investigation and management of acute transfusion reactions: Prepared by the BCSH Blood Transfusion Task Force.1 and Vlaar AP, Juffermans NP. Transfusion-related acute lung injury: A clinical review.23
Management
The management of suspected TRALI requires immediate cessation of the transfusion. The patient’s airway should be ensured, the arterial O2 saturation level obtained and high-flow O2 should be begun. Mechanical ventilation should be considered. The patient should be assessed clinically for cardiac overload and a CXR should be obtained. The rest of the management should be supportive. Although some case reports advocate the use of steroids, no evidence supports their use.16 The reaction must be reported to the blood bank to quarantine or recall the rest of components from the implicated donor and to make decisions regarding donor deferral. The donor deferral decision must be made by an expert in transfusion medicine. Currently, tests to investigate an implicated donor are not available in Oman, but should be considered whenever made available.
Prevention
No precautions are needed for future transfusions, providing that TACO has been ruled out. There are two groups of donors likely to be implicated in antibody-mediated TRALI: multiparous females and donors with a history of transfusions. In the light of this, some countries have undertaken policies which only accept male donors for plasma and platelet donations in order to minimise the risk of TRALI.11
Transfusion-Associated Circulatory Overload
TACO is a condition characterised by left ventricular failure and pulmonary oedema due to fluid overload. This occurs either during transfusion or within the following six hours, in transfusions with a rapid infusion rate or a high volume of transfused products. Risk factors include extremes of age (patients >60 years or <3 years), pre-existing cardiac and/or renal dysfunction, transfusions after an acute myocardial infarction, plasma transfusions, pre-existing positive fluid balances in the 24 hours prior to the transfusion and large-volume transfusions.32,33
Patients present with acute respiratory distress, dyspnoea, cyanosis, orthopnea, hypoxia, increase in systolic blood pressure (>20 mmHg above the baseline) and signs of cardiac overload (jugular venous pressure elevation and bilateral crepitation).1 Patients tend to respond to diuretics and worsen with fluid boluses. A CXR will show pulmonary oedema and cardiomegaly and an echocardiogram will be abnormal. There is some evidence suggesting that B-type natriuretic peptide (BNP) is a sensitive and specific marker of TACO.30 Until further evidence is available, BNP levels should be used as an adjunct marker to other features of volume overload in confirming the diagnosis of TACO.
Management
In suspected cases of TACO, management should begin by stopping the transfusion. Practitioners should ensure a patent airway, obtain the arterial O2 saturation level and start high-flow O2. Mechanical ventilation should be considered. In addition, cardiac overload should be assessed and a CXR obtained. Diuretics should be administered, such as furosemide via the IV route.
Prevention
In order to prevent recurrence, pre- or mid-transfusion diuretics should be considered in patients with a prior history or risk factors of TACO. Transfusion should recommence one unit at a time, with the patient being assessed after each unit. In high-risk patients, the transfusion can be performed at a slow rate. In patients with congestive heart failure (CHF), the transfusion should be postponed if possible until the CHF is deemed to be under control. When a transfusion is needed during an episode of CHF, it is important to request a split RBC unit to reduce the volume of infused blood in the four-hour time frame.33
Transfusion-Associated Dyspnoea
Transfusion-associated dyspnoea (TAD) is defined as any respiratory distress within 24 hours of transfusion that does not meet the criteria of TRALI, TACO or an allergic reaction and that cannot be explained by an underlying condition or any other known cause.34 TAD remains a clinical diagnosis and a diagnosis of exclusion.
Management
TAD should be managed by stopping the transfusion immediately. Management is supportive, after the patient has been assessed for potential causes of dyspnoea, including an AHTR, allergic TR, TRALI or TACO.
Prevention
No precautions are needed for future transfusions, provided that other transfusion-related causes of dyspnoea have been ruled out.
Acute Haemolytic Transfusion Reactions
An AHTR occurs secondarily to a mismatched transfusion due to ABO incompatibility. This can occur as a result of clerical error, improper sample labelling or an error in patient testing or identification. It can also occur as an anamnestic response caused by non-ABO antibodies in alloimmuised patients due to a prior pregnancy or transfusion. The latter can occur in alloimmunised patients with sickle cell disease, among others, who develop an anamnestic response of pre-existing antibodies following the transfusion of non-phenotype-matched RBC unit(s), leading to acute haemolysis. The binding of the antibodies to donor RBCs leads to RBC lysis, activation of complement and coagulation pathways and cytokine release. Complement activation induces haemolysis, while activation of the coagulation cascade and thrombin generation predisposes the patient to DIC.
The most common presentation of an AHTR is significant fever and chills, defined as a sustained fever ≥39 °C or a rise of ≥2 °C from baseline and/or a fever accompanied by systemic symptoms such as chills, rigors, myalgia, nausea or vomiting. Alternatively, fevers which are unresponsive to symptomatic measures should be considered as a sign of a possible AHTR.
The classic presenting features are intravascular haemolysis (e.g. a falling haemoglobin level with a positive direct antiglobulin test (DAT), a rise in serum lactate dehydrogenase and haemoglobinurea), pain at the infusion site, flank pain, feelings of impending doom, anxiety and facial flushing. Patients may also present with isolated hypotension. If this reaction is not recognised, patients can progress to acute renal failure, DIC and death.
Management
The management of suspected AHTRs should begin by stopping the transfusion immediately, establishing a new IV line and starting 0.9% normal saline. Saline boluses should be given to maintain urine output. The attending physician should be notified immediately and urine alkalinisation should be considered in the event of renal failure. As soon as a reaction is suspected, the hospital blood bank should be immediately informed by telephone as well as via the regular reaction reporting procedures. This is crucial in order to quarantine and recall other components that could have been issued to other patients. A post-transfusion blood sample and the first voided urine sample should be collected and sent to the blood bank, along with the implicated unit and the TR report. Coagulation, renal and liver function tests should be considered.
Prevention
Prevention of AHTRs is heavily related to adherence to clinical and laboratory policies with regards to patient identification and testing. At the time of the blood order/request proper patient identification and bedside sample labelling is a necessity to avoid sample mislabelling. In addition, and prior to commencing the transfusion, proper verification of the patient’s identity, the unit compatibility label and the request details is critical to detect errors and any ABO incompatibility. This should be performed at the patient’s bedside and in the presence of two nurses. However, it is also crucial to remember that non-ABO antibodies can be implicated in an AHTR. Therefore, pre-transfusion testing in the blood bank must include an antibody screen. If the antibody screen is positive, antibody identification should be carried out. Units selected for the transfusion should be antigen-negative for the implicated antibody and must be serologically cross-match compatible with the patient’s plasma using an indirect antiglobulin method.
Hypotensive Reactions
Hypotensive reactions are defined as hypotensive episodes with an isolated fall in systolic blood pressure of ≥30 mm occurring during or within one hour of completing the transfusion and a systolic blood pressure ≤80 mm, without any symptoms to suggest an allergic or anaphylactic reaction, other types of ATRs or other causes hypotension (e.g. blood loss).1 This reaction has been associated with bradykinin release upon exposure to filtered blood and a history of angiotensin-converting enzyme (ACE) inhibitors should therefore be checked.
Management
In cases of hypotensive reactions, the transfusion should be stopped. The patient should be placed in a recumbent position with their legs elevated, or in the recovery position if they are unconscious or nauseated. Normal saline boluses should be administered via an IV route. The transfusion should not be restarted. The reaction should be reported and the patient should be investigated as per the TR reporting format.
Prevention
In order to prevent further recurrence, patients should be given a trial of washed RBCs. In cases where ACE inhibitors have been implicated, halting the ACE inhibitors before a blood transfusion should be considered if clinically safe, otherwise alternative antihypertensives should be considered.1,12
Reporting Transfusion Reactions
The reporting of TRs to hospital blood banks is essential. It directs immediate action when haemolytic TRs, TRALI or septic reactions are suspected, and also contributes to the haemovigilance system. Reporting instances of TRs may prompt recall of related components from the same donation and may result in donor deferrals. For some reactions, action should be undertaken immediately so as to prevent harming other patients. The two classic reactions are haemolytic TRs and septic reactions.
The process of reporting TRs to the blood bank should follow the local procedures within an institution. However, there must be a standardised procedure in the blood banks on how to handle TR investigations. Reports should be reviewed by an expert in transfusion medicine in order to ensure a proper evaluation, management and the final classification of the reaction type.
Laboratory Investigations
The primary work-up for samples suspected of causing a TR is standardised. All units returned to the blood bank should undergo a second visual check for labelling, integrity and any evidence of haemolysis or bacterial contamination. In the event of a suspected haemolytic or septic reaction, the blood bank should recall and quarantine any associated components.
Reports of moderate or severe TRs should prompt testing to rule out AHTRs. This includes repeat ABO and rhesus (D) grouping of both the patient sample and the returned unit; repeat antibody screening of the pre- and post-transfusion samples; repeat cross-matching of both samples with the returned unit, and the performance of a DAT on the post-transfusion blood sample.1 In addition, the first voided post-transfusion urine sample should be assessed for the presence of haemoglobinuria. In addition to the above, and if there is any suspicion of bacterial contamination, blood cultures should be obtained from the patient and the implicated unit. A protocol should be established regarding the aseptic sampling of the components for cultures to minimise the risk of contamination.
Other basic investigations to be considered for moderate and severe TRs include a complete blood count, a coagulation profile and renal and liver function tests.
Haemovigilance
A haemovigilance scheme is a monitoring programme which ensure the safety of transfusions. It allows the evaluation of trends, identification of any practice concerns or training needs, the introduction of guidelines and the evaluation of corrective actions taken to reduce transfusion risks. Haemovigilance programmes have been developed in many countries and include either mandatory or voluntary reporting schemes. As part of a haemovigilance programme, a uniform and systematic method for the reporting and evaluation of TRs should be implemented.
Haemovigilance programmes should be overseen locally within individual institutions by the hospital transfusion committees. In addition, a haemovigilance programme needs to exist at a national level and all reported TRs should be reviewed by a national haemovigilance committee.
Conclusion
The prompt recognition, management and reporting of TRs are important aspects in ensuring patient safety during and after transfusions. TRs may have overlapping manifestations and it is therefore imperative that medical personnel be aware of the different types of reactions that can occur. This requires the continuous education of all healthcare professionals involved in the transfusion process as well as the development of standardised procedures. This paper has summarised the available literature on the recognition and management of different ATRs in order to provide local consensus guidelines for Oman. A national haemovigilance programme is required to set standards as well as ensuring training requirements on the proper handling and management of these reactions. Once such a programme has been developed in Oman, these guidelines may be used as a comprehensive source of information and guidance.
Appendix
A list of common medications that can be used for the management of different ATRs is available in the appendix after the references. This appendix should be used as a reference only.
Appendix:
List of common medications that can be used for the management of different acute transfusion reactions
| Medication | Adult doses35 | Paediatric and adolescent doses36 |
|---|---|---|
| Adrenaline, IM [1:1000 dilution, 1 mg/mL] | 0.5 mL (500 mcg)* | <6 years: 0.15 mL (150 mcg)* |
| 6–12 years: 0.3 mL (300 mcg)* | ||
| 12–18 years: 0.5 mL (500 mcg)* | ||
| Only 0.3 mL (300 mcg) should be given if the adolescent is small or pre-pubertal | ||
| Chlorphenamine, PO | 4 mg† | 1 month–2 years: 1 mg‡ |
| Maximum of 24 mg daily for adults and 12 mg daily for the elderly | 2–6 years: 1 mg† | |
| Maximum of 6 mg daily | ||
| 6–12 years: 2 mg† | ||
| Maximum of 12 mg daily | ||
| 12–18 years: 4 mg† | ||
| Maximum of 24 mg daily | ||
| Chlorphenamine, IM or slow IV | 10 mg† | 1–6 months: 250 mcg/kg/dose§ |
| Maximum of 40 mg daily | Maximum of 2.5 mg per dose | |
| 6 months–6 years: 2.5 mg§ | ||
| 6–12 years: 5 mg§ | ||
| 12–18 years: 10 mg§ | ||
| Furosemide | 20–50 mg, via IM or slow IV | 0.5–1.0 mg/kg, via IV only |
| Hydrocortisone, IM/IV | 100–500 mg¶ | <6 months: 25 mg¶ |
| 6 months–6 years: 50 mg¶ | ||
| 6–12 years: 100 mg¶ | ||
| 12–18 years: 200 mg¶ | ||
| Paracetamol/acetaminophen, PO | 500–1,000 mg† | 10–15 mg/kg† |
| Maximum of 4 g daily | Maximum of 60 mg/kg/day daily | |
| Paracetamol/acetaminophen, IV | <50 kg: 10–15 mg/kg/dose† | <10 kg: 7.5 mg/kg/dose† |
| Maximum of 60 mg/kg daily | Maximum of 30 mg/kg daily | |
| >50 kg: 1,000 mg† | 10–50 kg: 10–15 mg/kg/dose† | |
| Maximum of 4 g daily | Maximum of 60 mg/kg daily | |
| Pethidine, slow IV | 25–50 mg | Not recommended |
| Salbutamol, nebulised | 5 mg†† | <5 years: 2.5 mg†† |
| 6–12 years: 2.5–5 mg†† | ||
| 12–18 years: 5 mg†† |
IM = intramuscular; PO = per os; IV = intravenous.
Doses may be repeated if necessary at 5-min intervals according to the patient’s blood pressure, pulse and respiratory function.
Doses may be repeated every 4–6 hours.
Doses may be repeated if required up to two times in 24 hours.
Doses may be repeated if required up to four times in 24 hours.
Doses may be repeated if required up to three times in 24 hours and adjusted as per the response.
Repeat at 20–30 min intervals as necessary.
References
- 1.Tinegate H, Birchall J, Gray A, Haggas R, Massey E, Norfolk D, et al. Guideline on the investigation and management of acute transfusion reactions: Prepared by the BCSH Blood Transfusion Task Force. Br J Haematol. 2012;159:143–53. doi: 10.1111/bjh.12017. [DOI] [PubMed] [Google Scholar]
- 2.Knowles S, Cohen H, Watt A, Poles D, Jones H, Davies T, et al. Serious Hazards of Transfusion (SHOT): Annual Report 2010. From: www.shotuk.org/wp-content/uploads/2011/07/SHOT-2010-Report.pdf Accessed: Jan 2014. [Google Scholar]
- 3.Hillyer CD, Silberstein MD, Ness PM, Anderson KN. Blood Banking and Transfusion Medicine: Basic Principles and Practice. 1st ed. Oxford, UK: Churchill Livingstone; pp. 265–361. [Google Scholar]
- 4.Taylor C, Cohen H, Mold D, Jones H, Asher D, Cawley C, et al. Serious Hazards of Transfusion (SHOT): Annual 2009 Report. From: www.shotuk.org/wp-content/uploads/2010/03/SHOT-Report-2008.pdf Accessed: Jan 2014.
- 5.British Committee for Standards in Haematology Guideline on the Administration of Blood Components. From: www.bcshguidelines.com/documents/Admin_blood_components_bcsh_05012010.pdf Accessed: Mar 2014.
- 6.Gauvin F, Lacroix J, Robillard P, Lapointe H, Hume H. Acute transfusion reactions in the pediatric intensive care unit. Transfusion. 2006;46:1899–908. doi: 10.1111/j.1537-2995.2006.00995.x. [DOI] [PubMed] [Google Scholar]
- 7.Slonim AD, Joseph JG, Turenne WM, Sharangpani A, Luban NL. Blood transfusions in children: A multi-institutional analysis of practices and complications. Transfusion. 2008;48:73–80. doi: 10.1111/j.1537-2995.2007.01484.x. [DOI] [PubMed] [Google Scholar]
- 8.Pedrosa AK, Pinto FJ, Lins LD, Deus GM. Blood transfusion reactions in children: Associated factors. J Pediatr (Rio J) 2013;89:400–6. doi: 10.1016/j.jped.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Heddle NM, Klama L, Meyer R, Walker I, Boshkov L, Roberts R, et al. A randomized controlled trial comparing plasma removal with white cell reduction to prevent reactions to platelets. Transfusion. 1999;39:231–8. doi: 10.1046/j.1537-2995.1999.39399219278.x. [DOI] [PubMed] [Google Scholar]
- 10.Paglino JC, Pomper GJ, Fisch GS, Champion MH, Snyder EL. Reduction of febrile but not allergic reactions to RBCs and platelets after conversion to universal prestorage leukoreduction. Transfusion. 2004;44:16–24. doi: 10.1046/j.0041-1132.2004.00608.x. [DOI] [PubMed] [Google Scholar]
- 11.British Columbia Provincial Blood Coordinating Office, Provincial Health Services Authority Transfusion Medicine Medical Policy Manual. From: www.pbco.ca/index.php?option=com_content&task=category&id=58&Itemid=96 Accessed: May 2014.
- 12.Callum JL, Lin Y, Pinkerton PH. Bloody Easy 3: Blood Transfusions, Blood Alternatives and Transfusion Reactions: A Guide to Transfusion Medicine. 3rd ed. Toronto, Canada: Ontario Regional Blood Coordinating Network; 2011. [Google Scholar]
- 13.Lerner NB, Refaai MA, Blumberg N. Red cell transfusion. In: Kaushansky K, Lichtman MA, Beutler E, Kipps TJ, Seligsohn U, Prchal JT, editors. Williams Hematology. 8th ed. Columbus, Ohio: McGraw Hill Professional; 2010. pp. 2287–300. [Google Scholar]
- 14.Hirayama F. Current understanding of allergic transfusion reactions: Incidence, pathogenesis, laboratory tests, prevention and treatment. Brit J Haematol. 2013;160:434–44. doi: 10.1111/bjh.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.European Society for Immunodeficiencies Definition of IgA Deficiency. From: www.esid.org/content/download/198/910/file/IgA%20Deficiency.doc Accessed: Mar 2014. [Google Scholar]
- 16.Kennedy LD, Case LD, Hurd DD, Cruz JM, Pomper GJ. A prospective, randomized, double-blind controlled trial of acetaminophen and diphenhydramine pretransfusion medication versus placebo for the prevention of transfusion reactions. Transfusion. 2008;48:2285–91. doi: 10.1111/j.1537-2995.2008.01858.x. [DOI] [PubMed] [Google Scholar]
- 17.Sandler SG. How I manage patients suspected of having had an IgA anaphylactic transfusion reaction. Transfusion. 2006;46:10–13. doi: 10.1111/j.1537-2995.2006.00686.x. [DOI] [PubMed] [Google Scholar]
- 18.Lambin P, Le Pennec PY, Hauptmann G, Desaint O, Habibi B, Salmon C. Adverse transfusion reactions associated with a precipitating anti-C4 antibody of anti-Rodgers specificity. Vox Sang. 1984;47:242–9. doi: 10.1111/j.1423-0410.1984.tb01592.x. [DOI] [PubMed] [Google Scholar]
- 19.Westhoff CM, Sipherd BD, Wylie DE, Toalson LD. Severe anaphylactic reactions following transfusions of platelets to a patient with anti-Ch. Transfusion. 1992;32:576–9. doi: 10.1046/j.1537-2995.1992.32692367205.x. [DOI] [PubMed] [Google Scholar]
- 20.Blajchman M, Goldman M. Bacterial contamination of platelet concentrates: Incidence, significance, and prevention. Semin Hematol. 2011;38:20–6. doi: 10.1016/S0037-1963(01)90120-9. [DOI] [PubMed] [Google Scholar]
- 21.Hewitt P. Bacterial contamination. In: Murphy MF, Pamphilon DH, editors. Practical Transfusion Medicine. 3rd ed. Oxford, UK: Wiley-Blackwell; 2009. pp. 146–52. [Google Scholar]
- 22.Wallis JP, Sachs UJH. Transfusion-related acute lung injury. In: Simon TL, Snyder EL, Solheim BG, Stowell CP, Strauss RG, Solheim BG, et al., editors. Rossi’s Principles of Transfusion Medicine. 4th ed. Bethesda, Maryland: Wiley-Blackwell; 2009. pp. 870–84. [Google Scholar]
- 23.Vlaar AP, Juffermans NP. Transfusion-related acute lung injury: A clinical review. Lancet. 2013;382:984–94. doi: 10.1016/S0140-6736(12)62197-7. [DOI] [PubMed] [Google Scholar]
- 24.Kleinman S, Caulfield T, Chan P, Davenport R, McFarland J, McPhedran S, et al. Toward an understanding of transfusion related acute lung injury: Statement of a consensus panel. Transfusion. 2004;44:1774–89. doi: 10.1111/j.0041-1132.2004.04347.x. [DOI] [PubMed] [Google Scholar]
- 25.Bux J, Sachs UJ. The pathogenesis of transfusion-related acute lung injury (TRALI) Brit J Haematol. 2007;136:788–99. doi: 10.1111/j.1365-2141.2007.06492.x. [DOI] [PubMed] [Google Scholar]
- 26.Silliman CC. The two-event model of transfusion-related acute lung injury. Crit Care Med. 2006;34:S124–31. doi: 10.1097/01.CCM.0000214292.62276.8E. [DOI] [PubMed] [Google Scholar]
- 27.Sachs UJ. Recent insights into the mechanism of transfusion-related acute lung injury. Curr Opin Hematol. 2011;18:436–42. doi: 10.1097/MOH.0b013e32834bab01. [DOI] [PubMed] [Google Scholar]
- 28.Skeate RC, Eastlund T. Distinguishing between transfusion related acute lung injury and transfusion associated circulatory overload. Curr Opin Hematol. 2007;14:682–7. doi: 10.1097/MOH.0b013e3282ef195a. [DOI] [PubMed] [Google Scholar]
- 29.Li G, Daniels CE, Kojicic M, Krpata T, Wilson GA, Winters JL, et al. The accuracy of natriuretic peptides (brain natriuretic peptide and N-terminal pro-brain natriuretic) in the differentiation between transfusion-related acute lung injury and transfusion-related circulatory overload in the critically ill. Transfusion. 2009;49:13–20. doi: 10.1111/j.1537-2995.2008.01941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silliman CC, McLaughlin NJ. Transfusion-related acute lung injury. Blood Rev. 2006;20:139–59. doi: 10.1016/j.blre.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Sheppard CA, Lögdberg LE, Zimring JC, Hillyer CD. Transfusion-related acute lung injury. Hematol Oncol Clin North Am. 2007;21:163–76. doi: 10.1016/j.hoc.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 32.Growe GH, Petraszko TR, Bigham M. The approach taken to reducing the risk of transfusion related acute lung injury in Canada. Asian J Transfus Sci. 2008;2:84–6. doi: 10.4103/0973-6247.42696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alam A, Lin Y, Lima A, Hansen M, Callum JL. The prevention of transfusion-associated circulatory overload. Transfusion Med Rev. 2013;27:105–12. doi: 10.1016/j.tmrv.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 34.International Haemovigilance Network/International Society for Blood Transfusion Proposed Standard Definitions for Surveillance of Non-infectious Adverse Transfusion Reactions. From: www.ihn-org.com/wp-content/uploads/2011/06/ISBT-definitions-for-non-infectious-transfusion-reactions.pdf Accessed: Mar 2014.
- 35.British Medical Association, Royal Pharmaceutical Society of Great Britain . British National Formulary. 66th ed. London: Pharmaceutical Press; 2013. [Google Scholar]
- 36.British Medical Association, Royal Pharmaceutical Society, Royal College of Paediatrics & Child Health, Neonatal & Paediatric Pharmacists Group . British National Formulary for Children 2014–2015. London: Pharmaceutical Press; 2014. [Google Scholar]